Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment
Abstract
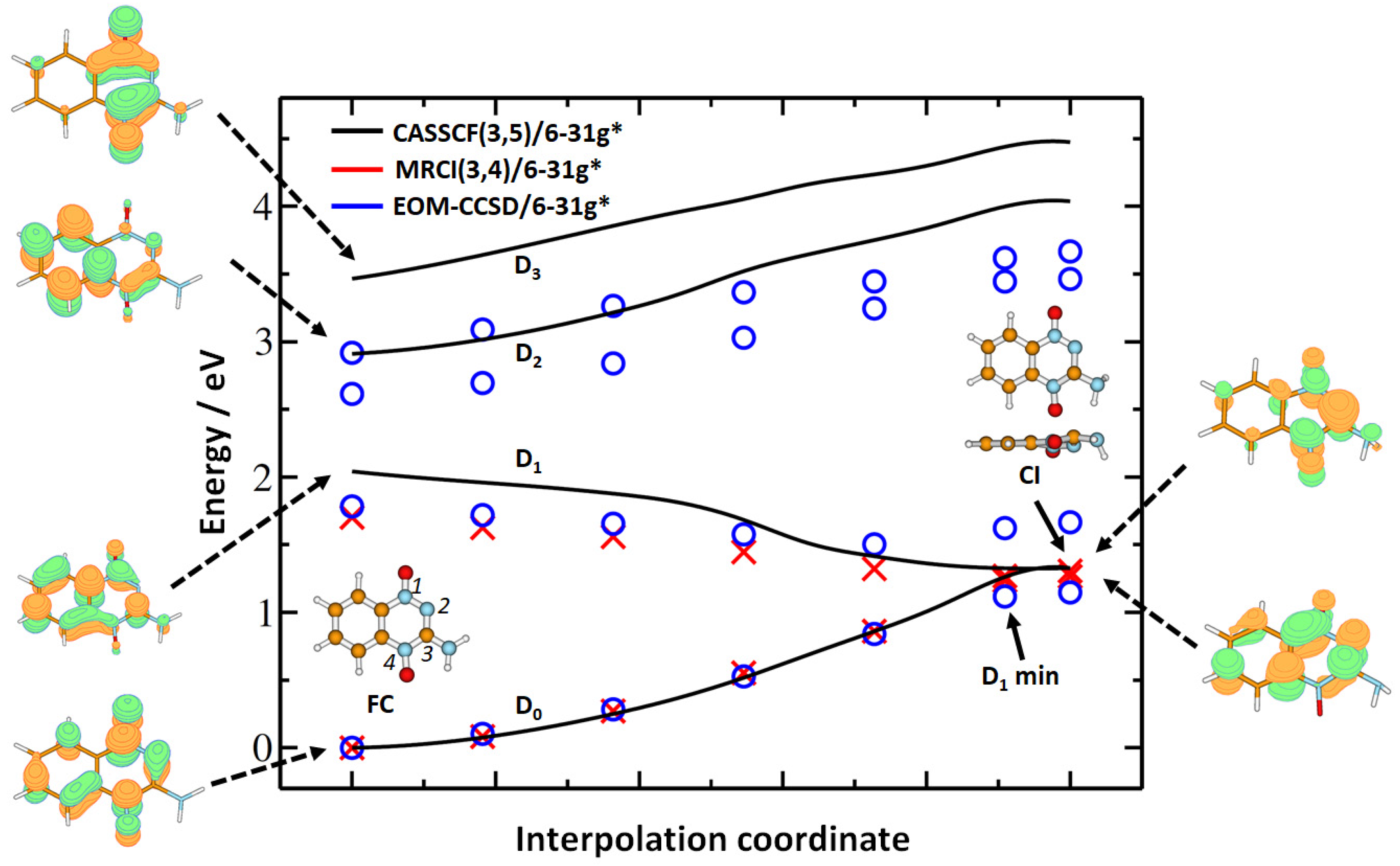
1. Introduction
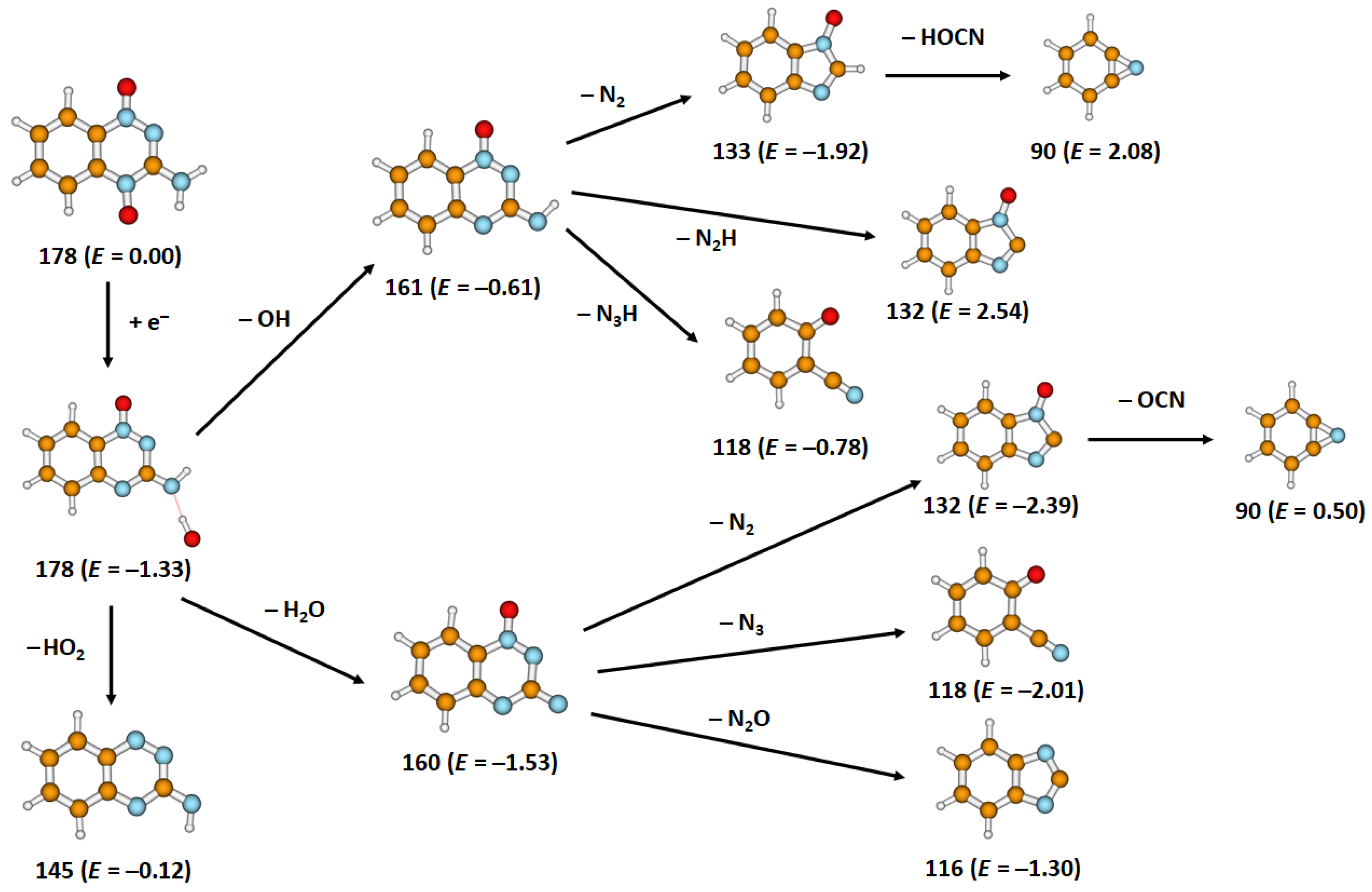
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oronsky, B.T.; Knox, S.J.; Scicinski, J. Six Degrees of Separation: The Oxygen Effect in the Development of Radiosensitizers. Transl. Oncol. 2011, 4, 189–198. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Raviraj, J.; Bokkasam, V.K.; Kumar, V.S.; Reddy, U.S.; Suman, V. Radiosensitizers, radioprotectors, and radiation mitigators. Indian J. Dent. Res. 2014, 25, 83. [Google Scholar] [CrossRef]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The concurrent chemoradiation paradigm—General principles. Nat. Clin. Pract. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Meißner, R.; Kočišek, J.; Feketeová, L.; Fedor, J.; Fárník, M.; Limão-Vieira, P.; Illenberger, E.; Denifl, S. Low-energy electrons transform the nimorazole molecule into a radiosensitiser. Nat. Commun. 2019, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Hansen, H.S.; Overgaard, M.; Bastholt, L.; Berthelsen, A.; Specht, L.; Lindeløv, B.; Jørgensen, K. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother. Oncol. 1998, 46, 135–146. [Google Scholar] [CrossRef]
- Hay, M.P.; Pruijn, F.B.; Gamage, S.A.; Liyanage, H.D.S.; Kovacs, M.S.; Patterson, A.V.; Wilson, W.R.; Brown, J.M.; Denny, W.A. DNA-Targeted 1,2,4-Benzotriazine 1,4-Dioxides: Potent Analogues of the Hypoxia-Selective Cytotoxin Tirapazamine. J. Med. Chem. 2004, 47, 475–488. [Google Scholar] [CrossRef]
