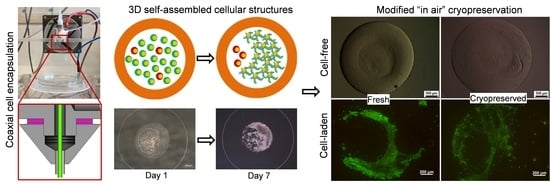
Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage
Abstract
1. Introduction
2. Results
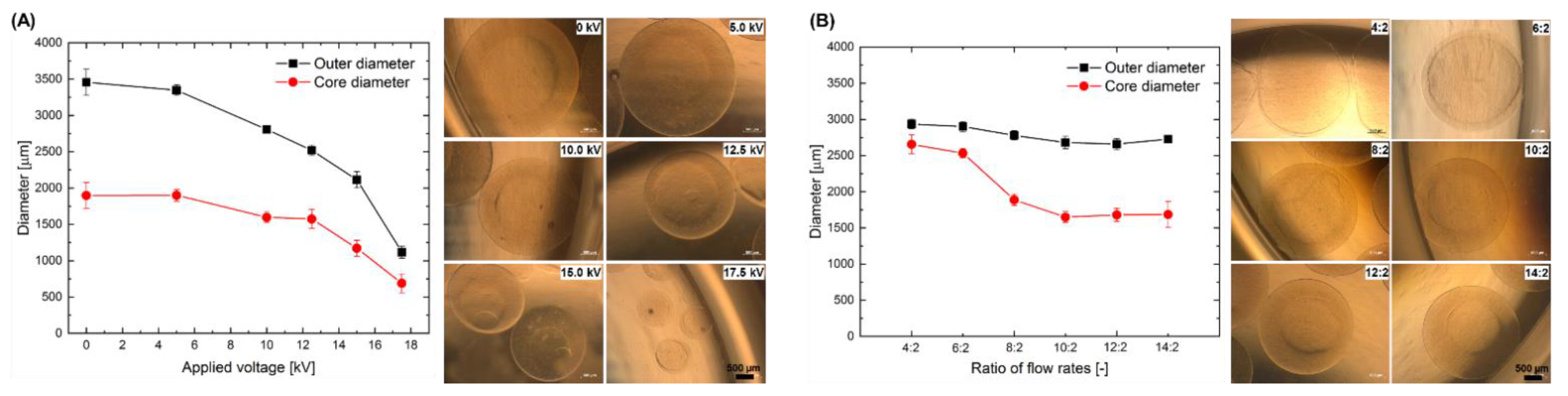
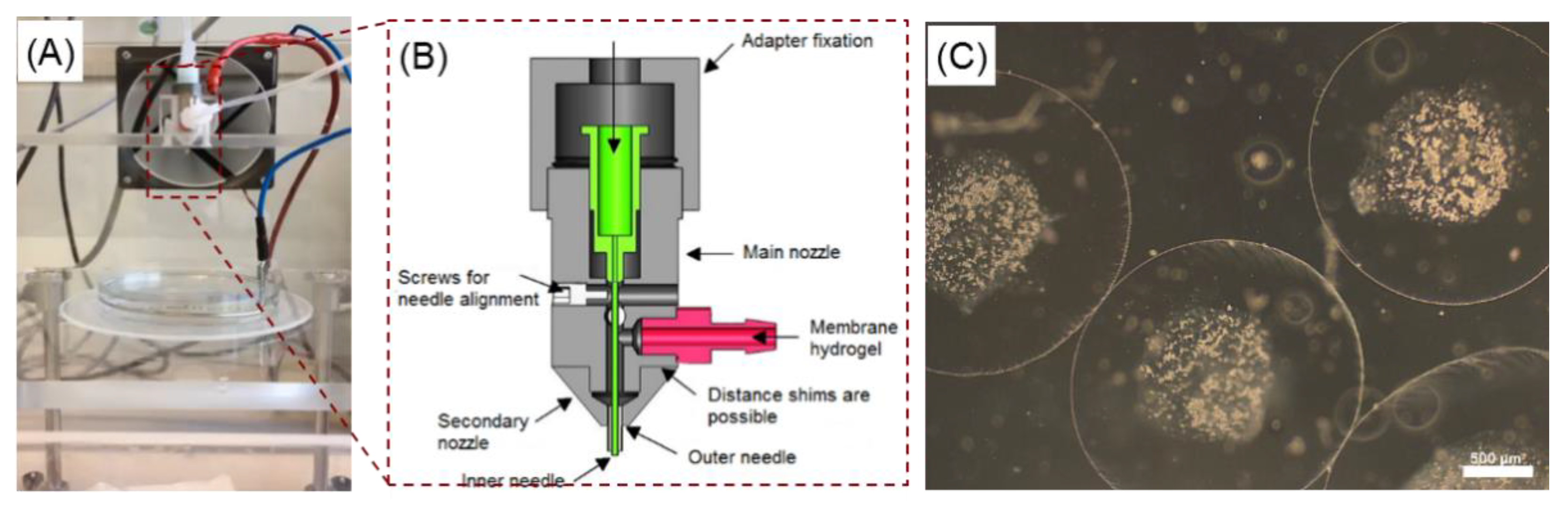
2.1. Effect of the Electrospraying Process Parameters on the Size of Core-Shell Capsules
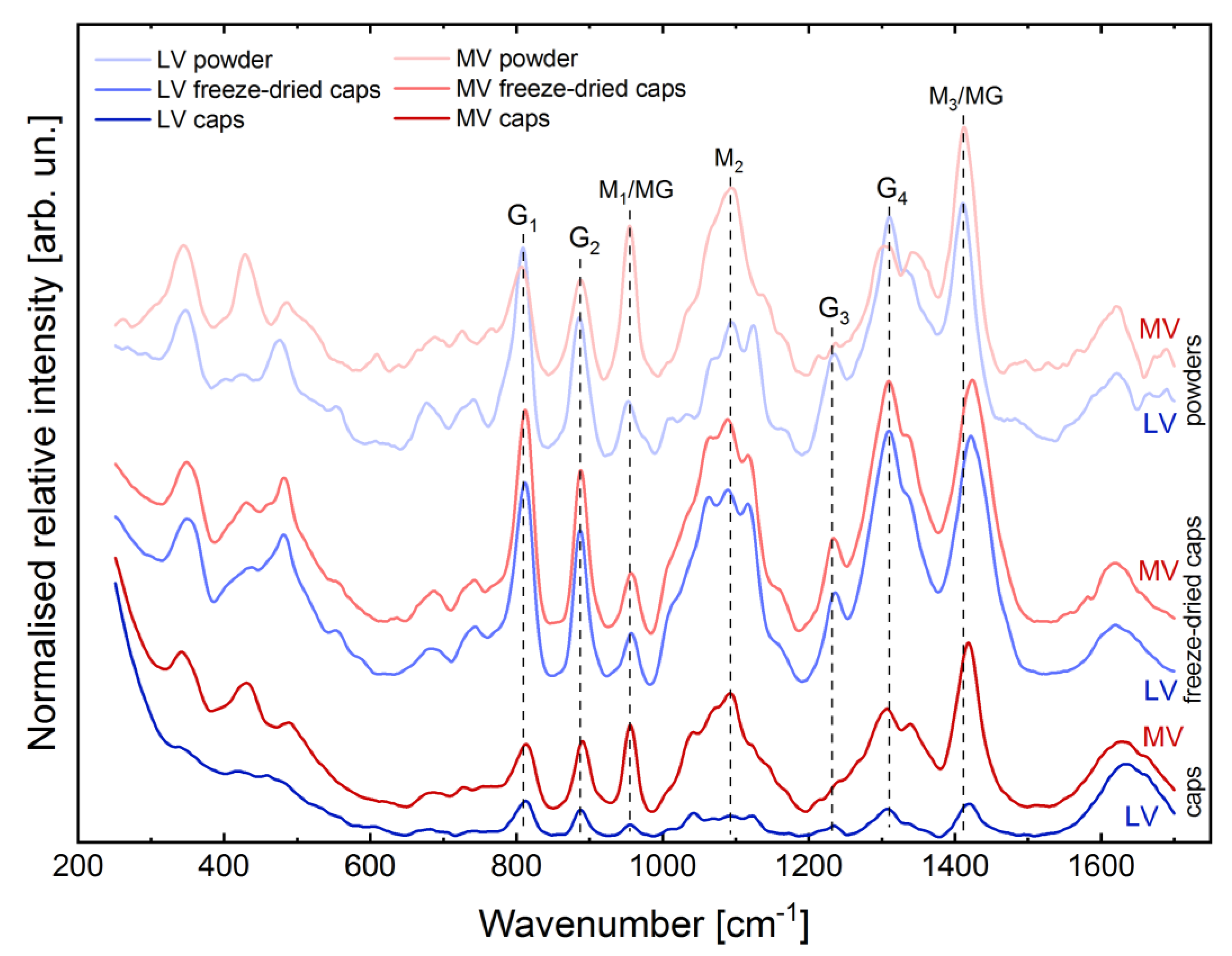
2.2. Evaluation of Structural Properties of Core-Shell Capsules
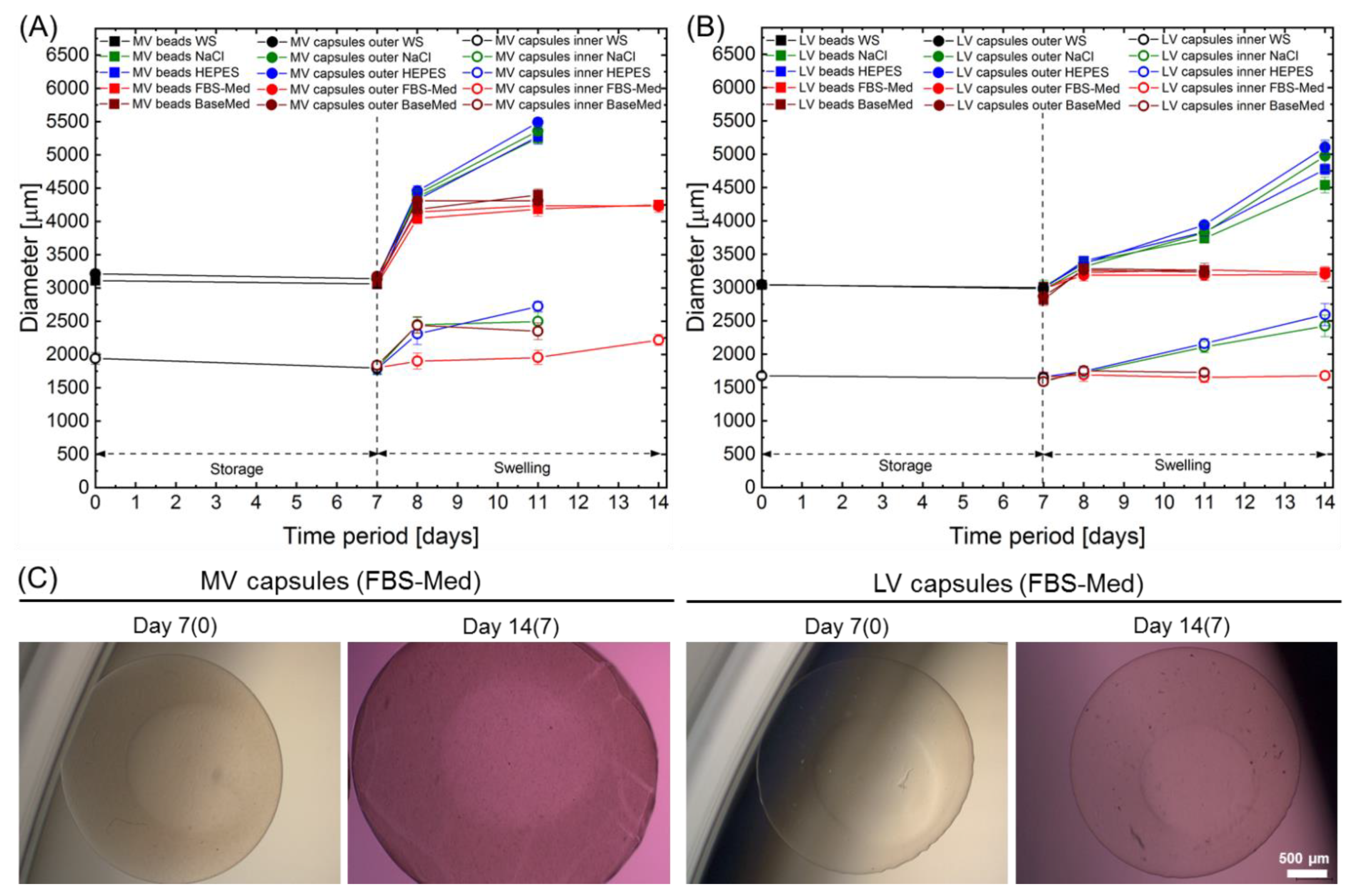
2.3. Storage Stability and Swelling Behaviour
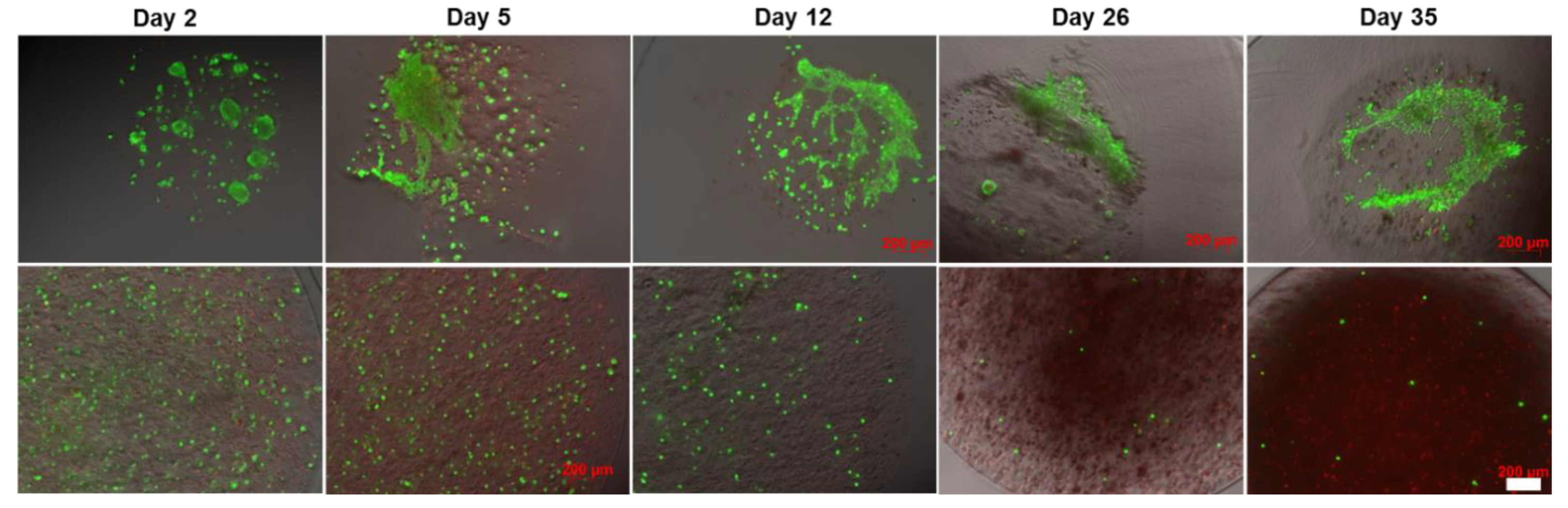
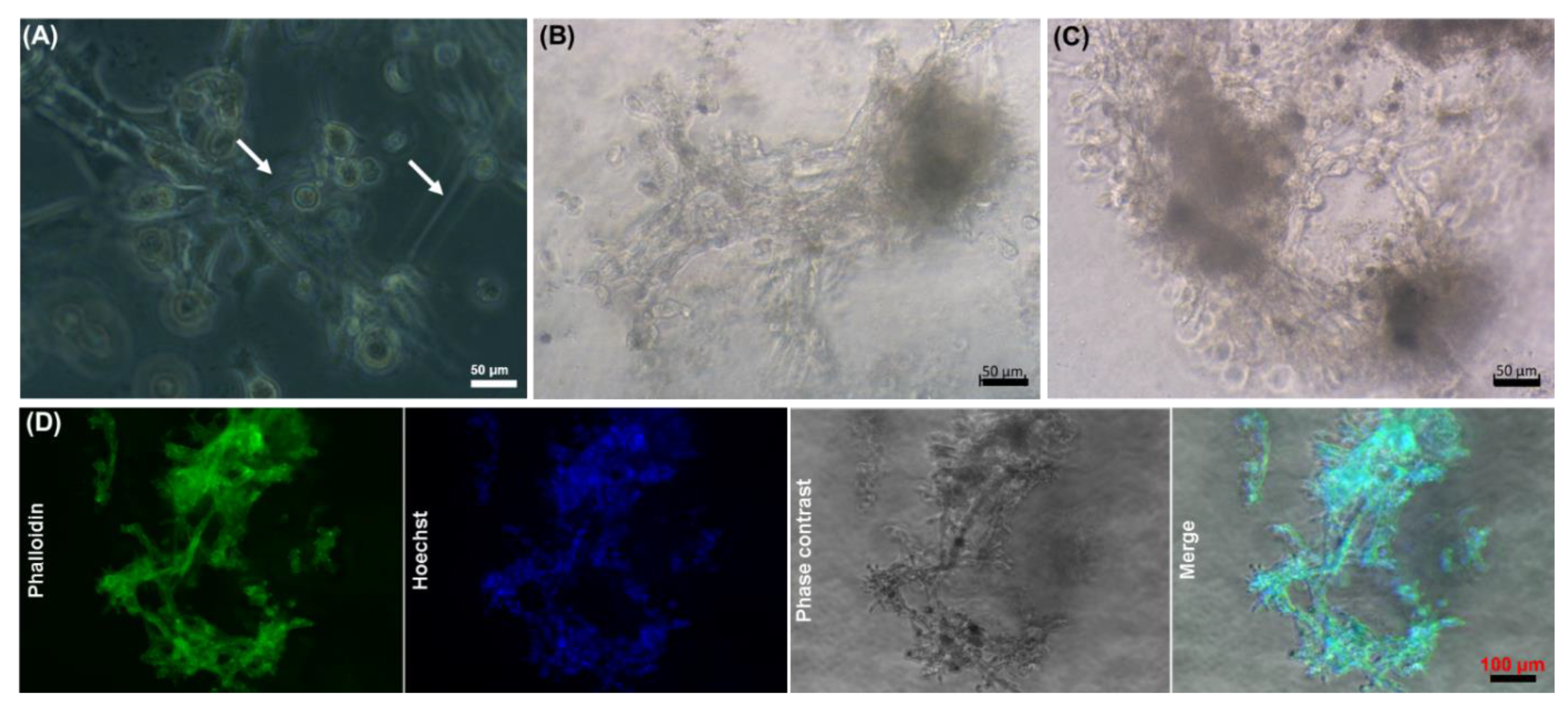
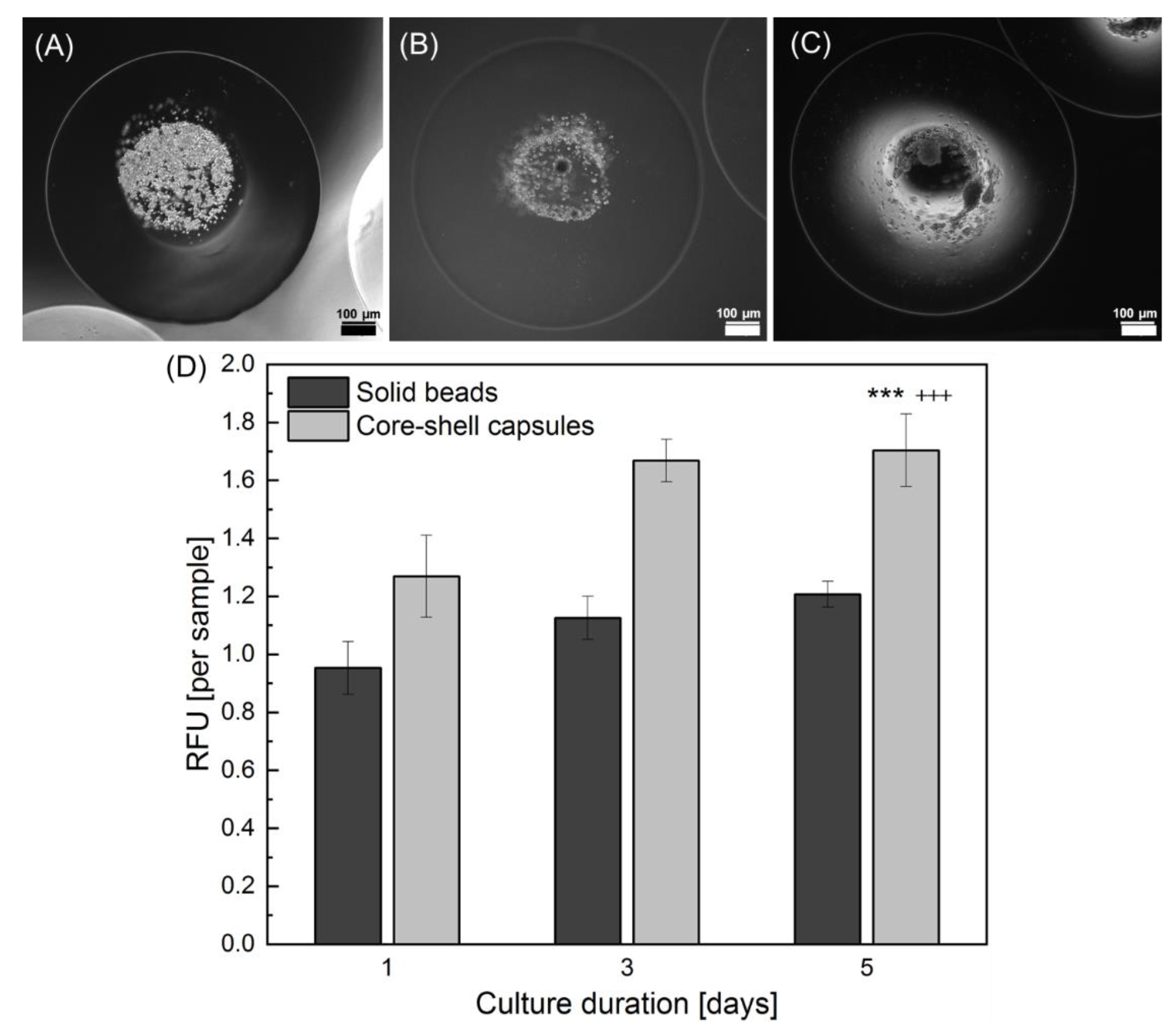
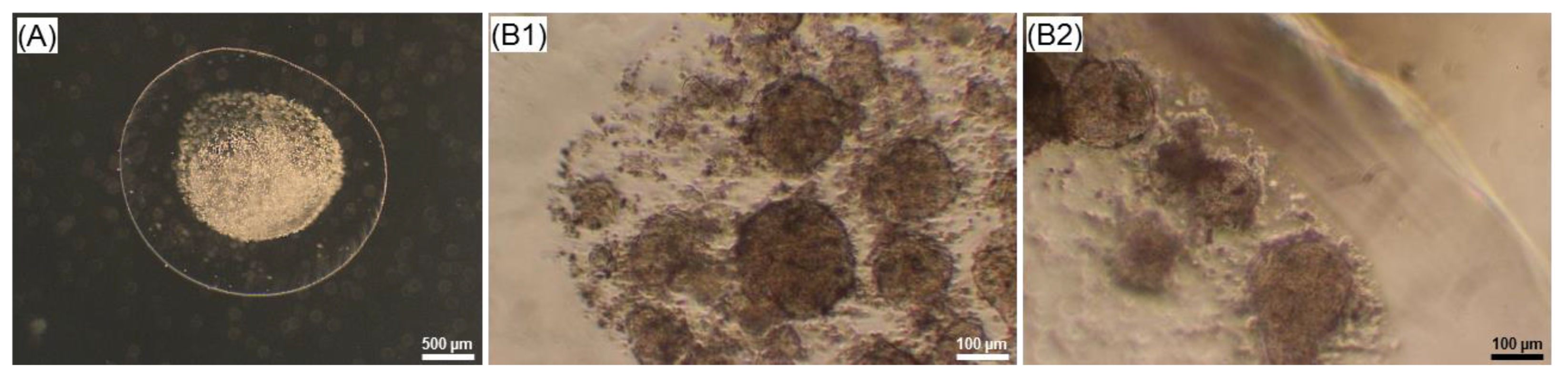
2.4. Effect of the Core-Shell Capsules and Solid Beads on the Cell Viability
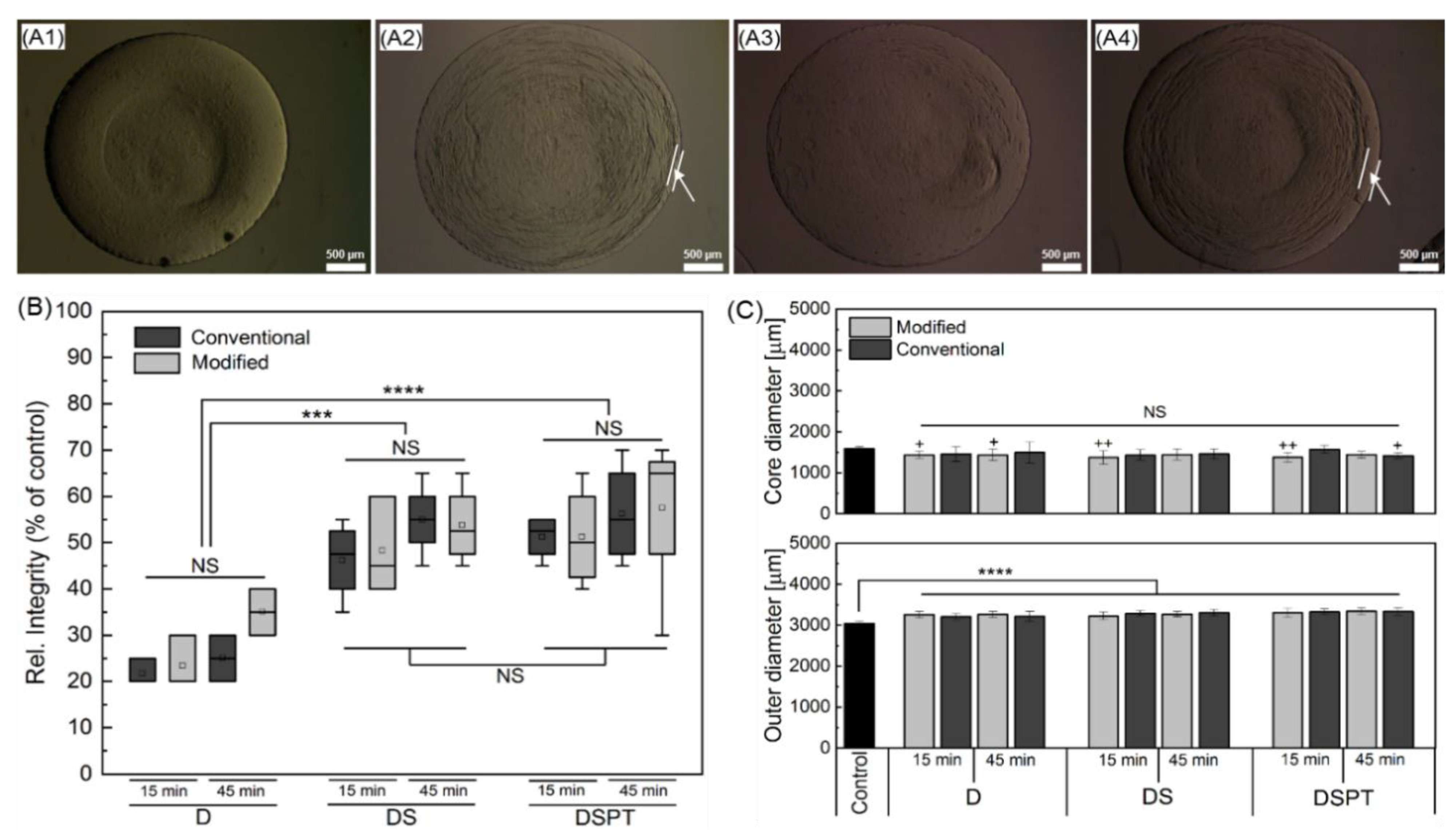
2.5. Selection of Parameters for the Cryopreservation of Cell-Free Core-Shell Capsules
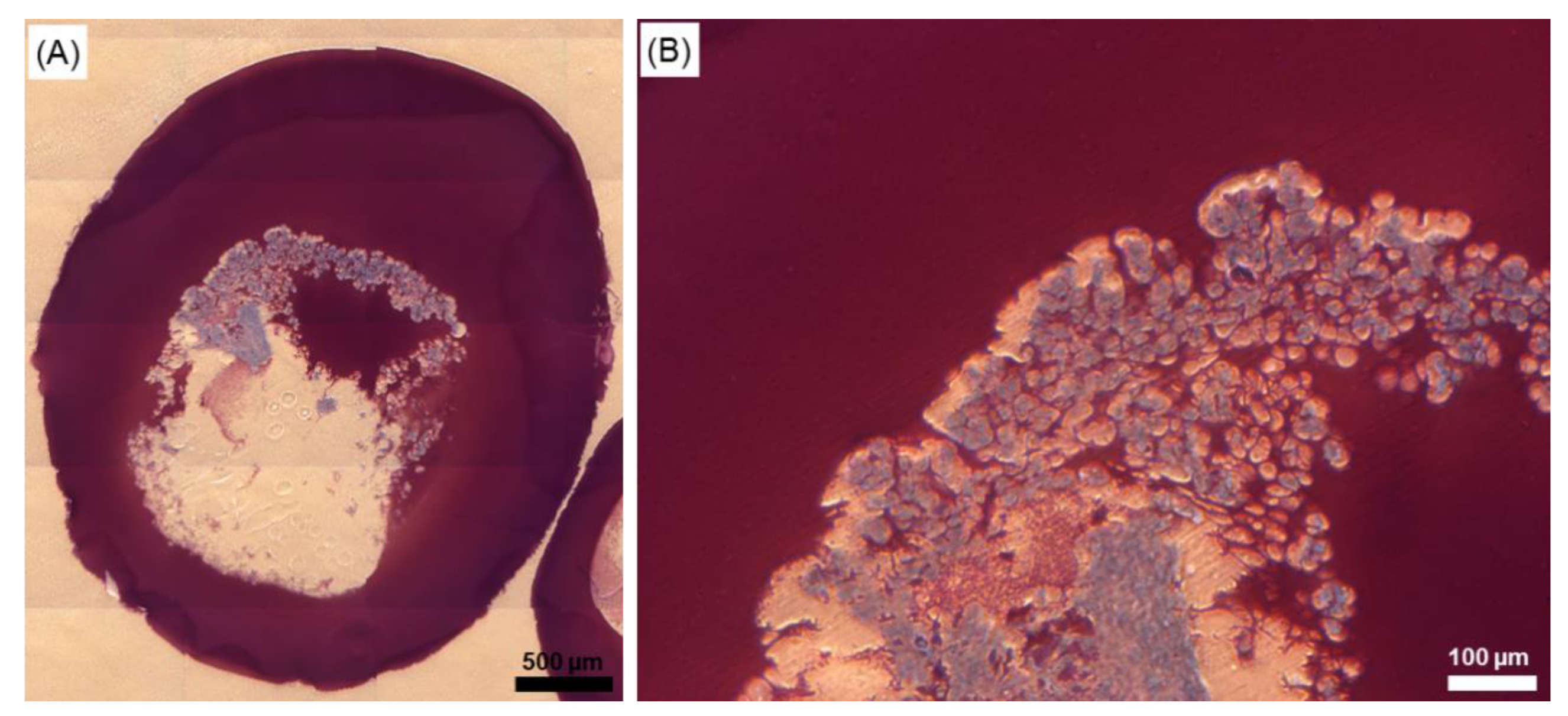
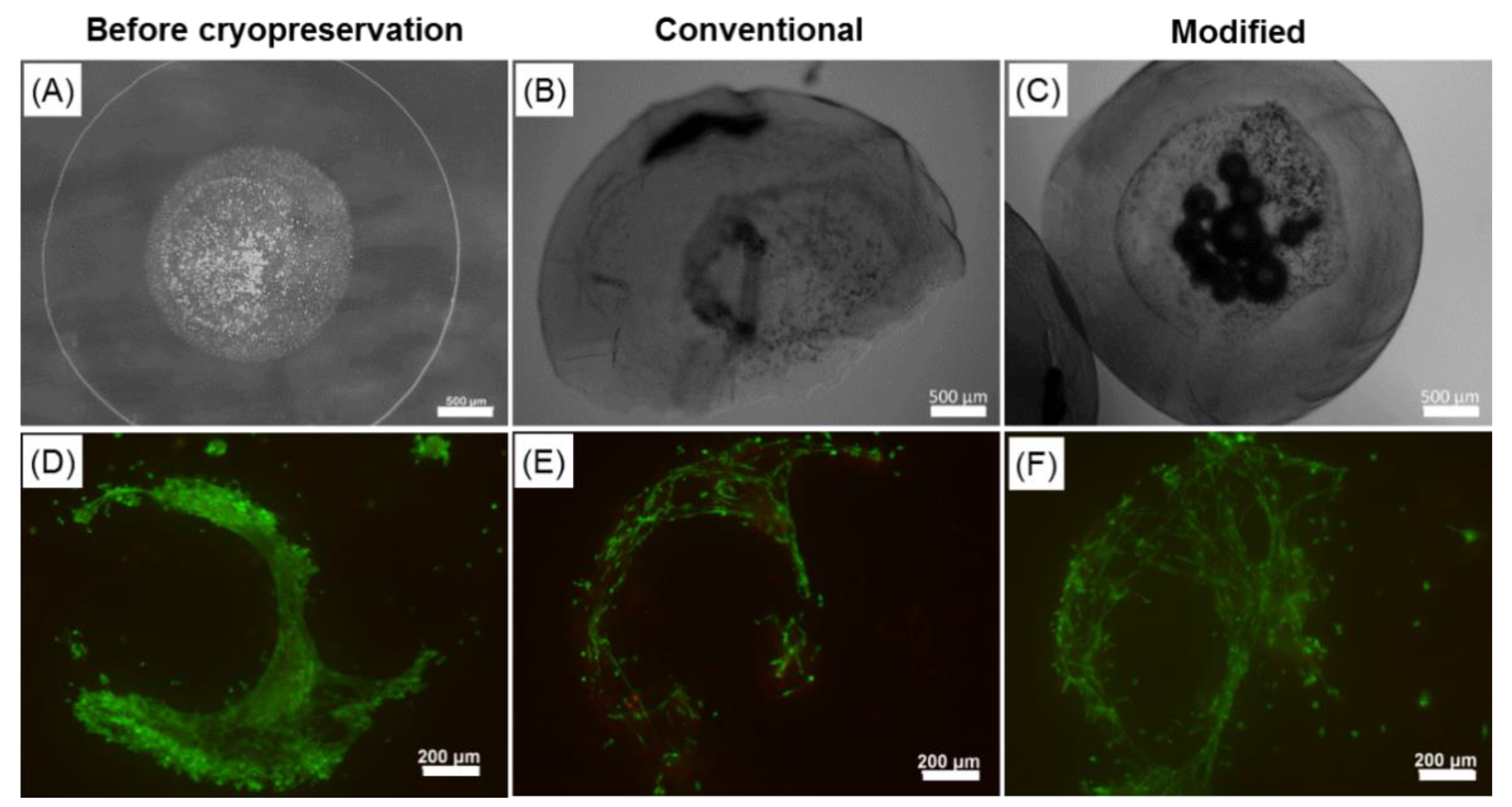
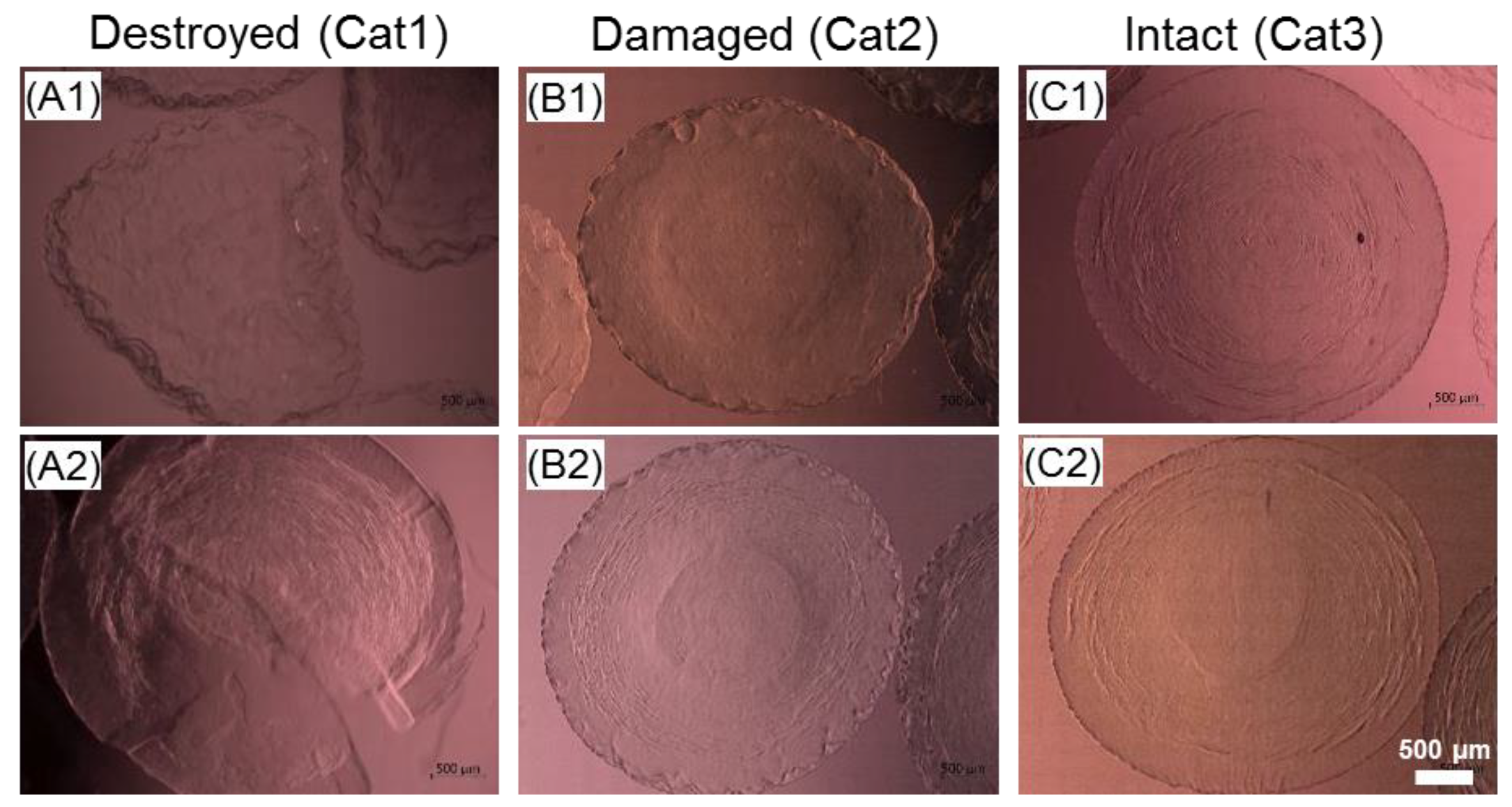
2.6. Effect of the Cryopreservation on Cell-Encapsulated Core-Shell Capsules
3. Discussion
3.1. Reproducible Production of Core-Shell Capsules Using Coaxial Electrospraying
3.2. Structural Peculiarities and Swelling Behaviour of the Core-Shell Capsules and Solid Beads
3.3. Core-Shell Capsules Are Superior to Solid Beads in Terms of Long-Term Cell Culture and Formation of 3D Self-Assembled Cellular Structures
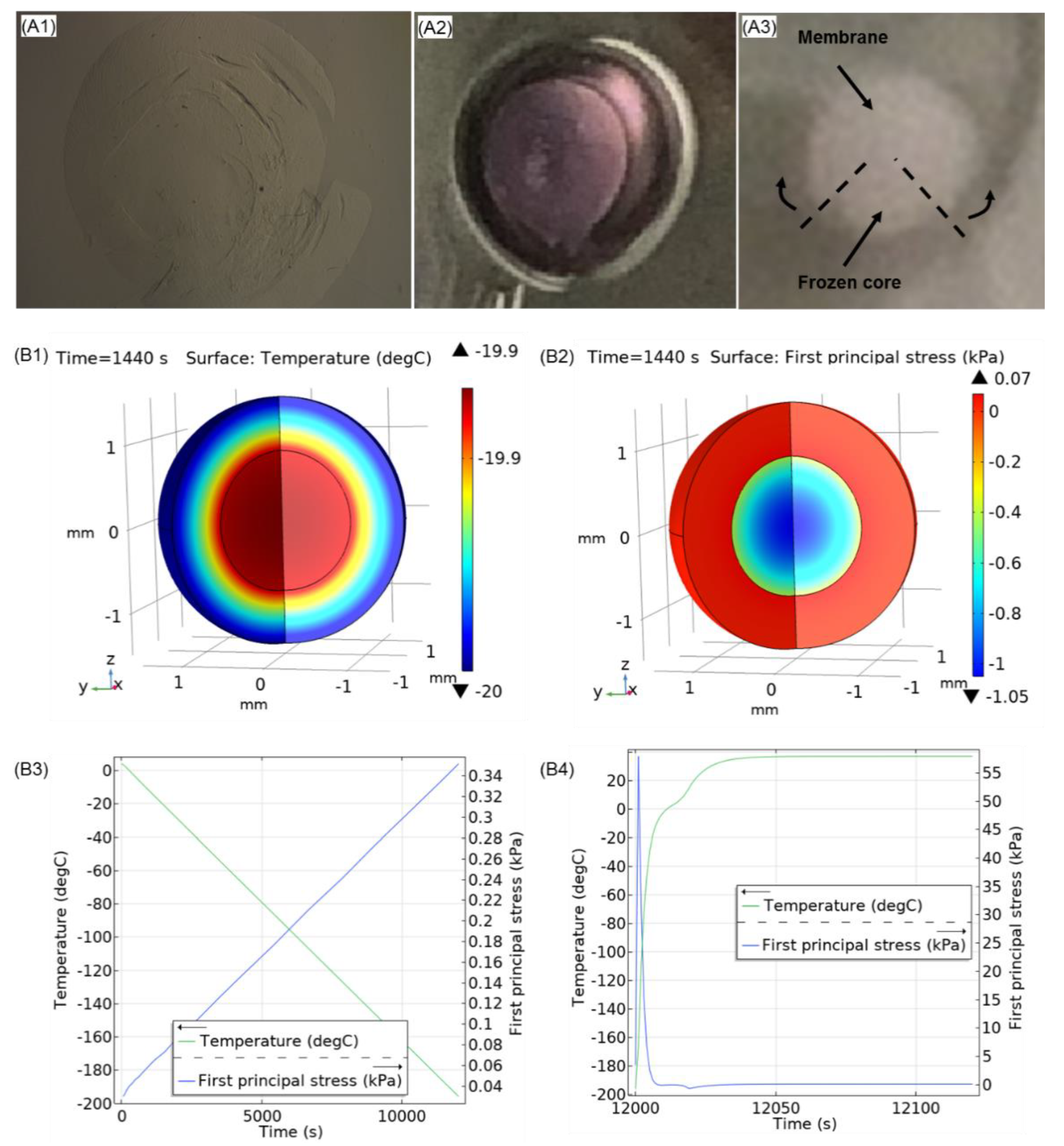
3.4. Steps towards Efficient Cryopreservation
3.5. Future Prospects and Limitations
3.5.1. Application of Alginates of Clinical Grade
3.5.2. Efficiency of Formation of Self-Organized Cellular Structures
3.5.3. Preservation of the Alginate Membrane Integrity and Mechanical Properties of Formed Hydrogel Constructs after Cryopreservation
3.5.4. Effect of Hydrogel Material Properties on the Encapsulated Cells
4. Materials and Methods
4.1. Preparation of Core-Shell Capsules and Solid Beads
4.2. RAMAN Spectroscopy, Water Content and Swelling Behaviour
4.3. Cell Culture and Encapsulation
4.4. Cryopreservation
4.4.1. Cryopreservation of Cell-Free Core-Shell Capsules
4.4.2. Cryopreservation of Cell-Laden Core-Shell Capsules
4.5. Analysis of the Cell Viability, Metabolic Activity, Cell–Cell and Cell-Scaffold Interactions
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, S.K.; Kinney, R.C.; Schwartz, Z.; Boyan, B.D. Microencapsulation of Stem Cells for Therapy. Methods Mol. Biol. 2017, 1479, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Kaczmarek-Pawelska, A. Alginate-Based Hydrogels in Regenerative Medicine. In Alginates—Recent Uses of This Natural Polymer; Pereira, L., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-641-5. [Google Scholar]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Donati, I.; Holtan, S.; Mørch, Y.A.; Borgogna, M.; Dentini, M.; Skjåk-Braek, G. New hypothesis on the role of alternating sequences in calcium-alginate gels. Biomacromolecules 2005, 6, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Sarker, B.; Boccaccini, A.R. Alginate Utilization in Tissue Engineering and Cell Therapy. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; pp. 121–155. ISBN 978-981-10-6909-3. [Google Scholar]
