Is High-Intensity Interval Training Suitable to Promote Neuroplasticity and Cognitive Functions after Stroke?
Abstract
1. Introduction
2. Methodological Considerations for Endurance Exercise Studies
2.1. Definition of Exercise Intensity
2.2. Timing of Endurance Training after Stroke
2.3. Blood Measurement of Neurotrophins after Training
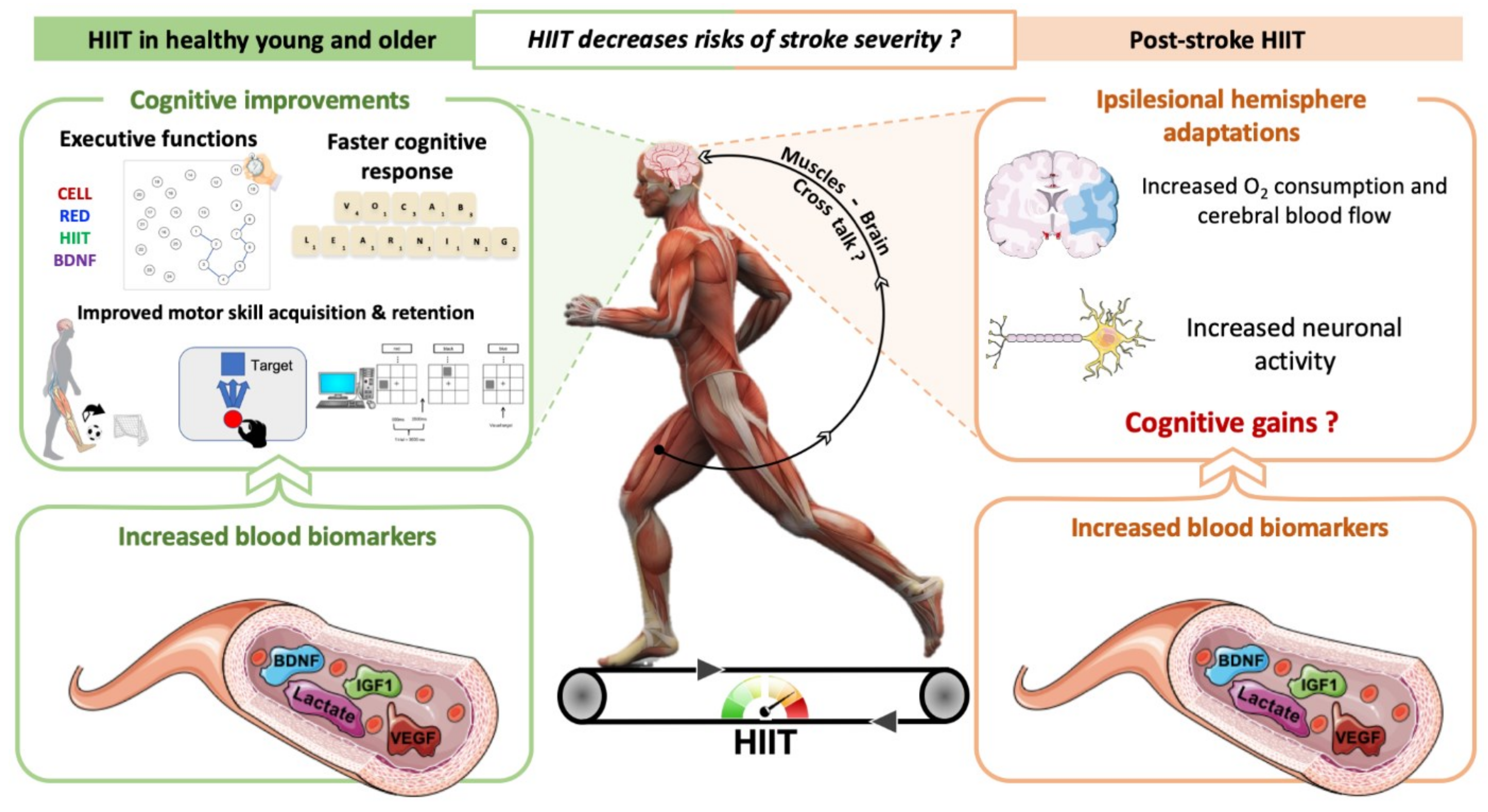
3. How Can HIIT Promote Neuroplasticity and Cognitive Benefits in Individuals with Stroke?
4. Do HIIT Promote Neuroplasticity and Cognitive Benefits in Healthy Individuals and Rodents? Comparison with MICT
4.1. In Humans
4.2. In Rodents
5. HIIT Could Contribute to Neuroplasticity and Cognitive Recovery after Stroke
5.1. Clinical Studies
5.2. In Rodents with Cerebral Ischemia
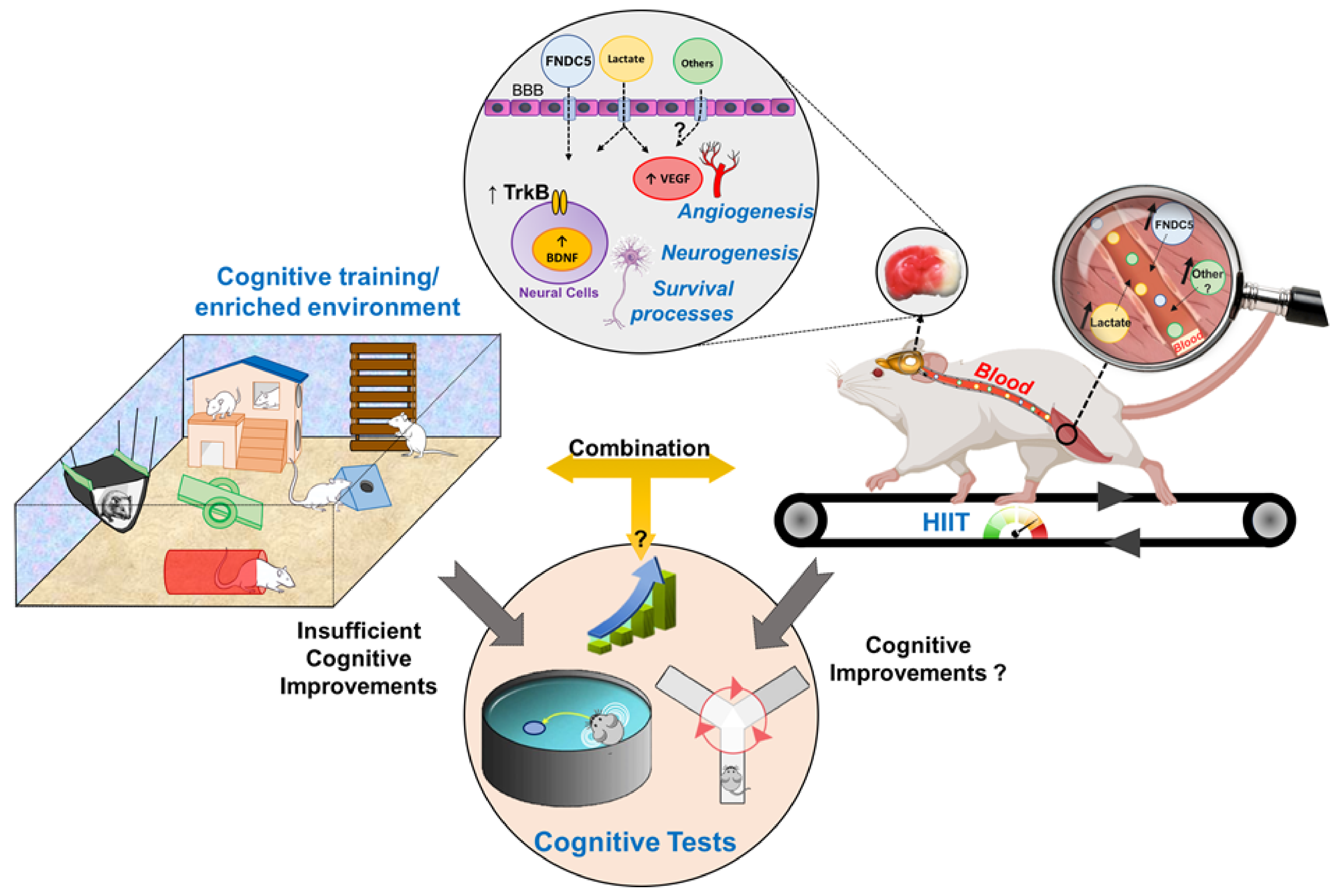
6. Perspectives
6.1. Is the Combination between HIIT and Cognitive Tasks Effective to Improve Cognitive Performance during the Stroke Rehabilitation?
