Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice
Abstract
1. Introduction
2. Results
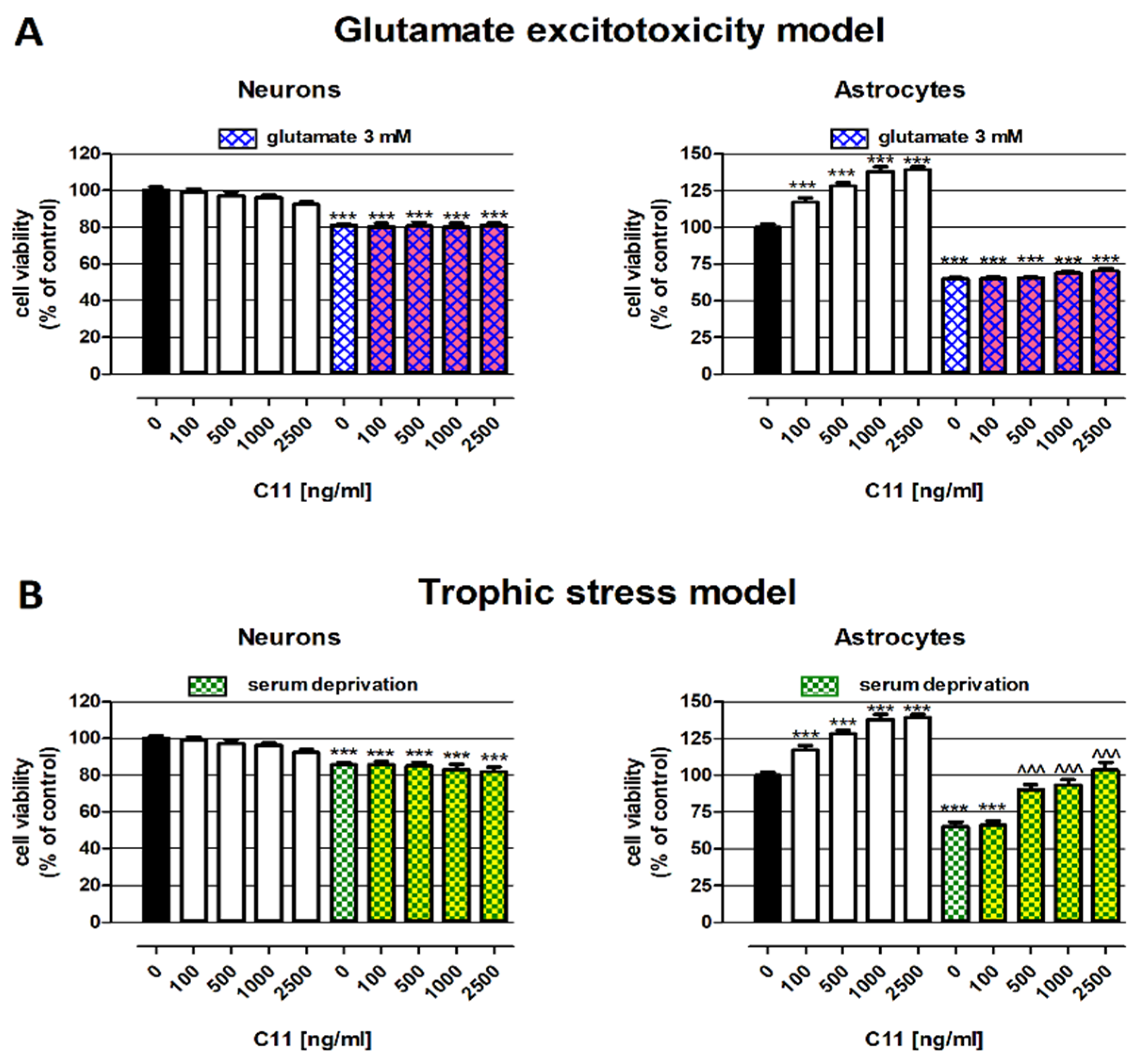
2.1. Protective Abilities of C11—In Vitro Studies
2.2. Cognitive Functions and Neurogenesis—In Vivo Studies
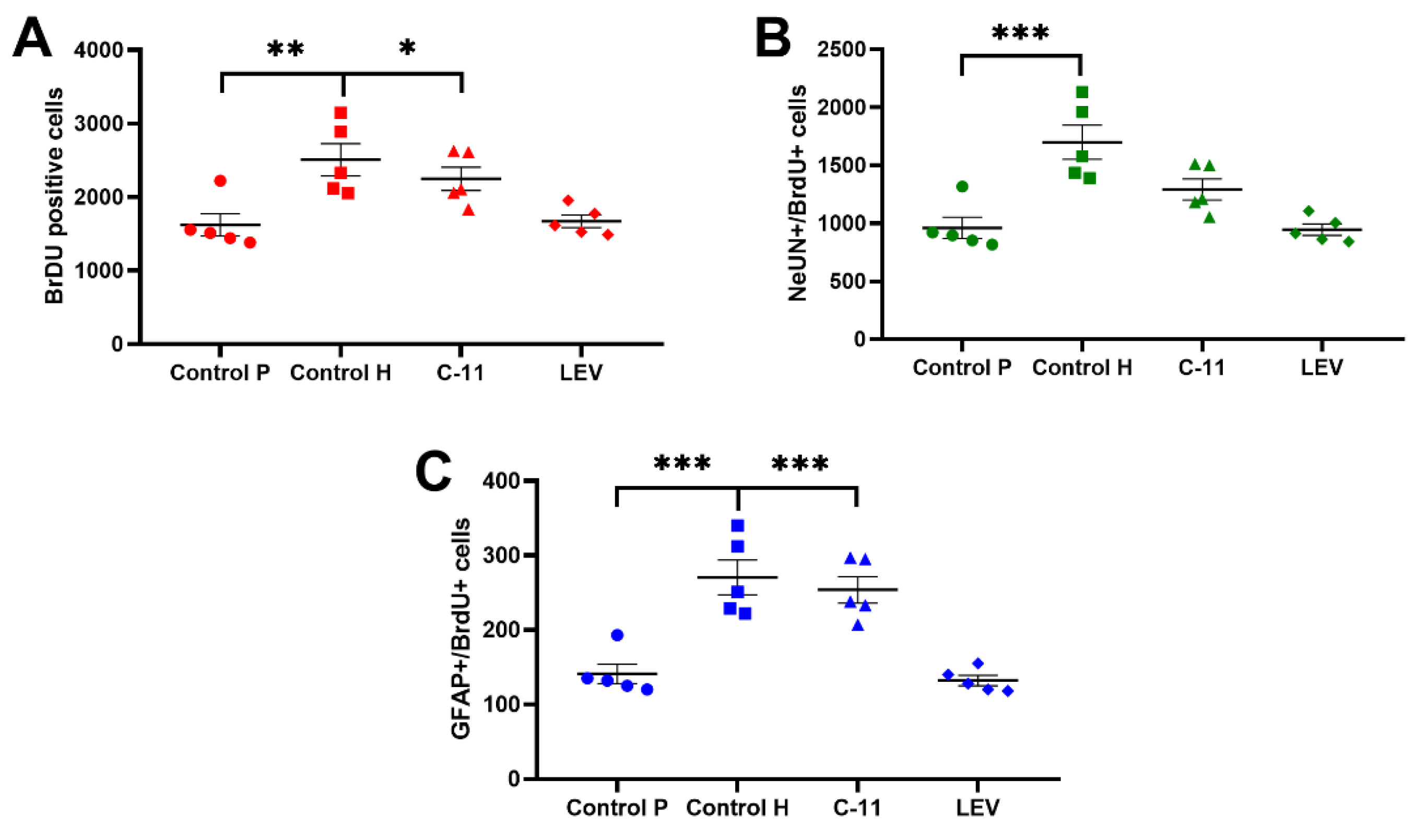
2.2.1. Evaluation of the Effects of Long-Term Administration of C11 and LEV on the Total Amount of Newborn Cells in the Dentate SGZ and Granular Cell Layer (GCL) of Mouse Hippocampus after PILO Induced SE
2.2.2. Evaluation of the Effects of Long-Term Administration of C11 and LEV on the Newborn Neurons in the Dentate SGZ and GCL of Mouse Hippocampus after PILO Induced SE
2.2.3. Evaluation of the Effects of Long-Term Administration of C11 and LEV on the Newborn Astrocytes in the Dentate SGZ and GCL of Mouse Hippocampus after PILO Induced SE
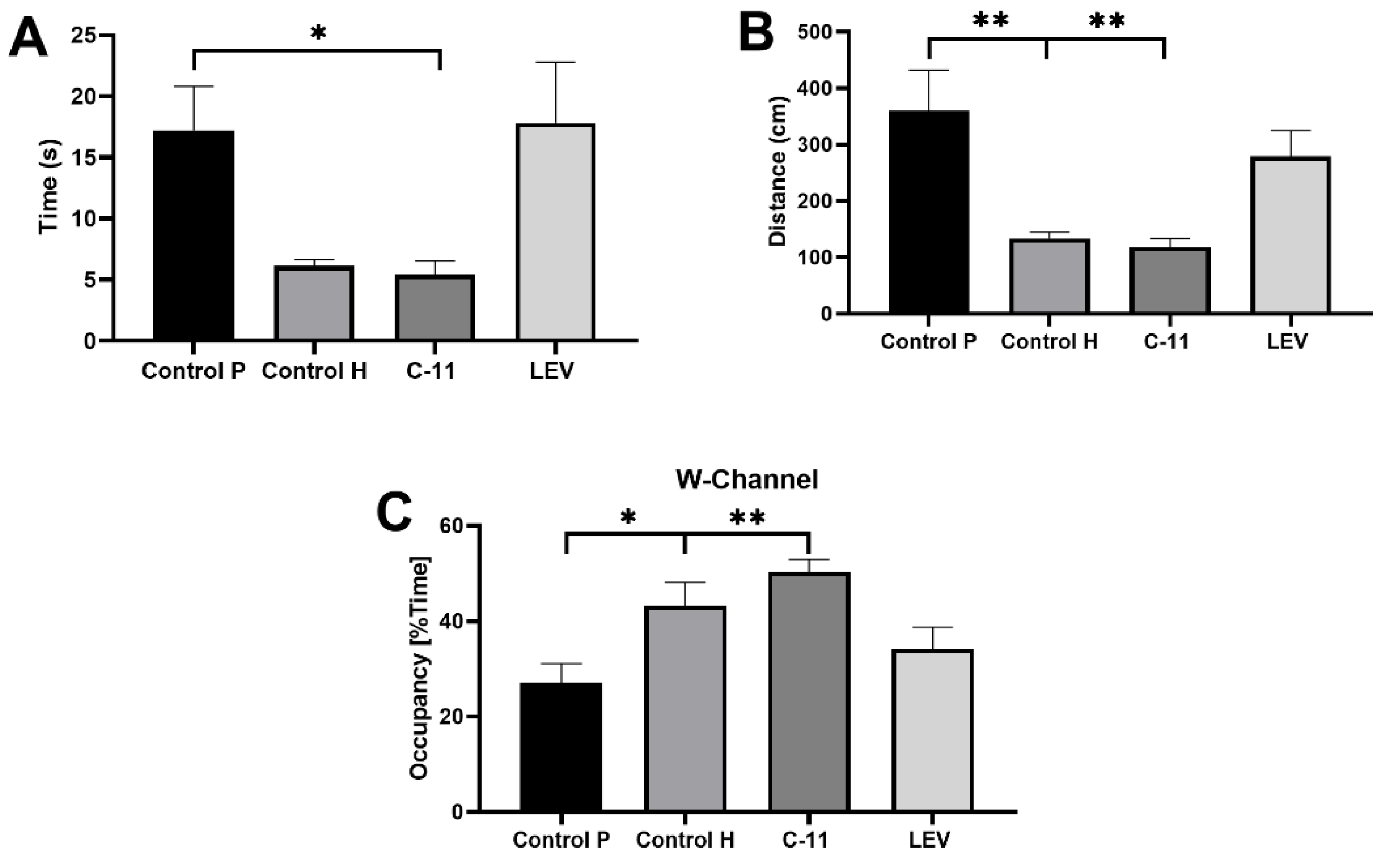
2.2.4. Evaluation of the Effects of Long-Term Administration of C11 and LEV on Mouse Spatial Learning and Memory after PILO Induced SE
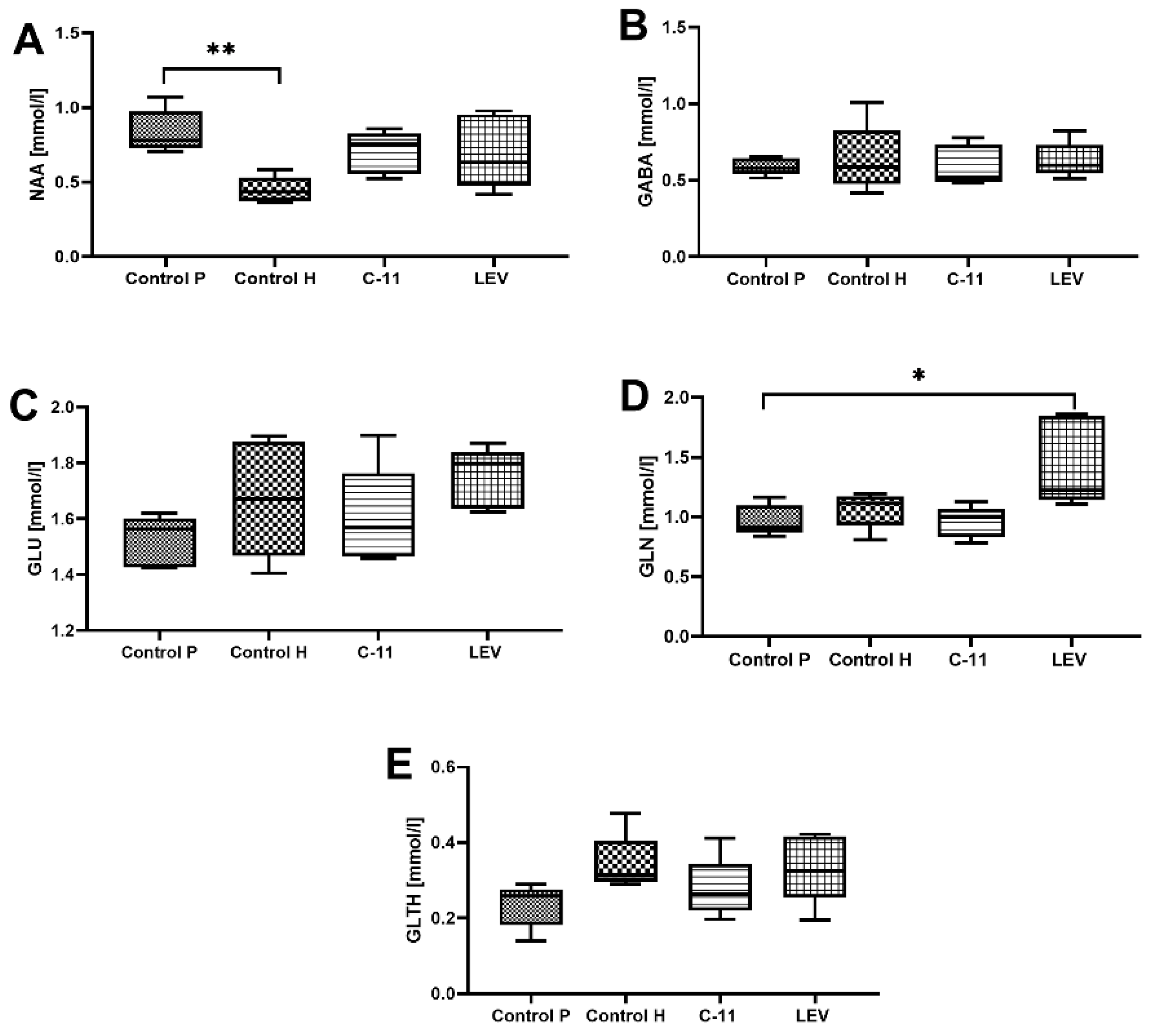
2.2.5. The Effect of Long-Term Treatment with C11 and LEV on the Level of NAA/Cr, GABA/Cr, Glc/Cr, Glt/Cr, and Gln/Cr in Mouse Brain after PILO-Induced SE
3. Discussion
4. Materials and Methods
4.1. In Vitro Study
4.1.1. Reagents
4.1.2. Neuroblastoma Cell Line
4.1.3. Differentiation of SH-SY5Y towards Neuronal Cells
4.1.4. Astroglia Cell Culture
4.1.5. Cell Viability Assessment—MTT Assay
4.2. In Vivo Study
4.2.1. Animals and Experimental Conditions
4.2.2. Status Epilepticus (SE) in Mice
4.2.3. Drugs
4.2.4. Drugs Administration
- PILO C11,
- PILO LEV,
- PILO Control group (PILO + water for injections + Tween 80), and
- Healthy Control group (water for injections + Tween 80)
4.2.5. Behavioral Study-Spatial Learning and Memory (MWM Test)
4.2.6. Magnetic Resonance Spectroscopy (MRS)
4.2.7. Brain Slice Preparation
4.2.8. Immunohistochemical Staining-Neurogenesis
4.2.9. Confocal Microscopy and Cell Counting