- Pravdyuk, A.I.; Petrenko, Y.A.; Fuller, B.J.; Petrenko, A.Y. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology 2013, 66, 215–222. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering Approaches for Bladder Regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Hydrogel Cryopreservation System: An Effective Method for Cell Storage. Int. J. Mol. Sci. 2018, 19, 3330. [Google Scholar] [CrossRef]
- Smidsrød, O.; Skjåk-Braek, G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990, 8, 71–78. [Google Scholar] [CrossRef]
- Bochenek, M.A.; Veiseh, O.; Vegas, A.J.; McGarrigle, J.J.; Qi, M.; Marchese, E.; Omami, M.; Doloff, J.C.; Mendoza-Elias, J.; Nourmohammadzadeh, M.; et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2018, 2, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; Paredes-Juarez, G.A.; Niclou, S.P.; De Vos, P. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J. Mech. Behav. Biomed. Mater. 2014, 37, 196–208. [Google Scholar] [CrossRef]
- de Vos, P.; Faas, M.M.; Strand, B.; Calafiore, R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 2006, 27, 5603–5617. [Google Scholar] [CrossRef]
- Safley, S.A.; Cui, H.; Cauffiel, S.; Tucker-Burden, C.; Weber, C.J. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine. J. Diabetes Sci. Technol. 2008, 2, 760–767. [Google Scholar] [CrossRef]
- Köllmer, M.; Appel, A.A.; Somo, S.I.; Brey, E.M. Long-Term Function of Alginate-Encapsulated Islets. Tissue Eng. Part B Rev. 2016, 22, 34–46. [Google Scholar] [CrossRef]
- Selden, C.; Bundy, J.; Erro, E.; Puschmann, E.; Miller, M.; Kahn, D.; Hodgson, H.; Fuller, B.; Gonzalez-Molina, J.; Le Lay, A.; et al. A clinical-scale BioArtificial Liver, developed for GMP, improved clinical parameters of liver function in porcine liver failure. Sci. Rep. 2017, 7, 14518. [Google Scholar] [CrossRef]
- Qi, M.; Strand, B.L.; Mørch, Y.; Lacík, I.; Wang, Y.; Salehi, P.; Barbaro, B.; Gangemi, A.; Kuechle, J.; Romagnoli, T.; et al. Encapsulation of human islets in novel inhomogeneous alginate-ca2+/ba2+ microbeads: In vitro and in vivo function. Artif. Cells Blood Substit. Immobil. Biotechnol. 2008, 36, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Najia, M.A.; Saeed, R.; McDevitt, T.C. Alginate encapsulation parameters influence the differentiation of microencapsulated embryonic stem cell aggregates. Biotechnol. Bioeng. 2014, 111, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-C.; Chen, W.-C.; Chiang, C.-W.; Chen, L.-Y.; Chang, Y.-C.; Chen, C.-H. Differentiation Effects of Platelet-Rich Plasma Concentrations on Synovial Fluid Mesenchymal Stem Cells from Pigs Cultivated in Alginate Complex Hydrogel. Int. J. Mol. Sci. 2015, 16, 18507–18521. [Google Scholar] [CrossRef]