6.2. Pre-Conditioning HIIT Might Reduce Poststroke Brain Damage
6.3. Is the HIIT Effectiveness on Neuroplasticity/Cognition Observed in Other Neurologic Disorders?
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Marzolini, S.; Oh, P.; McIlroy, W.; Brooks, D. The Feasibility of Cardiopulmonary Exercise Testing for Prescribing Exercise to People After. Stroke 2012, 43, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Coshall, C.; Rudd, A.G.; Wolfe, C.D.A. Cognitive Impairment after Stroke: Clinical Determinants and Its Associations with Long-Term Stroke Outcomes. J. Am. Geriatr. Soc. 2002, 50, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Mokashi, S.P.; Vivekanand, S. Relationship between cognitive deficits and the ability to perform the activities of daily living in stroke patients. Indian J. Occup. Ther. 2005, 37, 3–9. [Google Scholar]
- Kauranen, T.; Turunen, K.; Laari, S.; Mustanoja, S.; Baumann, P.; Poutiainen, E. The Severity of Cognitive Deficits Predicts Return to Work after a First-Ever Ischaemic Stroke. J. Neurol. Neurosurg. Psychiatry 2013, 84, 316–321. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bennett, S.; Koh, C.-L.; McKenna, K.T. Occupational Therapy for Cognitive Impairment in Stroke Patients. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Zheng, G.; Zhou, W.; Xia, R.; Tao, J.; Chen, L. Aerobic Exercises for Cognition Rehabilitation Following Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2016, 25, 2780–2789. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47. [Google Scholar] [CrossRef]
- Gillespie, D.C.; Bowen, A.; Chung, C.S.; Cockburn, J.; Knapp, P.; Pollock, A. Rehabilitation for Post-Stroke Cognitive Impairment: An Overview of Recommendations Arising from Systematic Reviews of Current Evidence. Clin. Rehabil. 2015, 29, 120–128. [Google Scholar] [CrossRef]
- Schulz, C.H.; Godwin, K.M.; Hersch, G.I.; Hyde, L.K.; Irabor, J.J.; Ostwald, S.K. Return to Work Predictors of Stroke Survivors and Their Spousal Caregivers. Work 2017, 57, 111–124. [Google Scholar] [CrossRef]
- El-Tamawy, M.S.S.; Abd-Allah, F.; Ahmed, S.M.; Darwish, M.H.; Khalifa, H.A. Aerobic Exercises Enhance Cognitive Functions and Brain Derived Neurotrophic Factor in Ischemic Stroke Patients. NeuroRehabilitation 2014, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, L.E.; Waiwood, A.M.; Cumming, T.B.; Marsland, A.L.; Bernhardt, J.; Erickson, K.I. Effects of Physical Activity on Post-Stroke Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Stroke 2018, 17, 3093–3100. [Google Scholar]
- Quaney, B.M.; Boyd, L.A.; McDowd, J.M.; Zahner, L.H.; He, J.; Mayo, M.S.; Macko, R.F. Aerobic Exercise Improves Cognition and Motor Function Poststroke. Neurorehabil. Neural Repair 2009, 23, 879–885. [Google Scholar] [CrossRef]
- Song, M.-K.; Kim, E.-J.; Kim, J.-K.; Lee, S.-G. Effects of Exercise Timing and Intensity on Neuroplasticity in a Rat Model of Cerebral Infarction. Brain Res. Bull. 2020, 160, 50–55. [Google Scholar] [CrossRef]
- Hong, M.; Kim, M.; Kim, T.-W.; Park, S.-S.; Kim, M.-K.; Park, Y.H.; Sung, Y.-H.; Shin, M.-S. Treadmill Exercise Improves Motor Function and Short-Term Memory by Enhancing Synaptic Plasticity and Neurogenesis in Photothrombotic Stroke Mice. Int. Neurourol. J. 2020, 24, S28–S38. [Google Scholar] [CrossRef]
- Ploughman, M.; Austin, M.W.; Glynn, L.; Corbett, D. The Effects of Poststroke Aerobic Exercise on Neuroplasticity: A Systematic Review of Animal and Clinical Studies. Transl. Stroke Res. 2015, 6, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Himi, N.; Takahashi, H.; Okabe, N.; Nakamura, E.; Shiromoto, T.; Narita, K.; Koga, T.; Miyamoto, O. Exercise in the Early Stage after Stroke Enhances Hippocampal Brain-Derived Neurotrophic Factor Expression and Memory Function Recovery. J. Stroke Cerebrovasc. Dis. 2016, 25, 2987–2994. [Google Scholar] [CrossRef]
- Constans, A.; Pin-barre, C.; Temprado, J.-J.; Decherchi, P.; Laurin, J. Influence of Aerobic Training and Combinations of Interventions on Cognition and Neuroplasticity after Stroke. Front. Aging Neurosci. 2016, 8, 164. [Google Scholar] [CrossRef]
- Cumming, T.B.; Tyedin, K.; Churilov, L.; Morris, M.E.; Bernhardt, J. The Effect of Physical Activity on Cognitive Function after Stroke: A Systematic Review. Int. Psychogeriatr. 2012, 24, 557–567. [Google Scholar] [CrossRef]
- Hicks, R.R.; Boggs, A.; Leider, D.; Kraemer, P.; Brown, R.; Scheff, S.W.; Seroogy, K.B. Effects of Exercise Following Lateral Fluid Percussion Brain Injury in Rats. Restor. Neurol. Neurosci. 1998, 12, 41–47. [Google Scholar] [PubMed]
- Ludyga, S.; Gerber, M.; Pühse, U.; Looser, V.N.; Kamijo, K. Systematic Review and Meta-Analysis Investigating Moderators of Long-Term Effects of Exercise on Cognition in Healthy Individuals. Nat. Hum. Behav. 2020, 4, 603–612. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Moon, S.; Lee, K.-H.; Jeon, H.-E.; Hwang, B.-H.; Beak, J.-W.; Joa, K.-L.; Kang, J.-H. Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion. Int. J. Mol. Sci. 2020, 21, 5842. [Google Scholar] [CrossRef]
- Tang, A.; Eng, J.; Krassioukov, A.; Tsang, T.; Liu-Ambrose, T. High- and Low-Intensity Exercise Do Not Improve Cognitive Function after Stroke: A Randomized Controlled Trial. J. Rehabil. Med. 2016, 48, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Carl, D.; Khoury, J.C.; Gerson, M.; Kissela, B.; et al. Effects of Exercise Intensity on Acute Circulating Molecular Responses Poststroke. Neurorehabil. Neural Repair 2020, 34, 222–234. [Google Scholar] [CrossRef]
- Calverley, T.A.; Ogoh, S.; Marley, C.J.; Steggall, M.; Marchi, N.; Brassard, P.; Lucas, S.J.E.; Cotter, J.D.; Roig, M.; Ainslie, P.N.; et al. HIITing the Brain with Exercise; Mechanisms, Consequences and Practical Recommendations. J. Physiol. 2020, 598, 2513–2530. [Google Scholar] [CrossRef] [PubMed]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training After Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabil. Neural Repair 2018, 32, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.; Eng, J.; Liu-Ambrose, T.; Richardson, J.; MacDermid, J.; Tang, A. Sex Differences in the Effects of Exercise on Cognition Post-Stroke: Secondary Analysis of a Randomized Controlled Trial. J. Rehabil. Med. 2020, 52, 1–8. [Google Scholar] [CrossRef]
