Impaired Butyrate Induced Regulation of T Cell Surface Expression of CTLA-4 in Patients with Ulcerative Colitis
Abstract
1. Introduction
2. Results
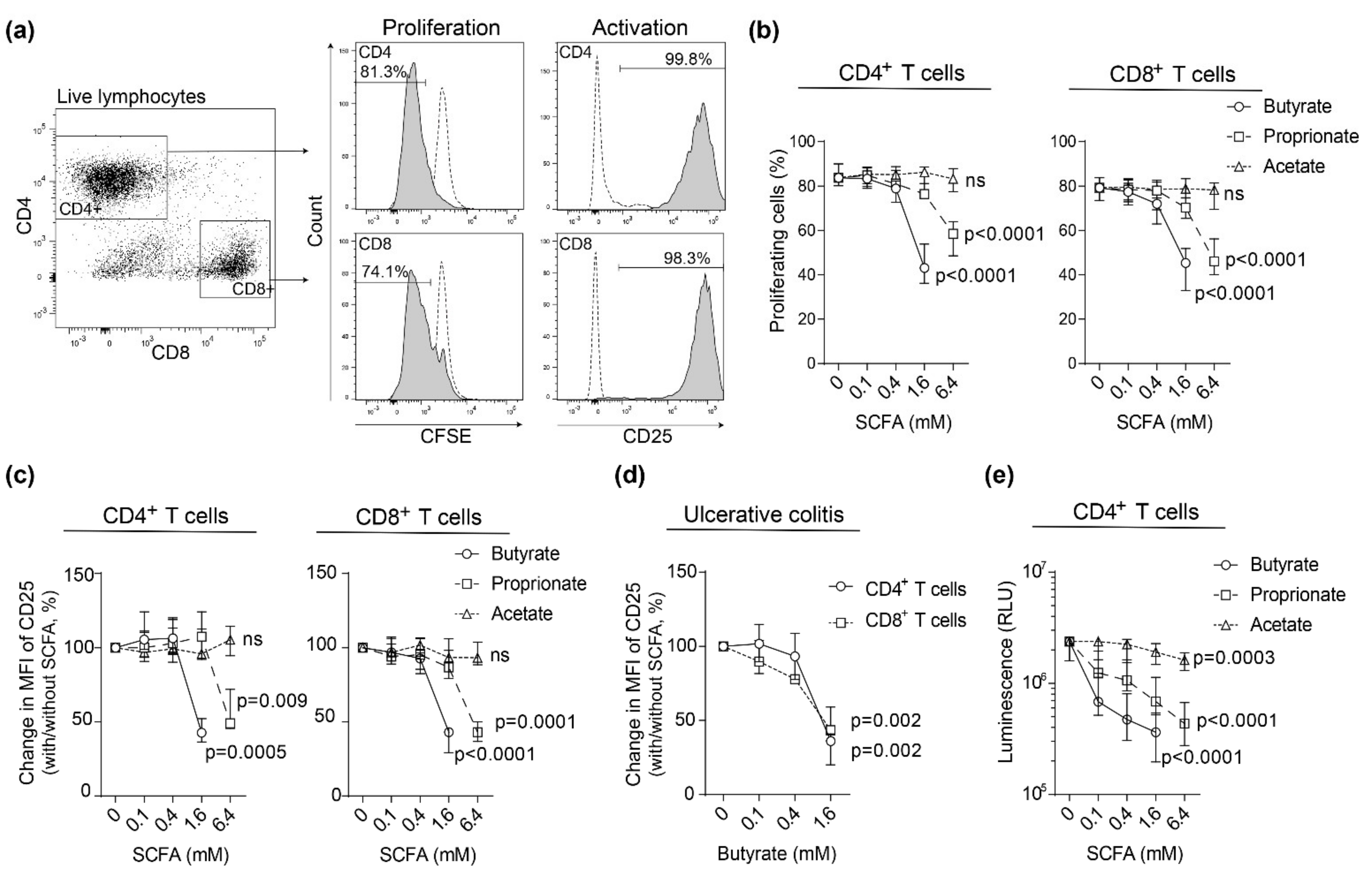
2.1. SCFAs Inhibit Blood T Cell Proliferation and Activation in Healthy Subjects and Patients with Active Ulcerative Colitis
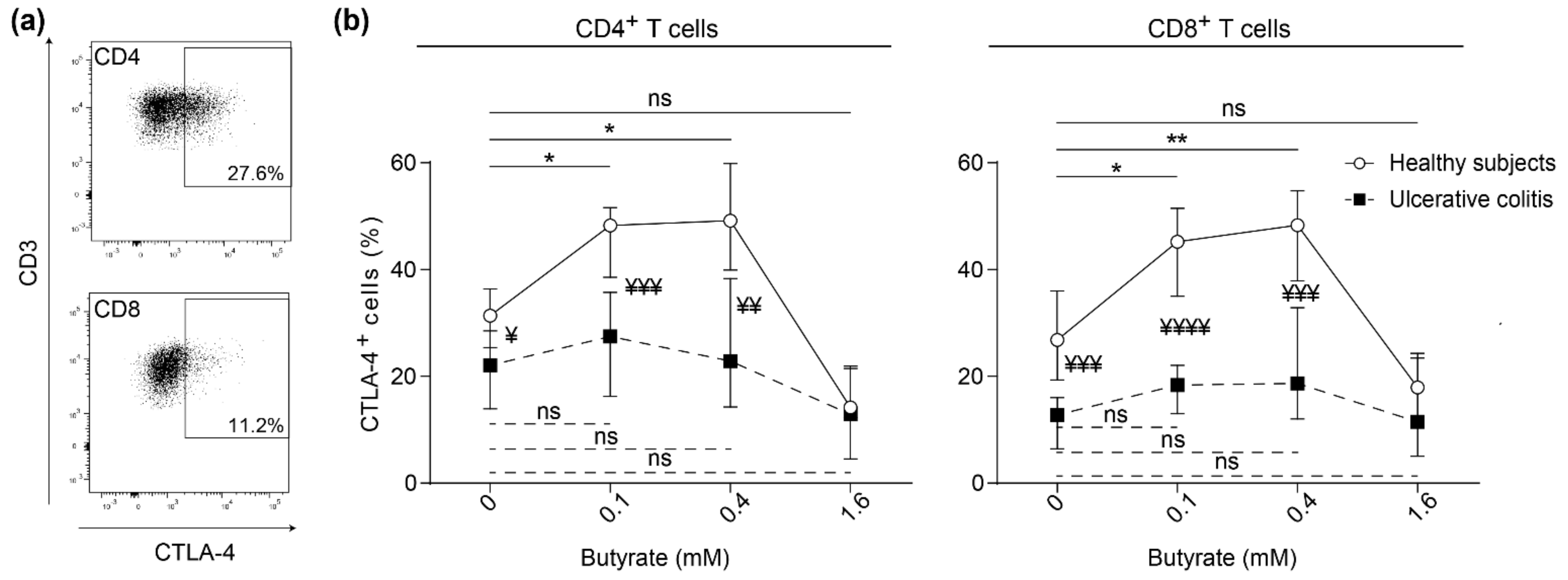
2.2. Butyrate Induces Surface Expression of CTLA-4 on Stimulated Blood T Cells from Healthy Subjects but not on Blood T Cells from Patients with Active Ulcerative Colitis
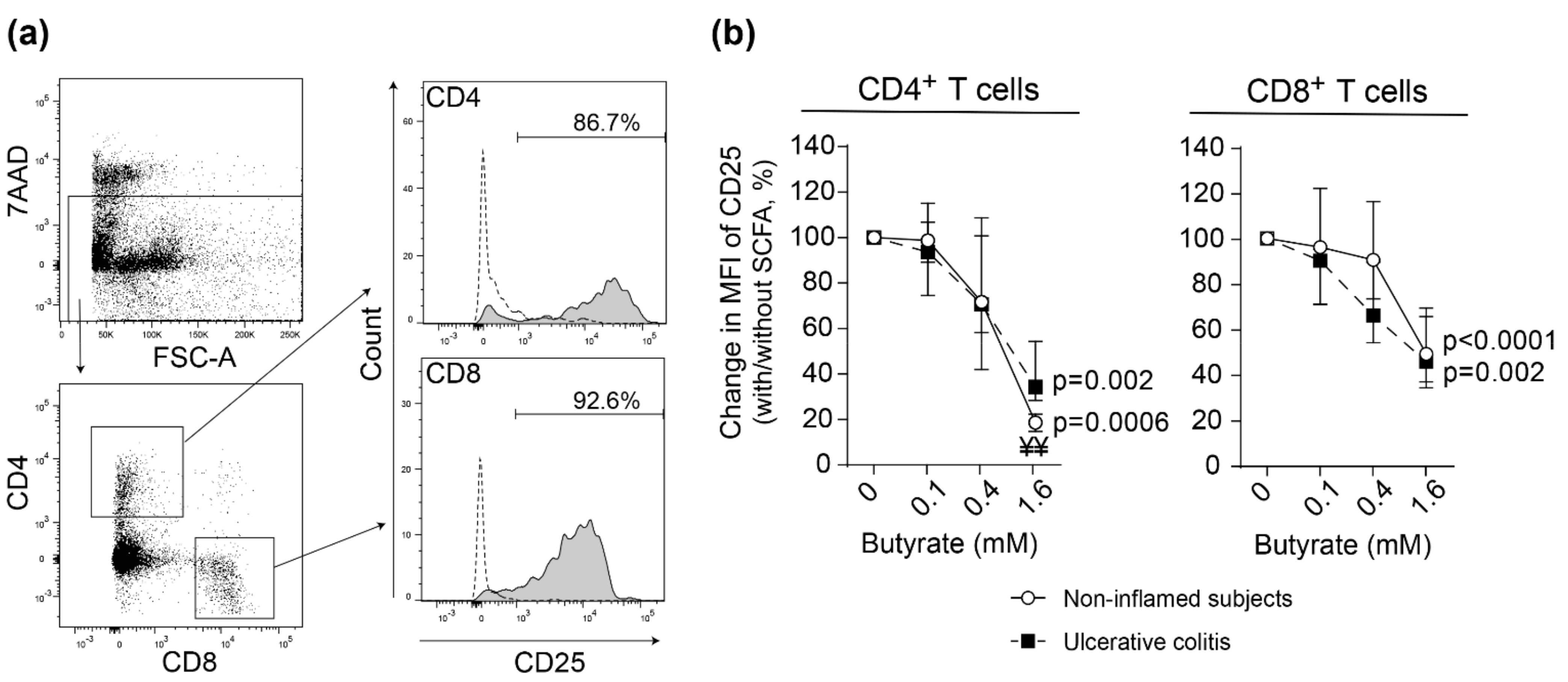
2.3. Butyrate Inhibits Lamina Propria T Cell Activation in Non-Inflamed Subjects and Patients with Active Ulcerative Colitis
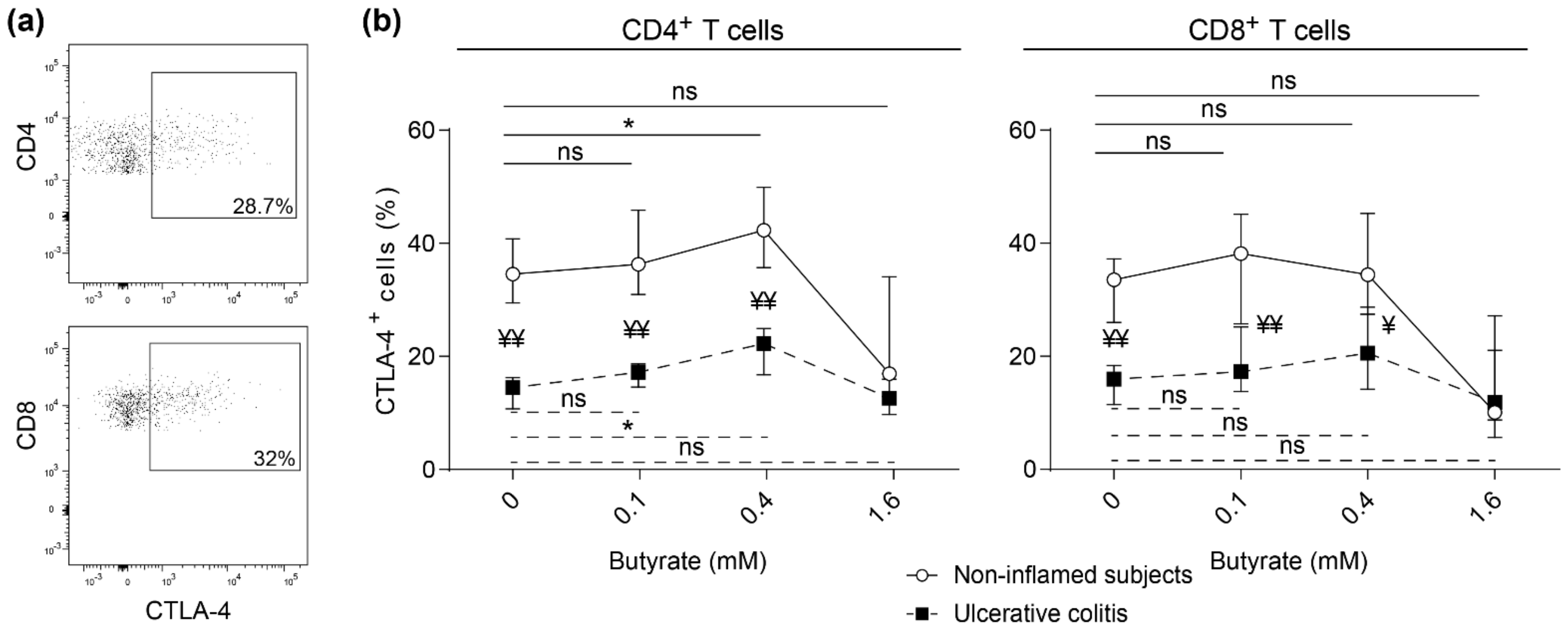
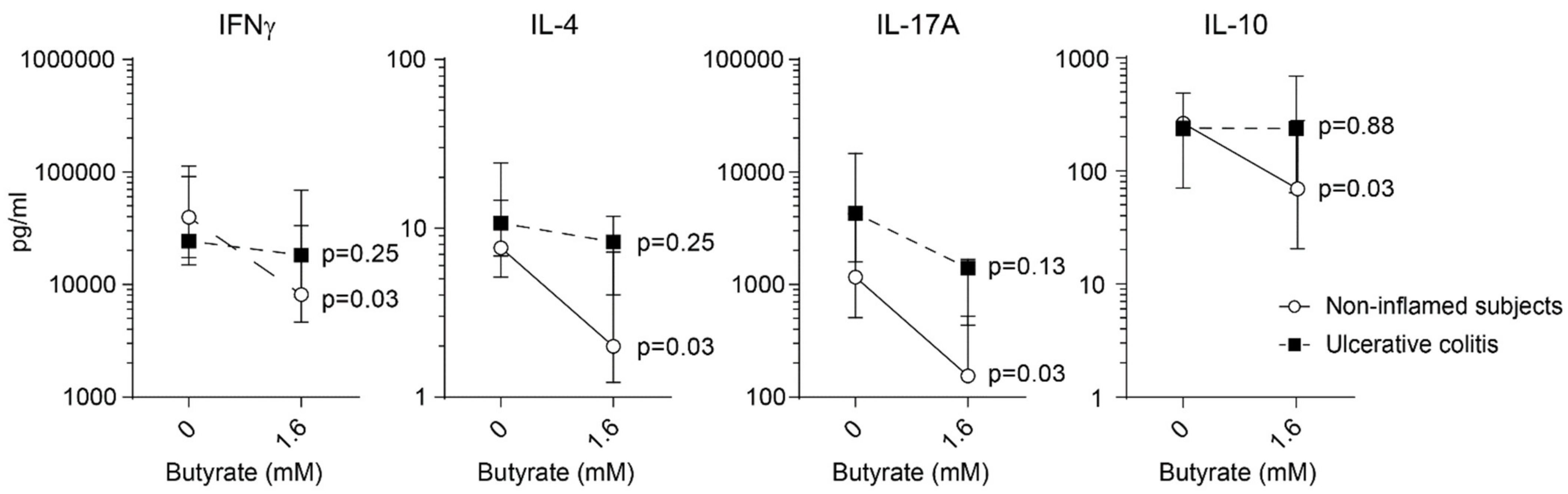
2.4. Impaired Butyrate Induced CTLA-4 Expression and Altered Cytokine Profile of Lamina Propria T Cells from Patients with Active Ulcerative Colitis
3. Discussion
4. Materials and Methods
4.1. Demographics of Participating Subjects and Sample Collection
4.2. Blood and Lamina Propria Cell Isolation
4.3. Cell Cultivation Assays
4.4. Histone Deacetylase (HDAC) I and II Measurement
4.5. Flow Cytometry
4.6. Cytokine Assays