- Chayosumrit, M.; Tuch, B.; Sidhu, K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials 2010, 31, 505–514. [Google Scholar] [CrossRef]
- Kim, W.S.; Mooney, D.J.; Arany, P.R.; Lee, K.; Huebsch, N.; Kim, J. Adipose tissue engineering using injectable, oxidized alginate hydrogels. Tissue Eng. Part A 2012, 18, 737–743. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Torres, A.L.; Barrias, C.C. Injectable Cell Delivery Systems Based on Alginate Hydrogels for Regenerative Therapies. Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. [Google Scholar]
- Kang, A.; Park, J.; Ju, J.; Jeong, G.S.; Lee, S.-H. Cell encapsulation via microtechnologies. Biomaterials 2014, 35, 2651–2663. [Google Scholar] [CrossRef]
- Gryshkov, O.; Pogozhykh, D.; Zernetsch, H.; Hofmann, N.; Mueller, T.; Glasmacher, B. Process engineering of high voltage alginate encapsulation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Hunckler, M.; Ramasubramanian, M.K.; Opara, E.C.; Katuri, K.C. Microfluidic Approach to Cell Microencapsulation. Methods Mol. Biol. 2017, 1479, 71–76. [Google Scholar] [CrossRef]
- Prüsse, U.; Bilancetti, L.; Bučko, M.; Bugarski, B.; Bukowski, J.; Gemeiner, P.; Lewińska, D.; Manojlovic, V.; Massart, B.; Nastruzzi, C.; et al. Comparison of different technologies for alginate beads production. Chem. Pap. 2008, 62. [Google Scholar] [CrossRef]
- Gryshkov, O.; Pogozhykh, D.; Hofmann, N.; Pogozhykh, O.; Mueller, T.; Glasmacher, B. Encapsulating non-human primate multipotent stromal cells in alginate via high voltage for cell-based therapies and cryopreservation. PLoS ONE 2014, 9, e107911. [Google Scholar] [CrossRef]
- Hu, M.; Zheng, G.; Zhao, D.; Yu, W. Characterization of the structure and diffusion behavior of calcium alginate gel beads. J. Appl. Polym. Sci. 2020, 137, 48923. [Google Scholar] [CrossRef]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Workman, V.L.; Dunnett, S.B.; Kille, P.; Palmer, D.D. Microfluidic chip-based synthesis of alginate microspheres for encapsulation of immortalized human cells. Biomicrofluidics 2007, 1, 14105. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, I.; Sakai, Y. Alginate Encapsulation of Pluripotent Stem Cells Using a Co-axial Nozzle. J. Vis. Exp. 2015, e52835. [Google Scholar] [CrossRef]
- Urbani, L.; Maghsoudlou, P.; Milan, A.; Menikou, M.; Hagen, C.K.; Totonelli, G.; Camilli, C.; Eaton, S.; Burns, A.; Olivo, A.; et al. Long-term cryopreservation of decellularised oesophagi for tissue engineering clinical application. PLoS ONE 2017, 12, e0179341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qin, T. Review: Vitreous Cryopreservation of Tissue-engineered Compositions for Tissue Repair. J. Med. Biol. Eng. 2013, 33, 125. [Google Scholar] [CrossRef]