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Castro, O.W.; Upadhya, D.; Kodali, M.; Shetty, A.K. Resveratrol for Easing Status Epilepticus Induced Brain Injury, Inflammation, Epileptogenesis, and Cognitive and Memory Dysfunction—Are We There Yet? Front. Neurol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Laxer, K.D.; Trinka, E.; Hirsch, L.J.; Cendes, F.; Langfitt, J.; Delanty, N.; Resnick, T.; Benbadis, S.R. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014, 37, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, K.; Andres-Mach, M.; Czuczwar, M.; Łuszczki, J.J.; Kruszyński, K.; Czuczwar, S.J. Mechanisms of epileptogenesis and preclinical approach to antiepileptogenic therapies. Pharmacol. Rep. 2018, 70, 284–293. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Fike, J.R.; Łuszczki, J.J. Neurogenesis in the epileptic brain: A brief overview from temporal lobe epilepsy. Pharmacol. Rep. 2011, 63, 1316–1323. [Google Scholar]
- Chen, J.; Quan, Q.-Y.; Yang, F.; Wang, Y.; Wang, J.-C.; Zhao, G.; Jiang, W. Effects of lamotrigine and topiramate on hippocampal neurogenesis in experimental temporal-lobe epilepsy. Brain Res. 2010, 1313, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, Y.; Maru, E.; Kudo, K.; Shibasaki, T.; Kato, N. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kainate-induced status epilepticus. Brain Res. 2010, 1352, 187–199. [Google Scholar] [CrossRef]
- Umka, J.; Mustafa, S.; Elbeltagy, M.; Thorpe, A.; Latif, L.; Bennett, G.; Wigmore, P. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 2010, 166, 15–22. [Google Scholar] [CrossRef]
- James, E.J.; Gu, J.; Ramirez-Vizcarrondo, C.M.; Hasan, M.; Truszkowski, T.L.; Tan, Y.; Oupravanh, P.M.; Khakhalin, A.S.; Aizenman, C.D. Valproate-induced neurodevelopmental deficits in Xenopuslaevis Tadpoles. J. Neurosci. 2015, 18, 3218–3229. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Haratym-Maj, A.; Zagaja, M.; Rola, R.; Maj, M.; Chrościńska-Krawczyk, M.; Luszczki, J.J. ACEA (a highly selective cannabinoid CB1 receptor agonist) stimulates hippocampal neurogenesis in mice treated with antiepileptic drugs. Brain Res. 2015, 