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Maynard, C.L.; Weaver, C.T. Intestinal effector T cells in health and disease. Immunity 2009, 31, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, A.J.; Cong, Y. Gut microbiota metabolite regulation of host defenses at mucosal surfaces: Implication in precision medicine. Precis. Clin. Med. 2019, 2, 110–119. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Segain, J.P.; de la Bletiere, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Q.; Dorfman, R.G.; Huang, X.; Fan, T.; Zhang, H.; Zhang, J.; Yu, C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Linsley, P.S.; Greene, J.L.; Tan, P.; Bradshaw, J.; Ledbetter, J.A.; Anasetti, C.; Damle, N.K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 1992, 176, 1595–1604. [Google Scholar] [CrossRef]
- Takahashi, T.; Tagami, T.; Yamazaki, S.; Uede, T.; Shimizu, J.; Sakaguchi, N.; Mak, T.W.; Sakaguchi, S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000, 192, 303–310. [Google Scholar] [CrossRef]
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.L.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol. 2006, 24, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Delladetsima, I.; Perdiki, M.; Siakavellas, S.I.; Goukos, D.; Papatheodoridis, G.V.; Daikos, G.L.; Gogas, H. Immunological Characteristics of Colitis Associated with Anti-CTLA-4 Antibody Therapy. Cancer Investig. 2017, 35, 443–455. [Google Scholar] [CrossRef]