- Shi, Q.; Xie, Y.; Wang, Y.; Li, S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: A systematic review and meta-anlaysis. Sci. Rep. 2017, 7, 8538. [Google Scholar] [CrossRef]
- Leonel, E.C.R.; Corral, A.; Risco, R.; Camboni, A.; Taboga, S.R.; Kilbride, P.; Vazquez, M.; Morris, J.; Dolmans, M.-M.; Amorim, C.A. Stepped vitrification technique for human ovarian tissue cryopreservation. Sci. Rep. 2019, 9, 20008. [Google Scholar] [CrossRef] [PubMed]
- Pegg, D.E. The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 2010, 60, S36–S44. [Google Scholar] [CrossRef]
- Huang, H.; Choi, J.K.; Rao, W.; Zhao, S.; Agarwal, P.; Zhao, G.; He, X. Alginate Hydrogel Microencapsulation Inhibits Devitrification and Enables Large-Volume Low-CPA Cell Vitrification. Adv. Funct. Mater. 2015, 25, 6839–6850. [Google Scholar] [CrossRef] [PubMed]
- Camboni, A.; van Langendonckt, A.; Donnez, J.; Vanacker, J.; Dolmans, M.M.; Amorim, C.A. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 2013, 67, 64–69. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Prokopyuk, O.; Kuleshova, L.; Goltsev, A.; Figueiredo, C.; Pogozhykh, D. Towards biobanking technologies for natural and bioengineered multicellular placental constructs. Biomaterials 2018, 185, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Correia, C.; Malpique, R.; Brito, C.; Jensen, J.; Bjorquist, P.; Carrondo, M.J.T.; Alves, P.M. Microencapsulation technology: A powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS ONE 2011, 6, e23212. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Fu, D.-J.; An, D.; Chiu, A.; Schwartz, R.; Nikitin, A.Y.; Ma, M. Scalable Production and Cryostorage of Organoids Using Core-Shell Decoupled Hydrogel Capsules. Adv. Biosyst. 2017, 1. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, X.; Zhu, K.; He, X. Hydrogel Encapsulation Facilitates Rapid-Cooling Cryopreservation of Stem Cell-Laden Core-Shell Microcapsules as Cell-Biomaterial Constructs. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, M.; Meinberg, H.; Büchs, J.; Koss, H.-J.; Ansorge-Schumacher, M.B. Method for quantitative determination of spatial polymer distribution in alginate beads using Raman spectroscopy. Appl. Spectrosc. 2005, 59, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Messmer, A.; Yeo, B.-S.; Zhang, W.; Zenobi, R. Towards chemical analysis of nanostructures in biofilms II: Tip-enhanced Raman spectroscopy of alginates. Anal. Bioanal. Chem. 2008, 391, 1907–1916. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Rogulska, O.Y.; Mutsenko, V.V.; Petrenko, A.Y. A sugar pretreatment as a new approach to the Me2SO- and xeno-free cryopreservation of human mesenchymal stromal cells. CryoLetters 2014, 35, 239–246. [Google Scholar]