- Brown, J.M. The Hypoxic Cell: A Target for Selective Cancer Therapy. Cancer Res. 1999, 59, 5863–5870. [Google Scholar]
- Junnotula, V.; Sarkar, U.; Sinha, S.; Gates, K.S. Initiation of DNA strand cleavage by 1, 2, 4-benzotriazine 1, 4-dioxide antitumor agents: Mechanistic insight from studies of 3-methyl-1, 2, 4-benzotriazine 1, 4-dioxide. J. Am. Chem. Soc. 2009, 131, 1015–1024. [Google Scholar] [CrossRef]
- Li, L.C.; Zha, D.; Zhu, Y.Q.; Xu, M.H.; Wong, N.B. Theoretical study of the mechanism of hydroxyl radical release from tirapazamine’s undergoing enzymatic catalysis. Chem. Phys. Lett. 2005, 408, 329–334. [Google Scholar] [CrossRef]
- Zagorevskii, D.; Song, M.; Breneman, C.; Yuan, Y.; Fuchs, T.; Gates, K.S.; Greenlief, C.M. A mass spectrometry study of tirapazamine and its metabolites: Insights into the mechanism of metabolic transformations and the characterization of reaction intermediates. J. Am. Soc. Mass Spectrom. 2003, 14, 881–892. [Google Scholar] [CrossRef][Green Version]
- Shinde, S.S.; Maroz, A.; Hay, M.P.; Patterson, A.V.; Denny, W.A.; Anderson, R.F. Characterization of radicals formed following enzymatic reduction of 3-substituted analogues of the hypoxia-selective cytotoxin 3-amino-1, 2, 4-benzotriazine 1, 4-dioxide (tirapazamine). J. Am. Chem. Soc. 2010, 132, 2591–2599. [Google Scholar] [CrossRef]
- Anderson, R.F.; Shinde, S.S.; Hay, M.P.; Gamage, S.A.; Denny, W.A. Activation of 3-amino-1, 2, 4-benzotriazine 1, 4-dioxide antitumor agents to oxidizing species following their one-electron reduction. J. Am. Chem. Soc. 2003, 125, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.F.; Shinde, S.S.; Hay, M.P.; Denny, W.A. Potentiation of the cytotoxicity of the anticancer agent tirapazamine by benzotriazine N-oxides: The role of redox equilibria. J. Am. Chem. Soc. 2006, 128, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.D.; Weinfeld, M. Dual action of tirapazamine in the induction of DNA strand breaks. Cancer Res. 1996, 56, 1584–1590. [Google Scholar] [PubMed]
- Daniels, J.S.; Gates, K.S. DNA cleavage by the antitumor agent 3-amino-1,2,4-benzotriazine 1,4-dioxide (SR4233): Evidence for involvement of hydroxyl radical. J. Am. Chem. Soc. 1996, 118, 3380–3385. [Google Scholar] [CrossRef]
- Adams, G.E.; Dewey, D.L. Hydrated electrons and radiobiological sensitization. Biochem. Biophys. Res. Commun. 1963, 12. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Production of low-energy electrons by ionizing radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Kumar, A.; Becker, D.; Adhikary, A.; Sevilla, M.D. Reaction of electrons with DNA: Radiation damage to radiosensitization. Int. J. Mol. Sci. 2019, 20, 3998. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Sanche, L. Precursors of solvated electrons in radiobiological physics and chemistry. Chem. Rev. 2012, 112, 5578–5602. [Google Scholar] [CrossRef] [PubMed]
- Boudaïffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science 2000, 287, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, F.; Denisov, S.A.; Adhikary, A.; Mostafavi, M. Reactivity of prehydrated electrons toward nucleobases and nucleotides in aqueous solution. Sci. Adv. 2017, 3, e1701669. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Baidoo, E.; Ameixa, J.; Ziegler, P.; Ferreira da Silva, F.; Ončák, M.; Denifl, S. Reactions in Tirapazamine Induced by the Attachment of Low-Energy Electrons: Dissociation Versus Roaming of OH. Angew. Chem.-Int. Ed. 2020, 59, 17177–17181. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Glaser, R.; Gates, K.S. On the reaction mechanism of tirapazamine reduction chemistry: Unimolecular N-OH homolysis, stepwise dehydration, or triazene ring-opening. Chem. Res. Toxicol. 2012, 25, 634–645. [Google Scholar] [CrossRef]
- Yin, J.; Glaser, R.; Gates, K.S. Electron and spin-density analysis of tirapazamine reduction chemistry. Chem. Res. Toxicol. 2012, 25, 620–633. [Google Scholar] [CrossRef]
- Chomicz, L.; Zdrowowicz, M.; Kasprzykowski, F.; Rak, J.; Buonaugurio, A.; Wang, Y.; Bowen, K.H. How to find out whether a 5-substituted uracil could be a potential DNA radiosensitizer. J. Phys. Chem. Lett. 2013, 4, 2853–2857. [Google Scholar] [CrossRef]
- Ameixa, J.; Arthur-Baidoo, E.; Meißner, R.; Makurat, S.; Kozak, W.; Butowska, K.; Ferreira Da Silva, F.; Rak, J.; Denifl, S. Low-energy electron-induced decomposition of 5-trifluoromethanesulfonyl-uracil: A potential radiosensitizer. J. Chem. Phys. 2018, 149, 164307. [Google Scholar] [CrossRef]
- Spisz, P.; Zdrowowicz, M.; Kozak, W.; Chomicz-Mańka, L.; Falkiewicz, K.; Makurat, S.; Sikorski, A.; Wyrzykowski, D.; Rak, J.; Arthur-Baidoo, E.; et al. Uracil-5-yl O-Sulfamate: An Illusive Radiosensitizer. Pitfalls in Modeling the Radiosensitizing Derivatives of Nucleobases. J. Phys. Chem. B 2020, 124, 5600–5613. [Google Scholar] [CrossRef] [PubMed]