- Breuer, R.I.; Soergel, K.H.; Lashner, B.A.; Christ, M.L.; Hanauer, S.B.; Vanagunas, A.; Harig, J.M.; Keshavarzian, A.; Robinson, M.; Sellin, J.H.; et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: A randomised, placebo controlled trial. Gut 1997, 40, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.; Vanhoutvin, S.A.; Troost, F.J.; Rijkers, G.; de Bruine, A.; Bast, A.; Venema, K.; Brummer, R.J. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig. Dis. Sci. 1996, 41, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, A.H.; Hiruki, T.; Brzezinski, A.; Baker, J.P. Treatment of left-sided ulcerative colitis with butyrate enemas: A controlled trial. Aliment. Pharmacol. Ther. 1996, 10, 729–736. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol. Investig. 2016, 45, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Brant, S.R.; Li, C.; Shrestha, U.K.; Jiang, T.; Zhou, F.; Jiang, Y.; Shi, X.; Zhao, Y.; Li, J.; et al. CTLA4 -1661A/G and 3′UTR long repeat polymorphisms are associated with ulcerative colitis and influence CTLA4 mRNA and protein expression. Genes Immun. 2010, 11, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, T.; Yao, L.; Ma, R.; Yang, S.; Yuan, F. Circulating follicular regulatory T cells could inhibit Ig production in a CTLA-4-dependent manner but are dysregulated in ulcerative colitis. Mol. Immunol. 2019, 114, 323–329. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Qureshi, O.S.; Gardner, D.; Hou, T.Z.; Briggs, Z.; Soskic, B.; Baker, J.; Raza, K.; Sansom, D.M. Vitamin D Antagonises the Suppressive Effect of Inflammatory Cytokines on CTLA-4 Expression and Regulatory Function. PLoS ONE 2015, 10, e0131539. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Ohman, L.; Dahlen, R.; Isaksson, S.; Sjoling, A.; Wick, M.J.; Sjovall, H.; Van Oudenhove, L.; Simren, M.; Strid, H. Serum IL-17A in newly diagnosed treatment-naive patients with ulcerative colitis reflects clinical disease severity and predicts the course of disease. Inflamm. Bowel Dis. 2013, 19, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Downey, J.; Smith, A.; Zinselmeyer, B.H.; Rush, C.; Brewer, J.M.; Wei, B.; Hogg, N.; Garside, P.; Rudd, C.E. Reversal of the TCR stop signal by CTLA-4. Science 2006, 313, 1972–1975. [Google Scholar] [CrossRef]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Kumari, R.; Ahuja, V.; Paul, J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. WJG 2013, 19, 3404–3414. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Galvez, J.; Rodriguez-Cabezas, M.E.; Zarzuelo, A. Effects of dietary fiber on inflammatory bowel disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Grahn, M.F.; Boyle, M.A.; Hutton, M.; Rogers, J.; Williams, N.S. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut 1994, 35, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Hiele, M.; Evenepoel, P.; Peeters, M.; Ghoos, Y.; Rutgeerts, P. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology 1998, 115, 584–590. [Google Scholar] [CrossRef]