- Mutsenko, V.; Knaack, S.; Lauterboeck, L.; Tarusin, D.; Sydykov, B.; Cabiscol, R.; Ivnev, D.; Belikan, J.; Beck, A.; Dipresa, D.; et al. Effect of ‘in air’ freezing on post-thaw recovery of Callithrix jacchus mesenchymal stromal cells and properties of 3D collagen-hydroxyapatite scaffolds. Cryobiology 2020, 92, 215–230. [Google Scholar] [CrossRef]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-Derived Polymeric Materials and Biomimetics: An Overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Gu, F.; Amsden, B.; Neufeld, R. Sustained delivery of vascular endothelial growth factor with alginate beads. J. Control. Release 2004, 96, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, X. Encapsulation of living cells in small (approximately 100 microm) alginate microcapsules by electrostatic spraying: A parametric study. J. Biomech. Eng. 2009, 131, 74515. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Chemometric prediction of alginate monomer composition: A comparative spectroscopic study using IR, Raman, NIR and NMR. Carbohydr. Polym. 2008, 72, 730–739. [Google Scholar] [CrossRef]
- Pielesz, A.; Bak, M.K.K. Raman spectroscopy and WAXS method as a tool for analysing ion-exchange properties of alginate hydrogels. Int. J. Biol. Macromol. 2008, 43, 438–443. [Google Scholar] [CrossRef]
- Pamies, R.; Schmidt, R.R.; Martínez, M.d.C.L.; La Torre, J.G.d. The influence of mono and divalent cations on dilute and non-dilute aqueous solutions of sodium alginates. Carbohydr. Polym. 2010, 80, 248–253. [Google Scholar] [CrossRef]
- Bruchet, M.; Mendelson, N.; Melman, A. Photochemical Patterning of Ionically Cross-Linked Hydrogels. Processes 2013, 1, 153–166. [Google Scholar] [CrossRef]
- Stokke, B.T.; Smidsrød, O.; Zanetti, F.; Strand, W.; Skjåk-Bræk, G. Distribution of uronate residues in alginate chains in relation to alginate gelling properties—2: Enrichment of β-d-mannuronic acid and depletion of α-l-guluronic acid in sol fraction. Carbohydr. Polym. 1993, 21, 39–46. [Google Scholar] [CrossRef]
- Draget, K.I.; Strand, B.; Hartmann, M.; Valla, S.; Smidsrød, O.; Skjåk-Bræk, G. Ionic and acid gel formation of epimerised alginates; the effect of AlgE4. Int. J. Biol. Macromol. 2000, 27, 117–122. [Google Scholar] [CrossRef]
- Chandía, N. Alginic acids in Lessonia trabeculata: Characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Campos-Vallette, M.M.; Chandía, N.P.; Clavijo, E.; Leal, D.; Matsuhiro, B.; Osorio-Román, I.O.; Torres, S. Characterization of sodium alginate and its block fractions by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2010, 758–763. [Google Scholar] [CrossRef]
- Ramos, P.E.; Silva, P.; Alario, M.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A.; Vicente, A.A. Effect of alginate molecular weight and M/G ratio in beads properties foreseeing the protection of probiotics. Food Hydrocoll. 2018, 77, 8–16. [Google Scholar] [CrossRef]