- Wen, D.; Utesch, T.; Wu, J.; Robertson, S.; Liu, J.; Hu, G.; Chen, H. Effects of Different Protocols of High Intensity Interval Training for VO2max Improvements in Adults: A Meta-Analysis of Randomised Controlled Trials. J. Sci. Med. Sport 2019, 22, 941–947. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle: Part I: Cardiopulmonary Emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle: Part II: Anaerobic Energy, Neuromuscular Load and Practical Applications. Sports Med. 2013, 43, 927–954. [Google Scholar] [CrossRef]
- Laursen, P.B.; Jenkins, D.G. The Scientific Basis for High-Intensity Interval Training: Optimising Training Programmes and Maximising Performance in Highly Trained Endurance Athletes. Sports Med. Auckl. NZ 2002, 32, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Askim, T.; Dahl, A.E.; Aamot, I.L.; Hokstad, A.; Helbostad, J.; Indredavik, B. High-Intensity Aerobic Interval Training for Patients 3-9 Months After Stroke. A Feasibility Study: Aerobic Interval Training After Stroke. Physiother. Res. Int. 2014, 19, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.; Dunning, K.; Carl, D.; Gerson, M.; Khoury, J.; Rockwell, B.; Keeton, G.; Westover, J.; Williams, A.; McCarthy, M.; et al. High-Intensity Interval Training and Moderate-Intensity Continuous Training in Ambulatory Chronic Stroke: A Feasibility Study. Phys. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Carl, D.L.; Boyne, P.; Rockwell, B.; Gerson, M.; Khoury, J.; Kissela, B.; Dunning, K. Preliminary Safety Analysis of High-Intensity Interval Training (HIIT) in Persons with Chronic Stroke. Appl. Physiol. Nutr. Metab. 2017, 42, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Fu, T.-C.; Huang, S.-C.; Chen, C.P.-C.; Wang, J.-S. Increased Serum Brain-Derived Neurotrophic Factor with High-Intensity Interval Training in Stroke Patients: A Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2020, S1877065720300889. [Google Scholar] [CrossRef] [PubMed]
- Olney, N.; Wertz, T.; LaPorta, Z.; Mora, A.; Serbas, J.; Astorino, T.A. Comparison of Acute Physiological and Psychological Responses Between Moderate-Intensity Continuous Exercise and Three Regimes of High-Intensity Interval Training. J. Strength Cond. Res. 2018, 32, 2130–2138. [Google Scholar] [CrossRef]
- Luo, L.; Li, C.; Deng, Y.; Wang, Y.; Meng, P.; Wang, Q. High-Intensity Interval Training on Neuroplasticity, Balance between Brain-Derived Neurotrophic Factor and Precursor Brain-Derived Neurotrophic Factor in Poststroke Depression Rats. J. Stroke Cerebrovasc. Dis. 2018, 28, 672–682. [Google Scholar] [CrossRef]
- Pin-Barre, C.; Constans, A.; Brisswalter, J.; Pellegrino, C.; Laurin, J. Effects of High- Versus Moderate-Intensity Training on Neuroplasticity and Functional Recovery After Focal Ischemia. Stroke 2017, 48, 2855–2864. [Google Scholar] [CrossRef]
- Pin-Barre, C.; Hugues, N.; Constans, A.; Berton, E.; Pellegrino, C.; Laurin, J. Effects of Different High-Intensity Interval Training Regimens on Endurance and Neuroplasticity After Cerebral Ischemia. Stroke 2021, 52, 1109–1114. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Chen, H.; Wu, C.-L.; Kuo, Y.-M.; Yu, L.; Huang, A.-M.; Wu, F.-S.; Chuang, J.-I.; Jen, C.J. Differential Effects of Treadmill Running and Wheel Running on Spatial or Aversive Learning and Memory: Roles of Amygdalar Brain-Derived Neurotrophic Factor and Synaptotagmin I: Treadmill Running and Wheel Running Affect Aversive Memory Differently. J. Physiol. 2009, 587, 3221–3231. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Wang, R.-Y.; Wang, P.S.-G. Early and Late Treadmill Training after Focal Brain Ischemia in Rats. Neurosci. Lett. 2003, 339, 91–94. [Google Scholar] [CrossRef]
- Billinger, S.A.; Boyne, P.; Coughenour, E.; Dunning, K.; Mattlage, A. Does Aerobic Exercise and the FITT Principle Fit into Stroke Recovery? Curr. Neurol. Neurosci. Rep. 2015, 15, 519. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-K.; Kim, E.-J.; Kim, J.-K.; Park, H.-K.; Lee, S.-G. Effect of Regular Swimming Exercise to Duration-Intensity on Neurocognitive Function in Cerebral Infarction Rat Model. Neurol. Res. 2019, 41, 37–44. [Google Scholar] [CrossRef]
- Gronwald, T.; de Bem Alves, A.C.; Murillo-Rodríguez, E.; Latini, A.; Schuette, J.; Budde, H. Standardization of Exercise Intensity and Consideration of a Dose–Response Is Essential. Commentary on “Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models”, by Lourenco et al., Published 2019 in Nature Medicine. J. Sport Health Sci. 2019, 8, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Constans, A.; Pin-Barre, C.; Molinari, F.; Temprado, J.-J.; Brioche, T.; Pellegrino, C.; Laurin, J. High-Intensity Interval Training Is Superior to Moderate Intensity Training on Aerobic Capacity in Rats: Impact on Hippocampal Plasticity Markers. Behav. Brain Res. 2021, 398, 112977. [Google Scholar] [CrossRef]
- Rist, P.M.; Lee, I.-M.; Kase, C.S.; Gaziano, J.M.; Kurth, T. Physical Activity and Functional Outcomes From Cerebral Vascular Events in Men. Stroke 2011, 42, 3352–3356. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Hennekens, C.H.; Berger, K.; Buring, J.E.; Manson, J.E. Exercise and Risk of Stroke in Male Physicians. Stroke 1999, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, G.G.; Gobatto, C.A.; Marcos-Pereira, M.; Dos Reis, I.G.M.; Verlengia, R. Interval versus Continuous Training with Identical Workload: Physiological and Aerobic Capacity Adaptations. Physiol. Res. 2015, 64, 209–219. [Google Scholar] [CrossRef]
- Fabre, C.; Masse-Biron, J.; Ahmaidi, S.; Adam, B.; Prefaut, C. Effectiveness of Individualized Aerobic Training at the Ventilatory Threshold in the Elderly. J. Gerontol. A. Biol. Sci. Med. Sci. 1997, 52A, B260–B266. [Google Scholar] [CrossRef]