- Roediger, W.E. The colonic epithelium in ulcerative colitis: An energy-deficiency disease? Lancet 1980, 2, 712–715. [Google Scholar] [CrossRef]
- De Preter, V.; Arijs, I.; Windey, K.; Vanhove, W.; Vermeire, S.; Schuit, F.; Rutgeerts, P.; Verbeke, K. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm. Bowel Dis. 2012, 18, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Diab, J.; Hansen, T.; Goll, R.; Stenlund, H.; Jensen, E.; Moritz, T.; Florholmen, J.; Forsdahl, G. Mucosal Metabolomic Profiling and Pathway Analysis Reveal the Metabolic Signature of Ulcerative Colitis. Metabolites 2019, 9, 291. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Isaksson, S.; Ohman, L. The Anti-inflammatory Immune Regulation Induced by Butyrate Is Impaired in Inflamed Intestinal Mucosa from Patients with Ulcerative Colitis. Inflammation 2020, 43, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Laeremans, T.; Vanhove, W.; Arnauts, K.; Ramalho, A.S.; Farre, R.; Cleynen, I.; Ferrante, M.; Vermeire, S. Butyrate does not protect against inflammation-induced loss of epithelial barrier function and cytokine production in primary cell monolayers from patients with ulcerative colitis. J. Crohn’s Colitis 2019, 13, 1351–1361. [Google Scholar] [CrossRef]