- Montanucci, P.; Terenzi, S.; Santi, C.; Pennoni, I.; Bini, V.; Pescara, T.; Basta, G.; Calafiore, R. Insights in Behavior of Variably Formulated Alginate-Based Microcapsules for Cell Transplantation. BioMed Res. Int. 2015, 2015, 965804. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Xiao, W.; Biswas, S.; Cong, Z.-Q.; Liu, X.-M.; Lam, K.S.; Liao, Y.-H.; Deng, W. Alginate Hydrogel Modified with a Ligand Interacting with α3β1 Integrin Receptor Promotes the Differentiation of 3D Neural Spheroids toward Oligodendrocytes in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 5821–5833. [Google Scholar] [CrossRef]
- Mitry, R.R.; Jitraruch, S.; Iansante, V.; Dhawan, A. Alginate Encapsulation of Human Hepatocytes and Assessment of Microbeads. Methods Mol. Biol. 2017, 1506, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Agarwal, P.; Rao, W.; Huang, H.; Zhang, R.; Liu, Z.; Yu, J.; Weisleder, N.; Zhang, W.; He, X. Coaxial electrospray of liquid core-hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr. Biol. 2014, 6, 874–884. [Google Scholar] [CrossRef]
- Tarusin, D.; Mazur, S.; Volkova, N.; Yu, P.; Zaikov, V.; Petrenko, A. Encapsulation of mesenchymal stromal cells in alginate microspheres. Biotechnol. Acta 2016, 9, 58–66. [Google Scholar] [CrossRef][Green Version]
- Gryshkov, O.; Hofmann, N.; Lauterboeck, L.; Pogozhykh, D.; Mueller, T.; Glasmacher, B. Multipotent stromal cells derived from common marmoset Callithrix jacchus within alginate 3D environment: Effect of cryopreservation procedures. Cryobiology 2015, 71, 103–111. [Google Scholar] [CrossRef]
- Mutsenko, V.; Tarusin, D.; Sydykov, B.; Zaragotas, D.; Simaioforidou, A.; Rozanski, C.; Gryshkov, O.; Wolkers, W.F.; Braslavsky, I.; Anastassopoulos, E.; et al. Study of natural ice-nucleating agents for cryopreservation of 3D tissue-engineered scaffolds. Cryobiology 2018, 85, 176. [Google Scholar] [CrossRef]
- Zaragotas, D.; Liolios, N.T.; Anastassopoulos, E. Supercooling, ice nucleation and crystal growth: A systematic study in plant samples. Cryobiology 2016, 72, 239–243. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, X.; Wang, X.; Chen, L.; Xie, H.; Wang, F.; Zheng, H.; Yu, W.; Ma, X. Improving stability and biocompatibility of alginate/chitosan microcapsule by fabricating bi-functional membrane. Macromol. Biosci. 2014, 14, 655–666. [Google Scholar] [CrossRef]
- Bhattacharya, S. Cryopretectants and Their Usage in Cryopreservation Process. In Cryopreservation Biotechnology in Biomedical and Biological Sciences; Bozkurt, Y., Ed.; IntechOpen: London, UK, 2018; ISBN 978-1-78984-680-5. [Google Scholar]
- Solanki, P.K.; Bischof, J.C.; Rabin, Y. Thermo-mechanical stress analysis of cryopreservation in cryobags and the potential benefit of nanowarming. Cryobiology 2017, 76, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.P.; Bischof, J.C.; Rabin, Y. Thermomechanical Stress in Cryopreservation Via Vitrification With Nanoparticle Heating as a Stress-Moderating Effect. J. Biomech. Eng. 2016, 138. [Google Scholar] [CrossRef] [PubMed]