- Choe, M.-A.; An, G.J.; Lee, Y.-K.; Im, J.H.; Choi-Kwon, S.; Heitkemper, M. Effect of Early Low-Intensity Exercise on Rat Hind-Limb Muscles Following Acute Ischemic Stroke. Biol. Res. Nurs. 2006, 7, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-C.; Yang, Y.-R.; Wang, R.-Y. Effects of Exercise Intensity on Spatial Memory Performance and Hippocampal Synaptic Plasticity in Transient Brain Ischemic Rats. PLoS ONE 2013, 8, e78163. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Hamakawa, M.; Ishida, A.; Tamakoshi, K.; Nakashima, H.; Ishida, K. Low-Speed Treadmill Running Exercise Improves Memory Function after Transient Middle Cerebral Artery Occlusion in Rats. Behav. Brain Res. 2013, 243, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate Threshold Concepts: How Valid Are They? Sports Med. Auckl. NZ 2009, 39, 469–490. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological Adaptations to Interval Training and the Role of Exercise Intensity: Training Adaptations and the Nature of the Stimulus. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical Activity and Exercise Recommendations for Stroke Survivors: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef] [PubMed]
- Risedal, A.; Zeng, J.; Johansson, B.B. Early Training May Exacerbate Brain Damage after Focal Brain Ischemia in the Rat. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1999, 19, 997–1003. [Google Scholar] [CrossRef]
- DeBow, S.B.; McKenna, J.E.; Kolb, B.; Colbourne, F. Immediate Constraint-Induced Movement Therapy Causes Local Hyperthermia That Exacerbates Cerebral Cortical Injury in Rats. Can. J. Physiol. Pharmacol. 2004, 82, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, S.; Robertson, A.D.; Oh, P.; Goodman, J.M.; Corbett, D.; Du, X.; MacIntosh, B.J. Aerobic Training and Mobilization Early Post-Stroke: Cautions and Considerations. Front. Neurol. 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.W.K.; Mak, M.K.Y. Speed-Dependent Treadmill Training Is Effective to Improve Gait and Balance Performance in Patients with Sub-Acute Stroke. J. Rehabil. Med. 2011, 43, 709–713. [Google Scholar] [CrossRef]
- Bernhardt, J. Efficacy and Safety of Very Early Mobilisation within 24 h of Stroke Onset (AVERT): A Randomised Controlled Trial. Lancet 2015, 386, 46–55. [Google Scholar] [CrossRef]
- Schmidt, A.; Wellmann, J.; Schilling, M.; Strecker, J.-K.; Sommer, C.; Schabitz, W.-R.; Diederich, K.; Minnerup, J. Meta-Analysis of the Efficacy of Different Training Strategies in Animal Models of Ischemic Stroke. Stroke 2014, 45, 239–247. [Google Scholar] [CrossRef]
- Hill, T.; Polk, J.D. BDNF, Endurance Activity, and Mechanisms Underlying the Evolution of Hominin Brains. Am. J. Phys. Anthropol. 2019, 168, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The Impact of Age, Weight and Gender on BDNF Levels in Human Platelets and Plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Banoujaafar, H.; Monnier, A.; Pernet, N.; Quirié, A.; Garnier, P.; Prigent-Tessier, A.; Marie, C. Brain BDNF Levels Are Dependent on Cerebrovascular Endothelium-Derived Nitric Oxide. Eur. J. Neurosci. 2016, 44, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, T.; Fujimura, H.; Altar, C.A.; Li, J.; Kambayashi, J.; Tandon, N.N.; Sun, B. Vascular Endothelial Cells Synthesize and Secrete Brain-Derived Neurotrophic Factor. FEBS Lett. 2000, 470, 113–117. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Gallmeier, E.; Behrens, L.; Leal, V.V.; Misgeld, T.; Klinkert, W.E.F.; Kolbeck, R.; Hoppe, E.; Oropeza-Wekerle, R.-L.; Bartke, I.; et al. Activated Human T Cells, B Cells, and Monocytes Produce Brain-Derived Neurotrophic Factor In Vitro and in Inflammatory Brain Lesions: A Neuroprotective Role of Inflammation? J. Exp. Med. 1999, 189, 865–870. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a Release of Brain-Derived Neurotrophic Factor from the Brain during Exercise: Brain-Derived Neurotrophic Factor Release during Exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Béjot, Y.; Mossiat, C.; Giroud, M.; Prigent-Tessier, A.; Marie, C. Circulating and Brain BDNF Levels in Stroke Rats. Relevance to Clinical Studies. PLoS ONE 2011, 6, e29405. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- So, J.H.; Huang, C.; Ge, M.; Cai, G.; Zhang, L.; Lu, Y.; Mu, Y. Intense Exercise Promotes Adult Hippocampal Neurogenesis But Not Spatial Discrimination. Front. Cell. Neurosci. 2017, 11, 13. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging Animal and Human Models of Exercise-Induced Brain Plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef]
- Vital, T.M.; Stein, A.M.; de Melo Coelho, F.G.; Arantes, F.J.; Teodorov, E.; Santos-Galduróz, R.F. Physical Exercise and Vascular Endothelial Growth Factor (VEGF) in Elderly: A Systematic Review. Arch. Gerontol. Geriatr. 2014, 59, 234–239. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Lee, J.; Kim, J.; Kim, G.; Kim, S.; Kim, J.; Chun, H.; Lee, J.H.; Lee, C.J.; Hwang, K.S. Ultra-Sensitive Detection of Brain-Derived Neurotrophic Factor (BDNF) in the Brain of Freely Moving Mice Using an Interdigitated Microelectrode (IME) Biosensor. Sci. Rep. 2016, 6, 33694. [Google Scholar] [CrossRef]
- Tisdall, M.M.; Smith, M. Cerebral Microdialysis: Research Technique or Clinical Tool. Br. J. Anaesth. 2006, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscle as a Secretory Organ. In Comprehensive Physiology; Terjung, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. c120033. ISBN 978-0-470-65071-4. [Google Scholar]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Cunningham, D.; Carl, D.; Jansen, C.; Khoury, J.C.; et al. Exercise Intensity Affects Acute Neurotrophic and Neurophysiological Responses Poststroke. J. Appl. Physiol. 2019, 126, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Coco, M.; Alagona, G.; Rapisarda, G.; Costanzo, E.; Calogero, R.A.; Perciavalle, V.; Perciavalle, V. Elevated Blood Lactate Is Associated with Increased Motor Cortex Excitability. Somatosens. Mot. Res. 2010, 27, 1–8. [Google Scholar] [CrossRef]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The Effect of Acute Exercise on Serum Brain-Derived Neurotrophic Factor Levels and Cognitive Function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Villringer, A.; Lehmann, N. Endurance Exercise as an “Endogenous” Neuro-Enhancement Strategy to Facilitate Motor Learning. Front. Hum. Neurosci. 2015, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Pilc, A. The Effect of Physical Activity on the Brain Derived Neurotrophic Factor: From Animal to Human Studies. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2010, 61, 533–541. [Google Scholar]
