Reduced Expression of Urokinase Plasminogen Activator in Brown Adipose Tissue of Obese Mouse Models
Abstract
1. Introduction
2. Results
2.1. The Different Presentations of Obesity and Metabolic Profiles in C57BL/6J and BALB/c Mice on HFD
2.2. Circulating uPA, Soluble uPAR (suPAR) and Plasminogen Activator Inhibitor-1 (PAI-1) Levels and Adiponectin in C57BL/6J and BALB/c Obese Mice
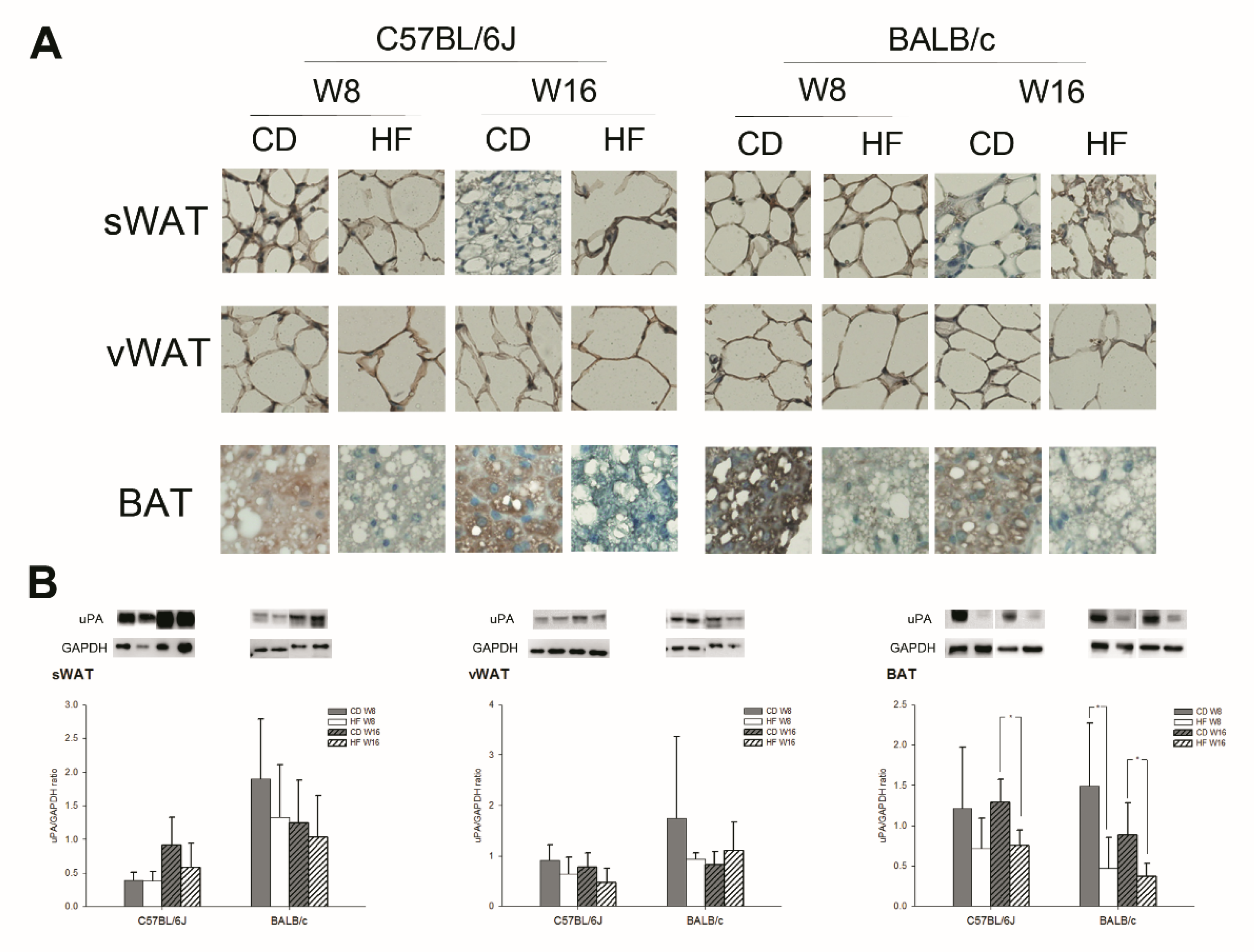
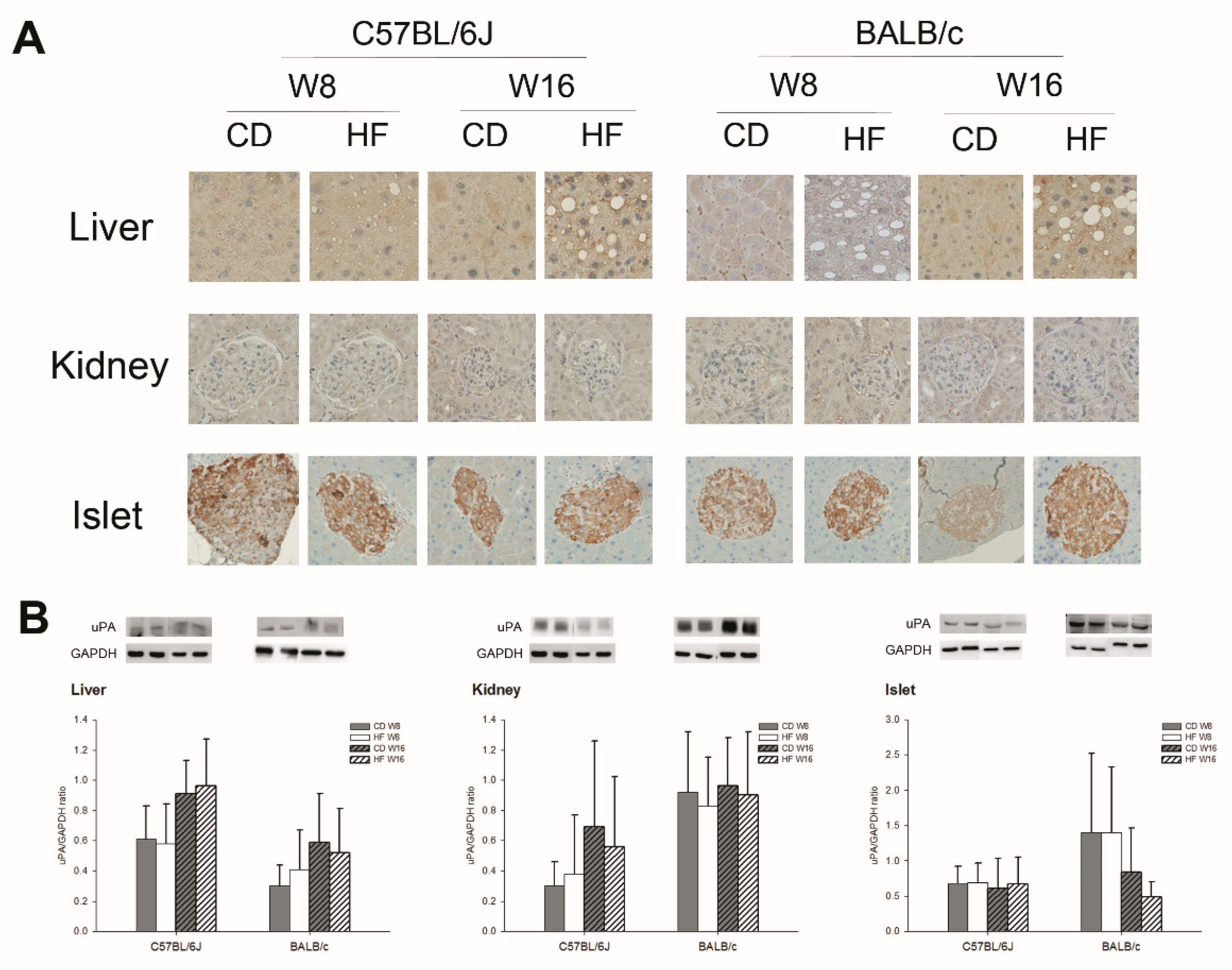
2.3. uPA Expression on Subcutaneous White Adipose Tissue (sWAT), Visceral White Adipose Tissue (vWAT), BAT, Liver, Kidney, and Pancreas in C57BL/6J and BALB/c Obese Mice
3. Discussion
4. Materials and Methods
4.1. Induced Obese Mouse Model
4.2. Measurement of uPA, suPAR, PAI-1 and Metabolic Biochemistry
4.3. IHC Stain and WB of sWAT, vWAT, BAT, Liver, Kidney, and Islet
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, K.C. Obesity and its related diseases in Taiwan. Obes. Rev. 2008, 9 (Suppl. S1), 32–34. [Google Scholar] [CrossRef]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef]
- Tsuboi, N.; Utsunomiya, Y.; Hosoya, T. Obesity-related glomerulopathy and the nephron complement. Nephrol. Dial. Transplant. 2013, 28 (Suppl. S4), 108–113. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Zafrir, B. Brown adipose tissue: Research milestones of a potential player in human energy balance and obesity. Horm. Metab. Res. 2013, 45, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.L.; Westermark, G.T.; Westermark, P.; Kahn, S.E. Islet amyloid: A critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 3629–3643. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef]
- Goossens, G.H. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 2008, 94, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Buechler, C.; Krautbauer, S.; Eisinger, K. Adipose tissue fibrosis. World J. Diabetes 2015, 6, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Mondino, A.; Blasi, F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 2004, 25, 450–455. [Google Scholar] [CrossRef]
- Gyetko, M.R.; Libre, E.A.; Fuller, J.A.; Chen, G.H.; Toews, G. Urokinase is required for T lymphocyte proliferation and activation in vitro. J. Lab. Clin. Med. 1999, 133, 274–288. [Google Scholar] [CrossRef]