1624, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Juliandi, B.; Tanemura, K.; Igarashi, K.; Tominaga, T.; Furukawa, Y.; Otsuka, M.; Moriyama, N.; Ikegami, D.; Abematsu, M.; Sanosaka, T.; et al. Reduced Adult Hippocampal Neurogenesis and Cognitive Impairments following Prenatal Treatment of the Antiepileptic Drug Valproic Acid. Stem Cell Rep. 2015, 5, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Matsuda, T.; Doi, H.; Nagaishi, Y.; Kato, K.; Nakashima, K. Ectopic neurogenesis induced by prenatal antiepileptic drug exposure augments seizure susceptibility in adult mice. Proc. Natl. Acad. Sci. USA 2018, 115, 4270–4275. [Google Scholar] [CrossRef] [PubMed]
- Sondossi, K.; Majdzadeh, M.; Ghaeli, P.; Ghahremani, M.H.; Shafaroodi, H.; Paknejad, B.; Ostad, S.N. Analysis of the antiepileptic, ethosuximide impacts on neurogenesis of rat forebrain stem cells. Fundam. Clin. Pharmacol. 2014, 28, 512–518. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Zagaja, M.; Haratym-Maj, A.; Parolaro, D.; Maj, M.; Haratym, J.; Dudra-Jastrzębska, M.; Łuszczki, J.J. A Long-Term Treatment with Arachidonyl-2′-Chloroethylamide Combined with Valproate Increases Neurogenesis in a Mouse Pilocarpine Model of Epilepsy. Int. J. Mol. Sci. 2017, 18, 900. [Google Scholar] [CrossRef] [PubMed]
- Bryda, J.; Zagaja, M.; Szewczyk, A.; Andres-Mach, M. Coumarins as potential supportive medication for the treatment of epilepsy. Acta Neurobiol. Exp. 2019, 79, 126–132. [Google Scholar] [CrossRef]
- Szewczyk, A.; Zagaja, M.; Bryda, J.; Kosikowska, U.; Stępień-Pyśniak, D.; Winiarczyk, S.; Andres-Mach, M. Topinambur—New possibilities for use in a supplementation diet. Ann. Agric. Environ. Med. 2019, 26, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Andres-Mach, M.; Patrzylas, P.; Pyrka, D.; Szpringer, M.; Florek-Łuszczki, M.; Żółkowska, D.; Skalicka-Woźniak, K.; Łuszczki, J.J. Influence of xanthotoxin (8-methoxypsoralen) on the anticonvulsant activity of various novel antiepileptic drugs against maximal electroshock-induced seizures in mice. Fitoterapia 2016, 115, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Haratym-Maj, A.; Szewczyk, A.; Rola, R.; Maj, M.; Łuszczki, J.J.; Andres-Mach, M. Levetiracetam combined with ACEA, highly selective cannabinoid CB1 receptor agonist changes neurogenesis in mouse brain. Neurosci. Lett. 2019, 696, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, K.; Obniska, J. Design, synthesis, and anticonvulsant activity of N-phenylamino derivatives of 3,3-dialkyl-pyrrolidine-2,5-diones and hexahydro-isoindole-1,3-diones. Bioorganic Med. Chem. 2008, 16, 4921–4931. [Google Scholar] [CrossRef]
- Obniska, J.; Kopytko, M.; Zagórska, A.; Chlebek, I.; Kaminski, K. Synthesis and Anticonvulsant Properties of New Mannich Bases Derived from 3-Aryl-pyrrolidine-2,5-diones. Part 1. Arch. Pharm. 2010, 343, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Obniska, J.; Chlebek, I.; Wiklik, B.; Rzepka, S. Design, synthesis and anticonvulsant properties of new N-Mannich bases derived from 3-phenylpyrrolidine-2,5-diones. Bioorganic Med. Chem. 2013, 21, 6821–6830. [Google Scholar] [CrossRef]
- Kamiński, K.; Zagaja, M.; Łuszczki, J.J.; Rapacz, A.; Andres-Mach, M.; Latacz, G.; Kieć-Kononowicz, K. Design, synthesis, and anticonvulsantactivity of newhybridcompoundsderived from 2-(2,5-dioxopyrrolidin-1-yl) propanamides and 2-(2,5-dioxopyrrolidin-1-yl) butanamides. J. Med. Chem. 2015, 58, 5274–5286. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, H.; Matagne, A.; Gobert, J.; Wülfert, E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur. J. Pharmacol. 1998, 353, 191–206. [Google Scholar] [CrossRef]
- Mazarati, A.M.; Baldwin, R.; Klitgaard, H.; Matagne, A.; Wasterlain, C.G. Anticonvulsant effects of levetiracetam and levetiracetam–diazepam combinations in experimental status epilepticus. Epilepsy Res. 2004, 58, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Al-Shorbagy, M.Y.; El Sayeh, B.M.; Abdallah, D.M. Additional Antiepileptic Mechanisms of Levetiracetam in Lithium-Pilocarpine Treated Rats. PLoS ONE 2013, 8, e76735. [Google Scholar] [CrossRef]