- Lisman, J.; Cooper, K.; Sehgal, M.; Silva, A.J. Memory Formation Depends on Both Synapse-Specific Modifications of Synaptic Strength and Cell-Specific Increases in Excitability. Nat. Neurosci. 2018, 21, 309–314. [Google Scholar] [CrossRef]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise Induces Cerebral VEGF and Angiogenesis via the Lactate Receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Strüder, H.K. Lactate Infusion at Rest Increases BDNF Blood Concentration in Humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.; Granata, C.; Barry, J.; Safdar, A.; Bishop, D.; Little, J.P. Impact of a Single Bout of High-Intensity Interval Exercise and Short-Term Interval Training on Interleukin-6, FNDC5, and METRNL MRNA Expression in Human Skeletal Muscle. J. Sport Health Sci. 2018, 7, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined Adult Neurogenesis and BDNF Mimic Exercise Effects on Cognition in an Alzheimer’s Mouse Model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef]
- Pin-Barre, C.; Pellegrino, C.; Laurin, F.; Laurin, J. Cerebral Ischemia Changed the Effect of Metabosensitive Muscle Afferents on Somatic Reflex Without Affecting Thalamic Activity. Front. Physiol. 2018, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Pin-Barre, C.; Laurin, J.; Felix, M.-S.; Pertici, V.; Kober, F.; Marqueste, T.; Matarazzo, V.; Muscatelli-Bossy, F.; Temprado, J.-J.; Brisswalter, J.; et al. Acute Neuromuscular Adaptation at the Spinal Level Following Middle Cerebral Artery Occlusion-Reperfusion in the Rat. PLoS ONE 2014, 9, e89953. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Adkins, D.L. Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection. Physiology 2015, 30, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Tennant, K.A. Thinking Outside the Brain: Structural Plasticity in the Spinal Cord Promotes Recovery from Cortical Stroke. Exp. Neurol. 2014, 254, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Proschinger, S.; Rademacher, A.; Schenk, A.; Bloch, W.; Warnke, C.; Gonzenbach, R.; Kool, J.; Bansi, J.; Zimmer, P. High-Intensity Interval Training Reduces Neutrophil-to-Lymphocyte Ratio in Persons with Multiple Sclerosis during Inpatient Rehabilitation. Mult. Scler. J. 2020, 135245852095138. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Ying, Z.; Gómez-Pinilla, F. Differential Effects of Acute and Chronic Exercise on Plasticity-Related Genes in the Rat Hippocampus Revealed by Microarray. Eur. J. Neurosci. 2002, 16, 1107–1116. [Google Scholar] [CrossRef]
- Zafra, F.; Castren, E.; Thoenen, H.; Lindholm, D. Interplay between Glutamate and Gamma-Aminobutyric Acid Transmitter Systems in the Physiological Regulation of Brain-Derived Neurotrophic Factor and Nerve Growth Factor Synthesis in Hippocampal Neurons. Proc. Natl. Acad. Sci. USA 1991, 88, 10037–10041. [Google Scholar] [CrossRef]
- Saucedo Marquez, C.M.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-Intensity Interval Training Evokes Larger Serum BDNF Levels Compared with Intense Continuous Exercise. J. Appl. Physiol. Bethesda Md 1985 2015, 119, 1363–1373. [Google Scholar] [CrossRef]
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.; Enikolopov, G.; et al. Neuronal Circuitry Mechanism Regulating Adult Quiescent Neural Stem-Cell Fate Decision. Nature 2012, 489, 150–154. [Google Scholar] [CrossRef]
- Stavrinos, E.L.; Coxon, J.P. High-Intensity Interval Exercise Promotes Motor Cortex Disinhibition and Early Motor Skill Consolidation. J. Cogn. Neurosci. 2017, 29, 593–604. [Google Scholar] [CrossRef]
- Blaesse, P.; Airaksinen, M.S.; Rivera, C.; Kaila, K. Cation-Chloride Cotransporters and Neuronal Function. Neuron 2009, 61, 820–838. [Google Scholar] [CrossRef]
- Goubert, E.; Altvater, M.; Rovira, M.-N.; Khalilov, I.; Mazzarino, M.; Sebastiani, A.; Schaefer, M.K.E.; Rivera, C.; Pellegrino, C. Bumetanide Prevents Brain Trauma-Induced Depressive-Like Behavior. Front. Mol. Neurosci. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, N.; Witte, O.W.; Frahm, C. Downregulation of Potassium Chloride Cotransporter KCC2 After Transient Focal Cerebral Ischemia. Stroke 2010, 41, e151–e159. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hotta, S.; Uchiyama, Y.; Kametaka, S. Role of the BDNF-TrkB Pathway in KCC2 Regulation and Rehabilitation Following Neuronal Injury: A Mini Review. Neurochem. Int. 2019, 128, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-Induced TrkB Activation down-Regulates the K+–Cl− Cotransporter KCC2 and Impairs Neuronal Cl− Extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Rasile, M.; Corradini, I.; Matteoli, M. Environmental Regulation of the Chloride Transporter KCC2: Switching Inflammation off to Switch the GABA On? Transl. Psychiatry 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Dennis, A.; Bandettini, P.A.; Johansen-Berg, H. The Effects of Aerobic Activity on Brain Structure. Front. Psychol. 2012, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be Smart, Exercise Your Heart: Exercise Effects on Brain and Cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Angevaren, M.; Verhaar, H.; Aufdemkampe, G.; Aleman, A.; Arens, K.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people. Cochrane Database Syst. Rev. 2005, CD005381. [Google Scholar]
- Tottori, N.; Morita, N.; Ueta, K.; Fujita, S. Effects of High Intensity Interval Training on Executive Function in Children Aged 8–12 Years. Int. J. Environ. Res. Public. Health 2019, 16, 4127. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Krueger, K.; Fromme, A.; Korsukewitz, C.; Floel, A.; et al. High Impact Running Improves Learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Kujach, S.; Olek, R.A.; Byun, K.; Suwabe, K.; Sitek, E.J.; Ziemann, E.; Laskowski, R.; Soya, H. Acute Sprint Interval Exercise Increases Both Cognitive Functions and Peripheral Neurotrophic Factors in Humans: The Possible Involvement of Lactate. Front. Neurosci. 2020, 13, 1455. [Google Scholar] [CrossRef] [PubMed]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.W.; Kiens, B.; Nielsen, J.B. Acute Exercise Improves Motor Memory: Exploring Potential Biomarkers. Neurobiol. Learn. Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Skriver, K.; Lundbye-Jensen, J.; Kiens, B.; Nielsen, J.B. A Single Bout of Exercise Improves Motor Memory. PLoS ONE 2012, 7, e44594. [Google Scholar] [CrossRef]