- Tomás, R.M.F.; Bailey, T.L.; Hasan, M.; Gibson, M.I. Extracellular Antifreeze Protein Significantly Enhances the Cryopreservation of Cell Monolayers. Biomacromolecules 2019, 20, 3864–3872. [Google Scholar] [CrossRef]
- Lauterboeck, L.; Hofmann, N.; Mueller, T.; Glasmacher, B. Active control of the nucleation temperature enhances freezing survival of multipotent mesenchymal stromal cells. Cryobiology 2015, 71, 384–390. [Google Scholar] [CrossRef]
- Morris, G.J.; Acton, E. Controlled ice nucleation in cryopreservation—A review. Cryobiology 2013, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.J. Technical Considerations in the Freezing, Low-Temperature Storage and Thawing of Stem Cells for Cellular Therapies. Transfus. Med. Hemother. 2019, 46, 134–150. [Google Scholar] [CrossRef]
- Chiu-Lam, A.; Staples, E.; Pepine, C.J.; Rinaldi, C. Perfusion, cryopreservation, and nanowarming of whole hearts using colloidally stable magnetic cryopreservation agent solutions. Sci. Adv. 2021, 7, eabe3005. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Pogozhykh, D.; Neehus, A.-L.; Hoffmann, A.; Blasczyk, R.; Müller, T. Molecular and cellular characteristics of human and non-human primate multipotent stromal cells from the amnion and bone marrow during long term culture. Stem Cell Res. Ther. 2015, 6, 150. [Google Scholar] [CrossRef]
- Ishikawa, D.; Diekmann, U.; Fiedler, J.; Just, A.; Thum, T.; Lenzen, S.; Naujok, O. miRNome Profiling of Purified Endoderm and Mesoderm Differentiated from hESCs Reveals Functions of miR-483-3p and miR-1263 for Cell-Fate Decisions. Stem Cell Rep. 2017, 9, 1588–1603. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; El Haj, A.J.; Then, K.Y.; Liu, K.-K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2005, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Knez, Ž.; Novak, Z. Formation of polysaccharide aerogels in ethanol. RSC Adv. 2015, 5, 77362–77371. [Google Scholar] [CrossRef]
- Kilbride, P.; Morris, G.J. Viscosities encountered during the cryopreservation of dimethyl sulphoxide systems. Cryobiology 2017, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, L.E.; Feig, J.S.G.; Schiffres, S.N.; Malen, J.A.; Rabin, Y. Large Thermal Conductivity Differences between the Crystalline and Vitrified States of DMSO with Applications to Cryopreservation. PLoS ONE 2015, 10, e0125862. [Google Scholar] [CrossRef]
- López Molina, J.A.; Rivera, M.J.; Berjano, E. Fourier, hyperbolic and relativistic heat transfer equations: A comparative analytical study. Proc. R. Soc. A 2014, 470, 20140547. [Google Scholar] [CrossRef]
| Property | Core-Shell Capsules (n = 15) | Solid Beads (n = 15) | ||
|---|---|---|---|---|
| LV | MV | LV | MV | |
| Hydrogel weight *, mg | 175.8 ± 2.7 | 225.7 ± 2.5 | 180.0 ± 12.2 | 212.6 ± 2.5 |
| Dry weight *, mg | 6.4 ± 0.3 | 9.3 ± 0.3 | 7.6 ± 0.3 | 10.4 ± 0.2 |