- Ptasińska, S.; Denifl, S.; Scheier, P.; Märk, T.D. Inelastic electron interaction (attachment/ionization) with deoxyribose. J. Chem. Phys. 2004, 120, 8505–8511. [Google Scholar] [CrossRef] [PubMed]
- Bald, I.; Langer, J.; Tegeder, P.; Ingólfsson, O. From isolated molecules through clusters and condensates to the building blocks of life. Int. J. Mass Spectrom. 2008, 277, 4–25. [Google Scholar] [CrossRef]
- Gorfinkiel, J.D.; Ptasinska, S. Electron scattering from molecules and molecular aggregates of biological relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef]
- Ribar, A.; Fink, K.; Probst, M.; Huber, S.E.; Feketeová, L.; Denifl, S. Isomer Selectivity in Low-Energy Electron Attachment to Nitroimidazoles. Chem.-Eur. J. 2017, 23, 12892–12899. [Google Scholar] [CrossRef]
- Ptasinska, S.; Denifl, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Bond- and site-selective loss of H atoms from nucleobases by very-low-energy electrons (<3 eV). Angew. Chem.-Int. Ed. 2005, 44, 6941–6943. [Google Scholar] [CrossRef]
- Gallup, G.A.; Aflatooni, K.; Burrow, P.D. Dissociative electron attachment near threshold, thermal attachment rates, and vertical attachment energies of chloroalkanes. J. Chem. Phys. 2003. [Google Scholar] [CrossRef]
- Meißner, R.; Feketeová, L.; Bayer, A.; Postler, J.; Limão-Vieira, P.; Denifl, S. Positive and negative ions of the amino acid histidine formed in low-energy electron collisions. J. Mass Spectrom. 2019, 54, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Jørgensen, P. Coupled cluster response functions. J. Chem. Phys. 1990, 93, 3333–3344. [Google Scholar] [CrossRef]