- Mang, C.S.; Snow, N.J.; Campbell, K.L.; Ross, C.J.D.; Boyd, L.A. A Single Bout of High-Intensity Aerobic Exercise Facilitates Response to Paired Associative Stimulation and Promotes Sequence-Specific Implicit Motor Learning. J. Appl. Physiol. 2014, 117, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Johnsen, L.K.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Intensity. PLoS ONE 2016, 11, e0159589. [Google Scholar] [CrossRef] [PubMed]
- Pyke, W.; Ifram, F.; Coventry, L.; Sung, Y.; Champion, I.; Javadi, A.-H. The Effects of Different Protocols of Physical Exercise and Rest on Long-Term Memory. Neurobiol. Learn. Mem. 2020, 167, 107128. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The Effects of Acute Exercise on Cognitive Performance: A Meta-Analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Afzalpour, M.E.; Chadorneshin, H.T.; Foadoddini, M.; Eivari, H.A. Comparing Interval and Continuous Exercise Training Regimens on Neurotrophic Factors in Rat Brain. Physiol. Behav. 2015, 147, 78–83. [Google Scholar] [CrossRef]
- E, L.; Burns, J.M.; Swerdlow, R.H. Effect of High-Intensity Exercise on Aged Mouse Brain Mitochondria, Neurogenesis, and Inflammation. Neurobiol. Aging 2014, 35, 2574–2583. [Google Scholar] [CrossRef]
- dos Santos, J.R.; Bortolanza, M.; Ferrari, G.D.; Lanfredi, G.P.; do Nascimento, G.C.; Azzolini, A.E.C.S.; Del Bel, E.; de Campos, A.C.; Faça, V.M.; Vulczak, A.; et al. One-Week High-Intensity Interval Training Increases Hippocampal Plasticity and Mitochondrial Content without Changes in Redox State. Antioxidants 2020, 9, 445. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in Vivo Correlate of Exercise-Induced Neurogenesis in the Adult Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.S.; Rocha-Vieira, E.; Freitas, D.A.; Soares, B.A.; Rocha-Gomes, A.; Riul, T.R.; Mendonça, V.A.; Lacerda, A.C.R.; Camargos, A.C.R.; Carvalho, L.E.D.; et al. A Single Session of High-Intensity Interval Exercise Increases Antioxidants Defenses in the Hippocampus of Wistar Rats. Physiol. Behav. 2019, 211, 112675. [Google Scholar] [CrossRef]
- Freitas, D.A.; Rocha-Vieira, E.; Soares, B.A.; Nonato, L.F.; Fonseca, S.R.; Martins, J.B.; Mendonça, V.A.; Lacerda, A.C.; Massensini, A.R.; Poortamns, J.R.; et al. High Intensity Interval Training Modulates Hippocampal Oxidative Stress, BDNF and Inflammatory Mediators in Rats. Physiol. Behav. 2018, 184, 6–11. [Google Scholar] [CrossRef]
- Freitas, D.A.; Rocha-Vieira, E.; De Sousa, R.A.L.; Soares, B.A.; Rocha-Gomes, A.; Chaves Garcia, B.C.; Cassilhas, R.C.; Mendonça, V.A.; Camargos, A.C.R.; De Gregorio, J.A.M.; et al. High-Intensity Interval Training Improves Cerebellar Antioxidant Capacity without Affecting Cognitive Functions in Rats. Behav. Brain Res. 2019, 376, 112181. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Pilc, A.; Majerczak, J.; Grandys, M.; Zapart-Bukowska, J.; Duda, K. Endurance Training Increases Plasma Brain-Derived Neurotrophic Factor Concentration in Young Healthy Men. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59 Suppl. S7, 119–132. [Google Scholar]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical Exercise Increases Adult Hippocampal Neurogenesis in Male Rats Provided It Is Aerobic and Sustained: Aerobic Exercise Promotes Adult Neurogenesis. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, D.A.; Cryan, J.F.; Nolan, Y.M. Born This Way: Hippocampal Neurogenesis across the Lifespan. Aging Cell 2019, 18, e13007. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.M.; de Jong, J.; de Kloet, E.R.; Vreugdenhil, E. Downregulation of BDNF MRNA and Protein in the Rat Hippocampus by Corticosterone. Brain Res. 1998, 813, 112–120. [Google Scholar] [CrossRef]
- Sale, M.V.; Ridding, M.C.; Nordstrom, M.A. Cortisol Inhibits Neuroplasticity Induction in Human Motor Cortex. J. Neurosci. 2008, 28, 8285–8293. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The Yin and Yang of Neurotrophin Action. Nat. Rev. Neurosci. 2005, 6, 603. [Google Scholar] [CrossRef] [PubMed]
- Lee, R. Regulation of Cell Survival by Secreted Proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Wang, Z.; Wang, Y.; Luo, L.; Cheng, J.; Chen, S.-F.; Liu, H.; Wan, Q.; Wang, Q. Exercise Ameliorates Post-Stroke Depression by Inhibiting PTEN Elevation-Mediated Upregulation of TLR4/NF-ΚB/NLRP3 Signaling in Mice. Brain Res. 2020, 1736, 146777. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; Szymanska, A.; Windle, V.; Corbett, D. Bi-Hemispheric Contribution to Functional Motor Recovery of the Affected Forelimb Following Focal Ischemic Brain Injury in Rats. Eur. J. Neurosci. 2005, 21, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Lei, M.; Tao, S.; Jie, L.T.; Qian, L.; Lin, F.Q.; Ping, W.X. Effects of Combined Intervention of Physical Exercise and Cognitive Training on Cognitive Function in Stroke Survivors with Vascular Cognitive Impairment: A Randomized Controlled Trial. Clin. Rehabil. 2019, 33, 54–63. [Google Scholar] [CrossRef]
- Boyne, P.; Scholl, V.; Doren, S.; Carl, D.; Billinger, S.A.; Reisman, D.S.; Gerson, M.; Kissela, B.; Vannest, J.; Dunning, K. Locomotor Training Intensity after Stroke: Effects of Interval Type and Mode. Top. Stroke Rehabil. 2020, 27, 483–493. [Google Scholar] [CrossRef]
- Eather, N.; Riley, N.; Miller, A.; Smith, V.; Poole, A.; Vincze, L.; Morgan, P.J.; Lubans, D.R. Efficacy and Feasibility of HIIT Training for University Students: The Uni-HIIT RCT. J. Sci. Med. Sport 2019, 22, 596–601. [Google Scholar] [CrossRef]
- Kobilo, T.; Liu, Q.-R.; Gandhi, K.; Mughal, M.; Shaham, Y.; van Praag, H. Running Is the Neurogenic and Neurotrophic Stimulus in Environmental Enrichment. Learn. Mem. 2011, 18, 605–609. [Google Scholar] [CrossRef]