- Wojda, E.; Wlaz, A.; Patsalos, P.N.; Luszczki, J.J. Isobolographic characterization of interactions of levetiracetam with the various antiepileptic drugs in the mouse 6Hz psychomotor seizure model. Epilepsy Res. 2009, 86, 163–174. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Luszczki, J.; Maj, M.; Rola, R.; Abram, M.; Kaminski, K. Evaluation of the impact of compound C11 a new anticonvulsant candidate on cognitive functions and hippocampal neurogenesis in mouse brain. Neuropharmacology 2020, 163, 107849. [Google Scholar] [CrossRef]
- Deckwerth, T.L.; Johnson, E.M. Neurotrophic factor deprivation-induced death. Ann. N. Y. Acad. Sci. 1993, 679, 121–131. [Google Scholar] [CrossRef]
- Weis, J.; Saxena, S.; Evangelopoulos, M.; Kruttgen, A. Trophic Factors in Neurodegenerative Disorders. IUBMB Life 2003, 55, 353–357. [Google Scholar] [CrossRef]
- Sheline, C.T.; Cai, A.-L.; Zhu, J.; Shi, C. Serum or target deprivation-induced neuronal death causes oxidative neuronal accumulation of Zn2+ and loss of NAD+. Eur. J. Neurosci. 2010, 32, 894–904. [Google Scholar] [CrossRef]
- Hansson, E.; Rönnbäck, L. Glial neuronal signaling in the central nervous system. FASEB J. 2003, 17, 341–348. [Google Scholar] [CrossRef]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Deneen, B. Glial Development: The Crossroads of Regeneration and Repair in the CNS. Neuron 2014, 83, 283–308. [Google Scholar] [CrossRef]
- Heysieattalab, S.; Sadeghi, L. Dynamic structural neuroplasticity during and after epileptogenesis in a pilocarpine rat model of epilepsy. Acta Epileptol. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Cho, K.-O.; Lybrand, Z.R.; Ito, N.; Brulet, R.; Tafacory, F.; Zhang, L.; Good, L.; Ure, K.; Kernie, S.G.; Birnbaum, S.G.; et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015, 6, 6606. [Google Scholar] [CrossRef]
- Ge, Y.-X.; Tian, X.-Z.; Lin, Y.-Y.; Liu, X.-Y. Chronic treatment with levetiracetam reverses deficits in hippocampal LTP in vivo in experimental temporal lobe epilepsy rats. Neurosci. Lett. 2016, 628, 194–200. [Google Scholar] [CrossRef]