| Water uptake (Wu), % | 96.4 ± 0.2 | 95.9 ± 0.2 | 95.8 ± 0.4 | 95.1 ± 0.1 |
| Water content (Wc), mgwater/mgdry | 26.7 ± 1.4 | 23.4 ± 0.9 | 22.7 ± 2.1 | 19.5 ± 0.3 |
| Test/Analysis | Alginate Concentration and Type, % | Type of Nozzles (Inner—Outer) | Voltage, kV | Spraying Distance, cm | Inner to Outer Nozzle Distance, mm | Flow Rates (Outer:Inner), mL/h:mL/h | Gelling Time, min |
|---|---|---|---|---|---|---|---|
| Density, viscosity, conductivity | 1.5% LV 2.0% LV | - | - | - | - | - | - |
| Main optimisation studies * | 1.5% LV caps | 21G–14G | 0–17.5 | 5–10 | 0–1.0 | 4:2–50:10 | 30 |
| 2.0% LV caps | 21G–14G | 0–1.0 | 4:2–50:10 | ||||
| 2.0% LV beads | 14G | - | |||||
| 2.0% MV caps | 21G–14G | 0–1.0 | 4:2–50:10 | ||||
| 2.0% MV beads | 14G | - | |||||
| RAMAN spectroscopy, water content, swelling | 2.0% LV caps | 21G–14G | 9.5 | 5 | 0.5 | 20:4 | 30 |
| 2.0% LV beads | 14G | 9.5 | 5 | - | 20 | ||
| 2.0% MV caps | 21G–14G | 6.5 | 5 | 0.5 | 20:4 | ||
| 2.0% MV beads | 14G | 6.5 | 5 | - | 20 | ||
| Fluorescent staining (cjaMSC-laden) | 2.0% LV caps | 21G–14G | 12 | 10 | 0.5 | 50:15 | 30 |
| 2.0% LV beads | 14G | 12 | 10 | - | 50 | ||
| 2.0% MV caps | 21G–14G | 10 | 10 | 0.5 | 50:10 | ||
| Cryopreservation (cell-free) | 2.0% LV caps 2.0% MV caps | 21G–14G | 10 | 5 | 0.5 | 40:8 | 30 |
| Histology, cryopreservation (cell-laden) | 2.0% LV caps | 21G–14G | 0 | 5 | 0.5 | 40:8 | 30 |
| Metabolic activity (hdMSC-laden) | 2.0% LV caps | 21G–14G | 7 | 4 | 0.5 | 40:10 | 15 |
| 2.0% LV beads | 14G | 7 | 4 | - | 40 | 15 | |
| hESCs encapsulation | 1.5% LV caps | 21G–14G | 18 | 10 | 0.5 | 40:10 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryshkov, O.; Mutsenko, V.; Tarusin, D.; Khayyat, D.; Naujok, O.; Riabchenko, E.; Nemirovska, Y.; Danilov, A.; Petrenko, A.Y.; Glasmacher, B. Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage. Int. J. Mol. Sci. 2021, 22, 3096. https://doi.org/10.3390/ijms22063096
Gryshkov O, Mutsenko V, Tarusin D, Khayyat D, Naujok O, Riabchenko E, Nemirovska Y, Danilov A, Petrenko AY, Glasmacher B. Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage. International Journal of Molecular Sciences. 2021; 22(6):3096. https://doi.org/10.3390/ijms22063096
Chicago/Turabian StyleGryshkov, Oleksandr, Vitalii Mutsenko, Dmytro Tarusin, Diaa Khayyat, Ortwin Naujok, Ekaterina Riabchenko, Yuliia Nemirovska, Arseny Danilov, Alexander Y. Petrenko, and Birgit Glasmacher. 2021. "Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage" International Journal of Molecular Sciences 22, no. 6: 3096. https://doi.org/10.3390/ijms22063096
APA StyleGryshkov, O., Mutsenko, V., Tarusin, D., Khayyat, D., Naujok, O., Riabchenko, E., Nemirovska, Y., Danilov, A., Petrenko, A. Y., & Glasmacher, B. (2021). Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage. International Journal of Molecular Sciences, 22(6), 3096. https://doi.org/10.3390/ijms22063096