- Ploughman, M.; Eskes, G.A.; Kelly, L.P.; Kirkland, M.C.; Devasahayam, A.J.; Wallack, E.M.; Abraha, B.; Hasan, S.M.M.; Downer, M.B.; Keeler, L.; et al. Synergistic Benefits of Combined Aerobic and Cognitive Training on Fluid Intelligence and the Role of IGF-1 in Chronic Stroke. Neurorehabil. Neural Repair 2019, 33, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Heisz, J.J.; Clark, I.B.; Bonin, K.; Paolucci, E.M.; Michalski, B.; Becker, S.; Fahnestock, M. The Effects of Physical Exercise and Cognitive Training on Memory and Neurotrophic Factors. J. Cogn. Neurosci. 2017, 29, 1895–1907. [Google Scholar] [CrossRef]
- Nepveu, J.-F.; Thiel, A.; Tang, A.; Fung, J.; Lundbye-Jensen, J.; Boyd, L.A.; Roig, M. A Single Bout of High-Intensity Interval Training Improves Motor Skill Retention in Individuals With Stroke. Neurorehabil. Neural Repair 2017, 31, 726–735. [Google Scholar] [CrossRef]
- Lin, K.; Huang, Y.; Hsieh, Y.; Wu, C. Potential Predictors of Motor and Functional Outcomes After Distributed Constraint-Induced Therapy for Patients With Stroke. Neurorehabil. Neural Repair 2009, 23, 336–342. [Google Scholar] [CrossRef]
- Madhavan, S.; Cleland, B.T.; Sivaramakrishnan, A.; Freels, S.; Lim, H.; Testai, F.D.; Corcos, D.M. Cortical Priming Strategies for Gait Training after Stroke: A Controlled, Stratified Trial. J. NeuroEngineering Rehabil. 2020, 17, 111. [Google Scholar] [CrossRef]
- Madhavan, S.; Stinear, J.W.; Kanekar, N. Effects of a Single Session of High Intensity Interval Treadmill Training on Corticomotor Excitability Following Stroke: Implications for Therapy. Neural Plast. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Barber, P.A.; Coxon, J.P.; Fleming, M.K.; Byblow, W.D. Priming the Motor System Enhances the Effects of Upper Limb Therapy in Chronic Stroke. Brain 2008, 131, 1381–1390. [Google Scholar] [CrossRef]
- Yen, C.-L.; Wang, R.-Y.; Liao, K.-K.; Huang, C.-C.; Yang, Y.-R. Gait Training—Induced Change in Corticomotor Excitability in Patients With Chronic Stroke. Neurorehabil. Neural Repair 2008, 22, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Beylin, A.; Tanapat, P.; Reeves, A.; Shors, T.J. Learning Enhances Adult Neurogenesis in the Hippocampal Formation. Nat. Neurosci. 1999, 2, 260–265. [Google Scholar] [CrossRef]
- Curlik, D.M.; Shors, T.J. Training Your Brain: Do Mental and Physical (MAP) Training Enhance Cognition through the Process of Neurogenesis in the Hippocampus? Neuropharmacology 2013, 64, 506–514. [Google Scholar] [CrossRef]
- Sakalem, M.E.; Seidenbecher, T.; Zhang, M.; Saffari, R.; Kravchenko, M.; Wördemann, S.; Diederich, K.; Schwamborn, J.C.; Zhang, W.; Ambrée, O. Environmental Enrichment and Physical Exercise Revert Behavioral and Electrophysiological Impairments Caused by Reduced Adult Neurogenesis: REDUCTION OF ADULT NEUROGENESIS AND ENVIRONMENTAL ENRICHMENT. Hippocampus 2017, 27, 36–51. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Jia, J. Exercise Preconditioning and Brain Ischemic Tolerance. Neuroscience 2011, 177, 170–176. [Google Scholar] [CrossRef]
- Rezaei, R.; Nasoohi, S.; Haghparast, A.; Khodagholi, F.; Bigdeli, M.R.; Nourshahi, M. High Intensity Exercise Preconditioning Provides Differential Protection against Brain Injury Following Experimental Stroke. Life Sci. 2018, 207, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hafez, S.; Khan, M.B.; Awad, M.E.; Wagner, J.D.; Hess, D.C. Short-Term Acute Exercise Preconditioning Reduces Neurovascular Injury After Stroke Through Induced ENOS Activation. Transl. Stroke Res. 2020, 11, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, S.; Asghar Ravasi, A. The Effect of Antecedent-Conditioning High-Intensity Interval Training on BDNF Regulation through PGC-1α Pathway Following Cerebral Ischemia. Brain Res. 2020, 1729, 146618. [Google Scholar] [CrossRef] [PubMed]
- Liebelt, B.; Papapetrou, P.; Ali, A.; Guo, M.; Ji, X.; Peng, C.; Rogers, R.; Curry, A.; Jimenez, D.; Ding, Y. Exercise Preconditioning Reduces Neuronal Apoptosis in Stroke by Up-Regulating Heat Shock Protein-70 (Heat Shock Protein-72) and Extracellular-Signal-Regulated-Kinase 1/2. Neuroscience 2010, 166, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, K.; Gibas, K.J. Ketogenic Diet, High Intensity Interval Training (HIIT) and Memory Training in the Treatment of Mild Cognitive Impairment: A Case Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Korman, N.; Armour, M.; Chapman, J.; Rosenbaum, S.; Kisely, S.; Suetani, S.; Firth, J.; Siskind, D. High Intensity Interval Training (HIIT) for People with Severe Mental Illness: A Systematic Review & Meta-Analysis of Intervention Studies– Considering Diverse Approaches for Mental and Physical Recovery. Psychiatry Res. 2020, 284, 112601. [Google Scholar] [CrossRef]
- Viana, R.B.; Gentil, P.; Naves, J.P.A.; Rebelo, A.C.S.; Santos, D.A.T.; Braga, M.A.O.; de Lira, C.A.B. Interval Training Improves Depressive Symptoms But Not Anxious Symptoms in Healthy Women. Front. Psychiatry 2019, 10, 661. [Google Scholar] [CrossRef]
- Li, B.; Liang, F.; Ding, X.; Yan, Q.; Zhao, Y.; Zhang, X.; Bai, Y.; Huang, T.; Xu, B. Interval and Continuous Exercise Overcome Memory Deficits Related to β-Amyloid Accumulation through Modulating Mitochondrial Dynamics. Behav. Brain Res. 2019, 376, 112171. [Google Scholar] [CrossRef]
- Koyuncuoğlu, T.; Sevim, H.; Çetrez, N.; Meral, Z.; Gönenç, B.; Kuntsal Dertsiz, E.; Akakın, D.; Yüksel, M.; Kasımay Çakır, Ö. High Intensity Interval Training Protects from Post Traumatic Stress Disorder Induced Cognitive Impairment. Behav. Brain Res. 2021, 397, 112923. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Barbieri, F.A.; Arthuso, F.Z.; Silva, F.A.; Moretto, G.F.; Imaizumi, L.F.I.; Ngomane, A.Y.; Guimarães, G.V.; Ciolac, E.G. High-Intensity Interval Versus Moderate-Intensity Continuous Training in Individuals With Parkinson’s Disease: Hemodynamic and Functional Adaptation. J. Phys. Act. Health 2020, 17, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.; Weston, K.L.; Gray, W.K.; O’Callaghan, A.; Oates, L.L.; Davidson, R.; Walker, R.W. High-Intensity Interval Training in People with Parkinson’s Disease: A Randomized, Controlled Feasibility Trial. Clin. Rehabil. 2019, 33, 428–438. [Google Scholar] [CrossRef]