- Shetty, A.K. Prospects of Levetiracetam as a Neuroprotective Drug against Status Epilepticus, Traumatic Brain Injury, and Stroke. Front. Neurol. 2013, 4, 172. [Google Scholar] [CrossRef]
- Santana-Gómez, C.E.; Valle-Dorado, M.G.; Domínguez-Valentín, A.E.; Hernández-Moreno, A.; Orozco-Suárez, S.; Rocha, L. Neuroprotective effects of levetiracetam, both alone and combined with propylparaben, in the long-term consequences induced by lithium-pilocarpine status epilepticus. Neurochem. Int. 2018, 120, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Shen, H.; Zhang, Y.; Zhu, X.; Wang, J.; Xu, P.; Jiang, D.; Yu, X. The antiepileptic drug levetiracetam promotes neuroblast differentiation and expression of superoxide dismutase in the mouse hippocampal dentate gyrus via PI3K/Aktsignalling. Neurosci. Lett. 2018, 662, 84–90. [Google Scholar] [CrossRef]
- Itoh, K.; Taniguchi, R.; Matsuo, T.; Oguro, A.; Vogel, C.F.; Yamazaki, T.; Ishihara, Y. Suppressive effects of levetiracetam on neuroinflammation and phagocytic microglia: A comparative study of levetiracetam, valproate and carbamazepine. Neurosci. Lett. 2019, 708, 134363. [Google Scholar] [CrossRef]
- Vyas, P.; Tulsawani, R.K.; Vohora, D. Loss of Protection by Antiepileptic Drugs in Lipopolysaccharide-primed Pilocarpine-induced Status Epilepticus is Mediated via Inflammatory Signalling. Neuroscience 2020, 442, 1–16. [Google Scholar] [CrossRef]
- Borges, K.; McDermott, D.; Irier, H.; Smith, Y.; Dingledine, R. Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus. Exp. Neurol. 2006, 201, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Liu, N.; Zheng, P.; Ma, L.; Guo, F.; Sun, T.; Zhou, R.; Yu, J. The Anticonvulsant Effects of Baldrinal on Pilocarpine-Induced convulsion in Adult Male Mice. Molecules 2019, 24, 1617. [Google Scholar] [CrossRef]
- Kamphuis, W.; Mamber, C.; Moeton, M.; Kooijman, L.; Sluijs, J.A.; Jansen, A.H.P.; Verveer, M.; De Groot, L.R.; Smith, V.D.; Rangarajan, S.; et al. GFAP Isoforms in Adult Mouse Brain with a Focus on Neurogenic Astrocytes and Reactive Astrogliosis in Mouse Models of Alzheimer Disease. PLoS ONE 2012, 7, e42823. [Google Scholar] [CrossRef] [PubMed]