- Stanton, J.F.; Bartlett, R.J. The equation of motion coupled-cluster method. A systematic biorthogonal approach to molecular excitation energies, transition probabilities, and excited state properties. J. Chem. Phys. 1993, 98, 7029–7039. [Google Scholar] [CrossRef]
- Krylov, A.I. Equation-of-motion coupled-cluster methods for open-shell and electronically excited species: The Hitchhiker’s guide to fock space. Annu. Rev. Phys. Chem. 2008, 59, 433–462. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Korona, T.; Lindh, R.; Mitrushenkov, A.; Rauhut, G.; et al. MOLPRO, version 2012.1; A package of ab initio programs; University of Cardiff Chemistry Consultants (UC3): Cardiff, UK, 2012.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016.


| Mass (u) | Anion | Neutral Counterparts | Resonance Positions (eV) | Thresholds (eV) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Exp | Theory | |||
| 178 | C7H6N4O2− | 0 | 0.1 | 0.3 | - | - | - | - | ~0 | −1.28 | |
| 177 a | C7H5N4O2− | H | 0 | 0.1 | 0.3 | 0.9 | - | - | - | ~0 | −0.92 |
| 162 a | C7H4N3O2− | NH2 | 0.3 | 0.7 | - | - | - | - | - | ~0 | −1.71 |
| 161 a | C7H5N4O− | OH | 0 | 0.1 | 0.3 | 0.9 | - | - | - | ~0 | −0.61 |
| 145 | C7H5N4− | HO2 | 0.4 | 1.0 | 1.7 | 4.5 | 5.3 | - | - | ~0 | −0.12 |
| 133 | C7H5N2O− | OH + N2 | 0.0 | 0.1 | 0.3 | 0.8 | 2.4 | - | - | ~0 | −1.92 |
| 132 | C7H4N2O− | H2O + N2/ OH + N2H | 0.0 | 0.1 | 0.3 | 0.8 | 2.4 | 2.8 | 3.2 | ~0 | −2.39/ 2.54 |