- O’Callaghan, A.; Harvey, M.; Houghton, D.; Gray, W.K.; Weston, K.L.; Oates, L.L.; Romano, B.; Walker, R.W. Comparing the Influence of Exercise Intensity on Brain-Derived Neurotrophic Factor Serum Levels in People with Parkinson’s Disease: A Pilot Study. Aging Clin. Exp. Res. 2020, 32, 1731–1738. [Google Scholar] [CrossRef]
| Studies | Participants | Aerobic Training | Results | |
|---|---|---|---|---|
| Intensity | Duration | |||
| Tang et al., 2016 [23] | n = 25 Age: 66 (62–71) years Timing after stroke: 3.5 (2.2–6.7) years | From 40 to 80% HRR | 60 min/session 3 sessions/week for 6 months | ➚ Short-term memory ⟺ Working memory, set shifting, conflict resolution |
| Boyne et al., 2019 [77] | n = 16 Age: 57.4 (37.7–72.1) years Timing after stroke: 6.5 (0.5–16.11) years | Treadmill HIIT: maximum tolerated speed 30 sec HI and 60 to 30 s LI Seated Stepper HIIT: maximal cadence with 50% of maximal resistance MICT: 45 ± 5% HRR | 25 min/session | ➚ serum BDNF Lower ➚ in serum BDNF after MICT |
| Boyne et al., 2020 [24] | n = 16 Age: 57.4 (37.7–72.1) years Timing after stroke: 6.5 (0.5–16.11) years | Treadmill HIIT: maximum tolerated speed 30 sec HI and 60 to 30 s LI Seated Stepper HIIT: maximal cadence with 50% of maximal resistance MICT: 45 ± 5% HRR | 25 min/session | ➚ VEGF, IGF-1 after HIIT ➚ Serum BDNF is correlated to ➚ blood lactate after HIIT |
| Hsu et al., 2020 [35] | n = 28 Age: HIIT: 58.5 (49.8–67.2) years MICT: 53.1 (46.2–60.0) years Timing after stroke: 38.5 (19.1–57.9) months | Bicycle ergometer HIIT: 3 min at 80% VO2peak separated by 3 min at 40% VO2peak Bicycle ergometer MICT: 60% VO2peak | 30 min/session Isocaloric 2 to 3/week 36 sessions | ➚ VO2peak after HIIT > MICT ➚ peak cardiac output ➚ △[HHB] and △[THB] after HIIT in lesioned hemisphere ➚ Serum BDNF after HIIT ➚ Dendritic growth with patient serum after HIIT |
| Studies | Participants | Aerobic Training | Results | |
|---|---|---|---|---|
| Intensity | Duration | |||
| Pin-Barre et al., 2017 [38] | Sprague-Dawley n = 70 Age: 2–3 months Method: tMCAO (120 min) Timing after stroke: 24–48 h | HIIT: 4 × (4 + 3 min active rest) 80% of Smax-SLT (week 1) 95% of Smax-SLT (2) MICT: 80% SLT | 28 min/session Isocaloric 5/week for 2 weeks | ➚ Endurance performance after HIIT ➘ Inflammation mainly in the lesioned hemisphere Restored NKCC1/KCC2 ratio in the contralesional hemisphere |
| Luo et al., 2018 [37] | Wistar n = 55 Age: 2–3 months Method: tMCAO (90 min) Timing after stroke: 28 days | HIIT: 4 × (4 + 3 min rest) SLT + 60–70% (Smax-SLT) MICT: 80–90% SLT | 28 min/session Isocaloric 5/week for 4 weeks | ➚ BDNF in ipsilesional CA1, CA3 and DG after HIIT ➚ mBDNF/proBDNF ratio in hippocampus after HIIT ➚ TrkB and NR2A expression after HIIT ➘ p75NTR and NR2B after HIIT |
| Li et al., 2020 [133] | C57BL/6J mice n = 5/group Age: 8–10 weeks Method: tMCAO (90 min) Timing after stroke: 28 days | HIIT: 4 × 4 (4 + 3 min rest) SLT + 60–70% (Smax-SLT) MICT: 80% SLT | 28 min/session Isocaloric HIIT: 5/week MICT: 7/week for 4 weeks | ➘ Neuronal death in DG after HIIT ➚ Neuroprotection through ➘ PTEN activity after HIIT ➘ Depression-like behavior after HIIT |
| Pin-Barre et al., 2021 [39] | Sprague-Dawley n = 42 Age: 2–3 months Method: tMCAO (120 min) Timing after stroke: 24–48 h | HIIT4: 4 x (4 + 3 min active rest) HIIT1: 1 + 1 min active rest 80% of Smax – SLT (1st week) 95% of Smax – SLT (2nd week) | 28 min/session Isocaloric 5/week for 2 weeks | Both HIIT does not reduce stroke-induced gliogenesis in the ipsilesional hesmisphere Both HIIT ➚ pTrkB in the contralesional hippocampus while HIIT4 only ➚ pTrkB in the contralesional cortex Both HIIT ➚ FNDC5 and Cyt C in the contralesional cortex |
| Studies | Participants | Aerobic Training | Combination | Results | |
|---|---|---|---|---|---|
| Intensity | Duration | ||||
| Madhavan et al., 2016 [144] | n = 11 Age: 58 Timing after stroke: 9 years | Incremental walking speed until 80% of the age-predicted HR (220-age) | 40 min/session 1 session | tDCS enhanced with a paretic ankle skill acquisition task (15 min) | ➘ CME of the paretic tibialis anterior after HIIT alone ➚ CME of the paretic tibialis anterior after the combination ➘ RPE after combination |
| Nepveu et al., 2017 [141] | n = 22 Age: 64.9 Timing after stroke: chronic stroke patients | HIIT: 3 × 3 min at 100% peak workload GXT interspersed with 2 × 2 min at 25% | 15 min/session 1 session | Time-on-target motor task ending 10 min before HIIT initiation Retention test 24 h after HIIT session | ➘ Tendency of SICI measured by TMS ➚ Skill retention after HIIT |
| Madhavan et al., 2020 [143] | n = 81 Age: 58.8 Timing after stroke: 5.5 years | Speed increment over 2 min to reach the maximal speed for 10 s Warm-up HR during recovery initiate a new interval | 40 min/day 3 days/week for 4 weeks | tDCS enhanced with a paretic ankle skill acquisition task (15 min) | CME with the combination Patients with ➚ CME increased walking speed more than others |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugues, N.; Pellegrino, C.; Rivera, C.; Berton, E.; Pin-Barre, C.; Laurin, J. Is High-Intensity Interval Training Suitable to Promote Neuroplasticity and Cognitive Functions after Stroke? Int. J. Mol. Sci. 2021, 22, 3003. https://doi.org/10.3390/ijms22063003
Hugues N, Pellegrino C, Rivera C, Berton E, Pin-Barre C, Laurin J. Is High-Intensity Interval Training Suitable to Promote Neuroplasticity and Cognitive Functions after Stroke? International Journal of Molecular Sciences. 2021; 22(6):3003. https://doi.org/10.3390/ijms22063003
Chicago/Turabian StyleHugues, Nicolas, Christophe Pellegrino, Claudio Rivera, Eric Berton, Caroline Pin-Barre, and Jérôme Laurin. 2021. "Is High-Intensity Interval Training Suitable to Promote Neuroplasticity and Cognitive Functions after Stroke?" International Journal of Molecular Sciences 22, no. 6: 3003. https://doi.org/10.3390/ijms22063003
APA StyleHugues, N., Pellegrino, C., Rivera, C., Berton, E., Pin-Barre, C., & Laurin, J. (2021). Is High-Intensity Interval Training Suitable to Promote Neuroplasticity and Cognitive Functions after Stroke? International Journal of Molecular Sciences, 22(6), 3003. https://doi.org/10.3390/ijms22063003