- Filibian, M.; Frasca, A.; Maggioni, D.; Micotti, E.; Vezzani, A.; Ravizza, T. In vivo imaging of glia activation using1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 2012, 53, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M.; Park, G.Y.; Im, K.C.; Kim, S.T.; Woo, C.-W.; Chung, J.H.; Kim, K.S.; Kim, J.S.; Shon, Y.-M.; Kim, Y.I.; et al. Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia 2012, 53, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-J.; Zheng, P.; Wright, D.K.; Dezsi, G.; Braine, E.; Nguyen, T.; Corcoran, N.M.; Johnston, L.A.; Hovens, C.M.; Mayo, J.N.; et al. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 2016, 139, 1919–1938. [Google Scholar] [CrossRef] [PubMed]
- Gröticke, I.; Hoffmann, K.; Löscher, W. Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp. Neurol. 2007, 207, 329–349. [Google Scholar] [CrossRef]
- Müller, C.J.; Gröticke, I.; Bankstahl, M.; Löscher, W. Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice. Exp. Neurol. 2009, 219, 284–297. [Google Scholar] [CrossRef]
- Pearson, J.N.; Schulz, K.M.; Patel, M. Specific alterations in the performance of learning and memory tasks in models of chemoconvulsant-induced status epilepticus. Epilepsy Res. 2014, 108, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.-K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-M.; Zhao, J.; Wu, W.-Y.; Fu, J.; Li, Z.-Y.; Zhang, M.; Lu, J. Post-status epilepticus treatment with the Fyn inhibitor, saracatinib, improves cognitive function in mice. BMC Neurosci. 2021, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Mogilski, S.; Pieróg, M.; Nieoczym, D.; Abram, M.; Szulczyk, B.; Lubelska, A.; Latacz, G.; Doboszewska, U.; Wlaź, P.; et al. KA-11, a Novel Pyrrolidine-2,5-dione Derived Broad-Spectrum Anticonvulsant: Its Antiepileptogenic, Antinociceptive Properties and in vitro Characterization. ACS Chem. Neurosci. 2018, 10, 636–648. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Nunes, F.M.; Cardoso, C.; Marques, G.; Rzeski, W. Neuroprotective properties of Cantharellus cibarius polysaccharide fractions in different in vitro models of neurodegeneration. Carbohydr. Polym. 