| 118 | C7H4NO− | OH + N3H/ H2O + N3 | 0.0 | 0.1 | 2.5 | 3.2 | 3.9 | - | - | ~0 | −0.78/ −2.01 |
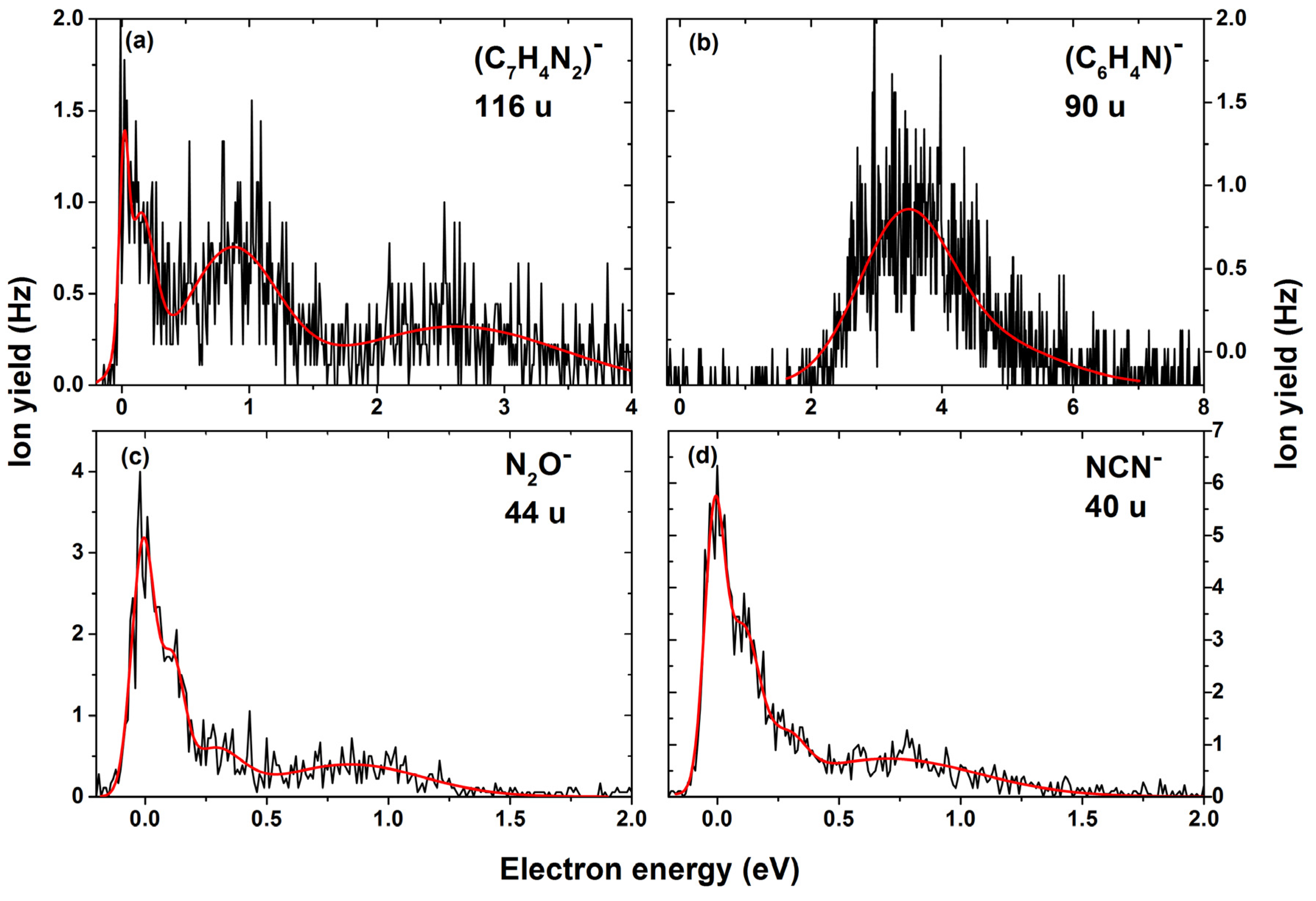
| 116 | C7H4N2− | H2O + N2O | 0.0 | 0.1 | 0.9 | 2.6 | - | - | - | ~0 | −1.30 |
| 90 | C6H4N− | OH + N2 + HOCN/H2O + N2 + NCO | 3.6 | 4.7 | - | - | - | - | - | 2.1 | 2.08/ 0.50 |
| 44 | N2O− | C7H6N2O | 0.0 | 0.1 | 0.3 | 0.9 | - | - | - | ~0 | −0.12 |
| 40 | NCN− | C6H6N2O2 | 0.0 | 0.1 | 0.3 | 0.7 | - | - | - | ~0 | −0.79 |
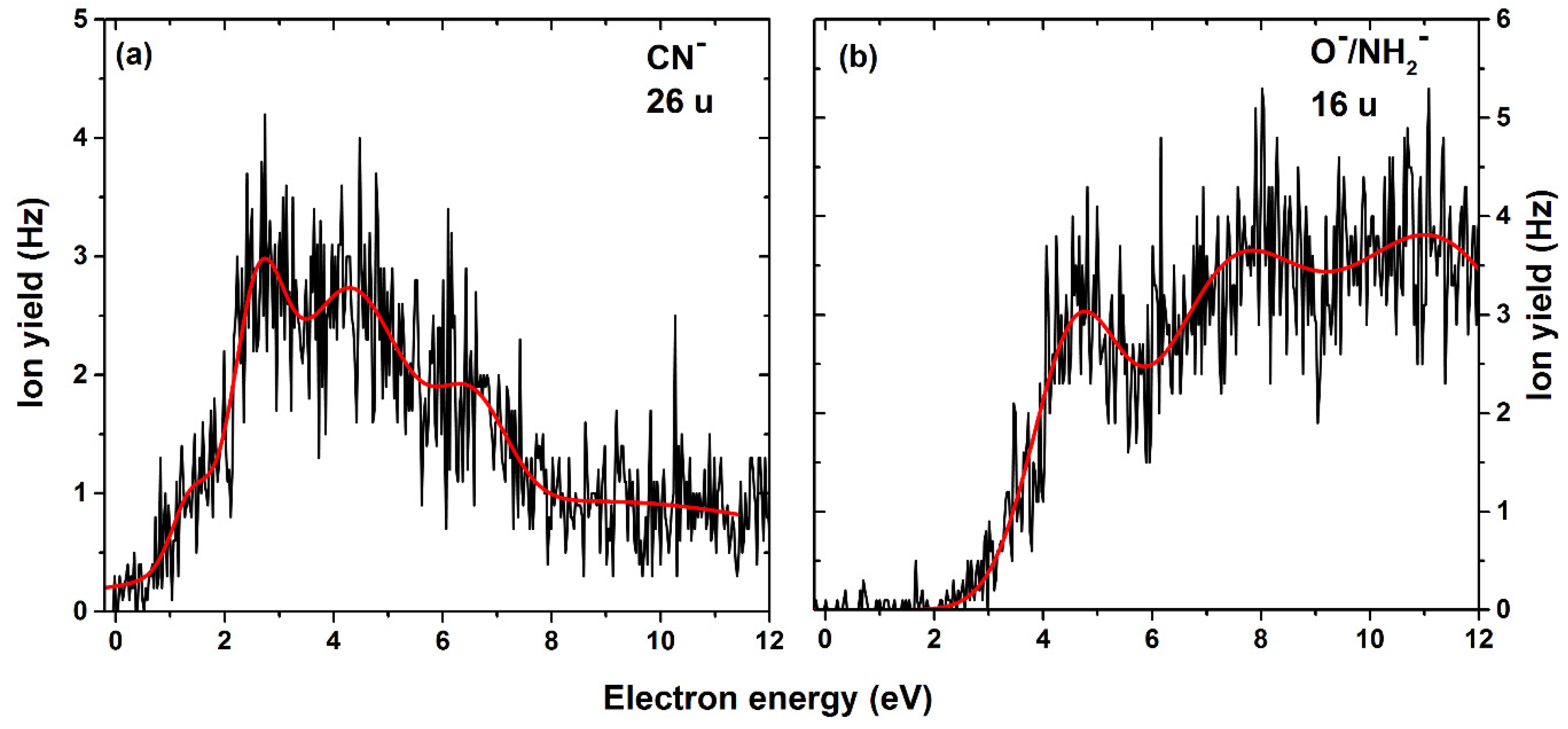
| 26 | CN− | C6H6N3O2/ C6H6NO2 + N2 | 1.4 | 2.6 | 4.2 | 6.5 | - | - | - | 0.7 | 0.58/ −2.64 |
| 16 | O− NH2− | C7H6N4O C7H4N3O2 | 4.6 | 7.3 | 11.1 | - | - | - | - | 2.7 | 0.81 1.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthur-Baidoo, E.; Ameixa, J.; Ončák, M.; Denifl, S. Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment. Int. J. Mol. Sci. 2021, 22, 3159. https://doi.org/10.3390/ijms22063159
Arthur-Baidoo E, Ameixa J, Ončák M, Denifl S. Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment. International Journal of Molecular Sciences. 2021; 22(6):3159. https://doi.org/10.3390/ijms22063159
Chicago/Turabian StyleArthur-Baidoo, Eugene, Joao Ameixa, Milan Ončák, and Stephan Denifl. 2021. "Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment" International Journal of Molecular Sciences 22, no. 6: 3159. https://doi.org/10.3390/ijms22063159
APA StyleArthur-Baidoo, E., Ameixa, J., Ončák, M., & Denifl, S. (2021). Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment. International Journal of Molecular Sciences, 22(6), 3159. https://doi.org/10.3390/ijms22063159