- Linsley, P.S.; Bradshaw, J.; Greene, J.; Peach, R.; Bennett, K.L.; Mittler, R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996, 4, 535–543. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnusson, M.K.; Vidal, A.; Maasfeh, L.; Isaksson, S.; Malhotra, R.; Olsson, H.K.; Öhman, L. Impaired Butyrate Induced Regulation of T Cell Surface Expression of CTLA-4 in Patients with Ulcerative Colitis. Int. J. Mol. Sci. 2021, 22, 3084. https://doi.org/10.3390/ijms22063084
Magnusson MK, Vidal A, Maasfeh L, Isaksson S, Malhotra R, Olsson HK, Öhman L. Impaired Butyrate Induced Regulation of T Cell Surface Expression of CTLA-4 in Patients with Ulcerative Colitis. International Journal of Molecular Sciences. 2021; 22(6):3084. https://doi.org/10.3390/ijms22063084
Chicago/Turabian StyleMagnusson, Maria K., Alexander Vidal, Lujain Maasfeh, Stefan Isaksson, Rajneesh Malhotra, Henric K. Olsson, and Lena Öhman. 2021. "Impaired Butyrate Induced Regulation of T Cell Surface Expression of CTLA-4 in Patients with Ulcerative Colitis" International Journal of Molecular Sciences 22, no. 6: 3084. https://doi.org/10.3390/ijms22063084
APA StyleMagnusson, M. K., Vidal, A., Maasfeh, L., Isaksson, S., Malhotra, R., Olsson, H. K., & Öhman, L. (2021). Impaired Butyrate Induced Regulation of T Cell Surface Expression of CTLA-4 in Patients with Ulcerative Colitis. International Journal of Molecular Sciences, 22(6), 3084. https://doi.org/10.3390/ijms22063084