2018, 197, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J.; Gartner, J.G.; Burnham, W.M. Epileptiform activity and neural plasticity in limbic structures. Brain Res. 1972, 47, 262–268. [Google Scholar] [CrossRef]
- Duarte, J.M.; Lei, H.; Mlynárik, V.; Gruetter, R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage 2012, 61, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Lin, S.; Ho, A.; Johnson, T.D.; Wang, P.C.; Scafidi, J.; Tu, T. Comparison of in vivo and in situ detection of hippocampal metabolites in mouse brain using 1 H-MRS. NMR Biomed. 2021, 34, e4451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gu, G.-J.; Zhang, Q.; Liu, J.-H.; Zhang, B.; Guo, Y.; Wang, M.-Y.; Gong, Q.-Y.; Xu, J.-R. NSCs promote hippocampal neurogenesis, metabolic changes and synaptogenesis in APP/PS1 transgenic mice. Hippocampus 2017, 27, 1250–1263. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Szala-Rycaj, J.; Lemieszek, M.K.; Maj, M.; Abram, M.; Kaminski, K. Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice. Int. J. Mol. Sci. 2021, 22, 3240. https://doi.org/10.3390/ijms22063240
Andres-Mach M, Szewczyk A, Zagaja M, Szala-Rycaj J, Lemieszek MK, Maj M, Abram M, Kaminski K. Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice. International Journal of Molecular Sciences. 2021; 22(6):3240. https://doi.org/10.3390/ijms22063240
Chicago/Turabian StyleAndres-Mach, Marta, Aleksandra Szewczyk, Mirosław Zagaja, Joanna Szala-Rycaj, Marta Kinga Lemieszek, Maciej Maj, Michał Abram, and Krzysztof Kaminski. 2021. "Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice" International Journal of Molecular Sciences 22, no. 6: 3240. https://doi.org/10.3390/ijms22063240
APA StyleAndres-Mach, M., Szewczyk, A., Zagaja, M., Szala-Rycaj, J., Lemieszek, M. K., Maj, M., Abram, M., & Kaminski, K. (2021). Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice. International Journal of Molecular Sciences, 22(6), 3240. https://doi.org/10.3390/ijms22063240