- Kawao, N.; Nagai, N.; Tamura, Y.; Horiuchi, Y.; Okumoto, K.; Okada, K.; Suzuki, Y.; Umemura, K.; Yano, M.; Ueshima, S.; et al. Urokinase-type plasminogen activator and plasminogen mediate activation of macrophage phagocytosis during liver repair in vivo. Thromb. Haemost. 2012, 107, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Chang, L.C.; Lin, Y.F.; Hung, Y.J.; Pei, D.; Chu, N.F.; Chen, J.S. Urokinase plasminogen activator receptor and its soluble form in common biopsy-proven kidney diseases and in staging of diabetic nephropathy. Clin. Biochem. 2015, 48, 1324–1329. [Google Scholar] [CrossRef]
- Stewart, D.; Fulton, W.B.; Wilson, C.; Monitto, C.L.; Paidas, C.N.; Reeves, R.H.; De Maio, A. Genetic contribution to the septic response in a mouse model. Shock 2002, 18, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004, 22, 460–466. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Jovicic, N.; Jeftic, I.; Jovanovic, I.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS ONE 2015, 10, e0134089. [Google Scholar] [CrossRef]
- Mazar, A.P.; Henkin, J.; Goldfarb, R.H. The urokinase plasminogen activator system in cancer: Implications for tumor angiogenesis and metastasis. Angiogenesis 1999, 3, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Pawlak, D.; Myśliwiec, M. Urokinase-type plasminogen activator and metalloproteinase-2 are independently related to the carotid atherosclerosis in haemodialysis patients. Thromb. Res. 2008, 121, 543–548. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Glogar, D.; Weidinger, F.; Domanovits, H.; Laggner, A.; Wojta, J.; Zorn, G.; Iordanova, N.; Huber, K. Association between plasmin activation system and intravascular ultrasound signs of plaque instability in patients with unstable angina and non-st-segment elevation myocardial infarction. Am. Heart J. 2004, 147, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Dellas, C.; Schremmer, C.; Hasenfuss, G.; Konstantinides, S.V.; Schäfer, K. Lack of urokinase plasminogen activator promotes progression and instability of atherosclerotic lesions in apolipoprotein E-knockout mice. Thromb. Haemost. 2007, 98, 220–227. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, X.; Lu, X.; Chen, G.; Ye, X.; Huang, J. Evaluation of plasma urokinase-type plasminogen activator and urokinase-type plasminogen-activator receptor in patients with acute and chronic hepatitis B. Thromb. Res. 2009, 123, 537–542. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Alessi, M.C.; Mavri, A.; Morange, P.E. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J. Thromb. Haemost. 2003, 1, 1575–1579. [Google Scholar] [CrossRef]
- Chen, J.S.; Wu, C.Z.; Chu, N.F.; Chang, L.C.; Pei, D.; Lin, Y.F. Association among fibrinolytic proteins, metabolic syndrome components, insulin secretion and resistance in schoolchildren. Int. J. Endocrinol. 2015, 2015, 170987. [Google Scholar] [CrossRef]
- Morange, P.E.; Lijnen, H.R.; Alessi, M.C.; Kopp, F.; Collen, D.; Juhan-Vague, I. Influence of PAI-1 on adipose tissue growth and metabolic parameters in a murine model of diet-induced obesity. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Alomar, S.Y. Oxygen deprivation and the cellular response to hypoxia in adipocytes—Perspectives on white and brown adipose tissues in obesity. Front. Endocrinol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Greenfield, J.R.; Ho, K.K.; Fulham, M.J. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E601–E606. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, M.; Calderon-Dominguez, M.; Bustos, E.; Ramos, P.; Casals, N.; Serra, D.; Viana, M.; Herrero, L. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci. Rep. 2017, 7, 16082. [Google Scholar] [CrossRef]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
| C57BL/6J | BALB/c | |||||||
|---|---|---|---|---|---|---|---|---|
| W8 | W16 | W8 | W16 | |||||
| CD | HFD | CD | HFD | CD | HFD | CD | HFD | |
| Body weight (g) | 25.80 ± 0.94 | 29.46 ± 1.32 *** | 27.04 ± 2.14 | 39.1 ± 1.86 *** | 29.26 ± 2.05 | 30.22 ± 1.23 | 30.04 ± 3.30 | 33.95 ± 2.73 * |
| BG (mmol/L) | 6.97 ± 1.22 | 9.24 ± 3.21 | 7.43 ± 0.90 | 9.50 ± 3.96 | 5.08 ± 1.00 | 6.36 ± 2.23 | 5.40 ± 1.25 | 6.96 ± 1.73 * |
| TC (mmol/L) | 2.60 ± 0.03 | 3.12 ± 0.35 * | 2.59 ± 0.01 | 3.68 ± 0.58 * | 4.94 ± 0.44 | 4.92 ± 0.81 | 3.42 ± 0.06 | 4.24 ± 1.36 |
| TG (mmol/L) | 1.11 ± 0.26 | 1.19 ± 0.09 | 1.07 ± 0.19 | 1.34 ± 0.13 * | 1.06 ± 0.09 | 1.07 ± 0.14 | 0.85 ± 0.11 | 0.71 ± 0.08 * |
| HOMA-IR | 2.58 ± 1.00 | 5.42 ± 2.88 | 2.96 ± 1.03 | 8.05 ± 1.61 *** | 1.80 ± 1.06 | 5.16 ± 3.75 | 1.76 ± 1.11 | 9.21 ± 5.92 * |
| HOMA-ß | 26.42 ± 3.09 | 23.27 ± 6.06 | 41.30 ± 17.45 | 34.71 ± 18.95 | 258.4 ± 151.1 | 618.4 ± 502.5 | 366.8 ± 458.9 | 122.4 ± 64.8 |
| FENa | 1.00 ± 0.76 | 0.69 ± 0.48 | 1.08 ± 0.70 | 0.33 ± 0.24 ** | 0.81 ± 0.78 | 0.84 ± 0.83 | 1.14 ± 0.54 | 0.53 ± 0.37 * |
| uPA (μg/mL) | 0.87 ± 0.35 | 2.56 ± 0.65 * | 1.24 ± 0.55 | 1.92 ± 0.19 | 1.88 ± 0.48 | 3.34 ± 0.84 * | 1.31 ± 0.37 | 2.52 ± 0.95 |
| suPAR (ng/mL) | 3.26 ± 0.19 | 3.43 ± 0.36 | 2.56 ± 0.74 | 2.27 ± 0.53 | 1.67 ± 0.60 | 2.22 ± 0.66 | 1.64 ± 0.56 | 3.09 ± 1.23 |
| PAI-1 (ng/mL) | 4.95 ± 0.77 | 4.06 ± 0.45 | 3.86 ± 0.58 | 7.76 ± 1.01 | 28.80 ± 2.03 | 36.22 ± 4.39 * | 22.54 ± 2.12 | 56.16 ± 4.60 *** |
| Adiponectin | 7.92 ± 0.43 | 9.06 ± 0.68 | 8.86 ± 0.45 | 6.98 ± 0.45 * | 9.12 ± 0.38 | 6.96 ± 0.13 ** | 8.87 ± 0.45 | 8.31 ± 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Z.; Chang, L.-C.; Cheng, C.-W.; Fang, T.-C.; Lin, Y.-F.; Pei, D.; Chen, J.-S. Reduced Expression of Urokinase Plasminogen Activator in Brown Adipose Tissue of Obese Mouse Models. Int. J. Mol. Sci. 2021, 22, 3407. https://doi.org/10.3390/ijms22073407
Wu C-Z, Chang L-C, Cheng C-W, Fang T-C, Lin Y-F, Pei D, Chen J-S. Reduced Expression of Urokinase Plasminogen Activator in Brown Adipose Tissue of Obese Mouse Models. International Journal of Molecular Sciences. 2021; 22(7):3407. https://doi.org/10.3390/ijms22073407
Chicago/Turabian StyleWu, Chung-Ze, Li-Chien Chang, Chao-Wen Cheng, Te-Chao Fang, Yuh-Feng Lin, Dee Pei, and Jin-Shuen Chen. 2021. "Reduced Expression of Urokinase Plasminogen Activator in Brown Adipose Tissue of Obese Mouse Models" International Journal of Molecular Sciences 22, no. 7: 3407. https://doi.org/10.3390/ijms22073407
APA StyleWu, C.-Z., Chang, L.-C., Cheng, C.-W., Fang, T.-C., Lin, Y.-F., Pei, D., & Chen, J.-S. (2021). Reduced Expression of Urokinase Plasminogen Activator in Brown Adipose Tissue of Obese Mouse Models. International Journal of Molecular Sciences, 22(7), 3407. https://doi.org/10.3390/ijms22073407






