EPIP-Evoked Modifications of Redox, Lipid, and Pectin Homeostasis in the Abscission Zone of Lupine Flowers
Abstract
1. Introduction
2. Results
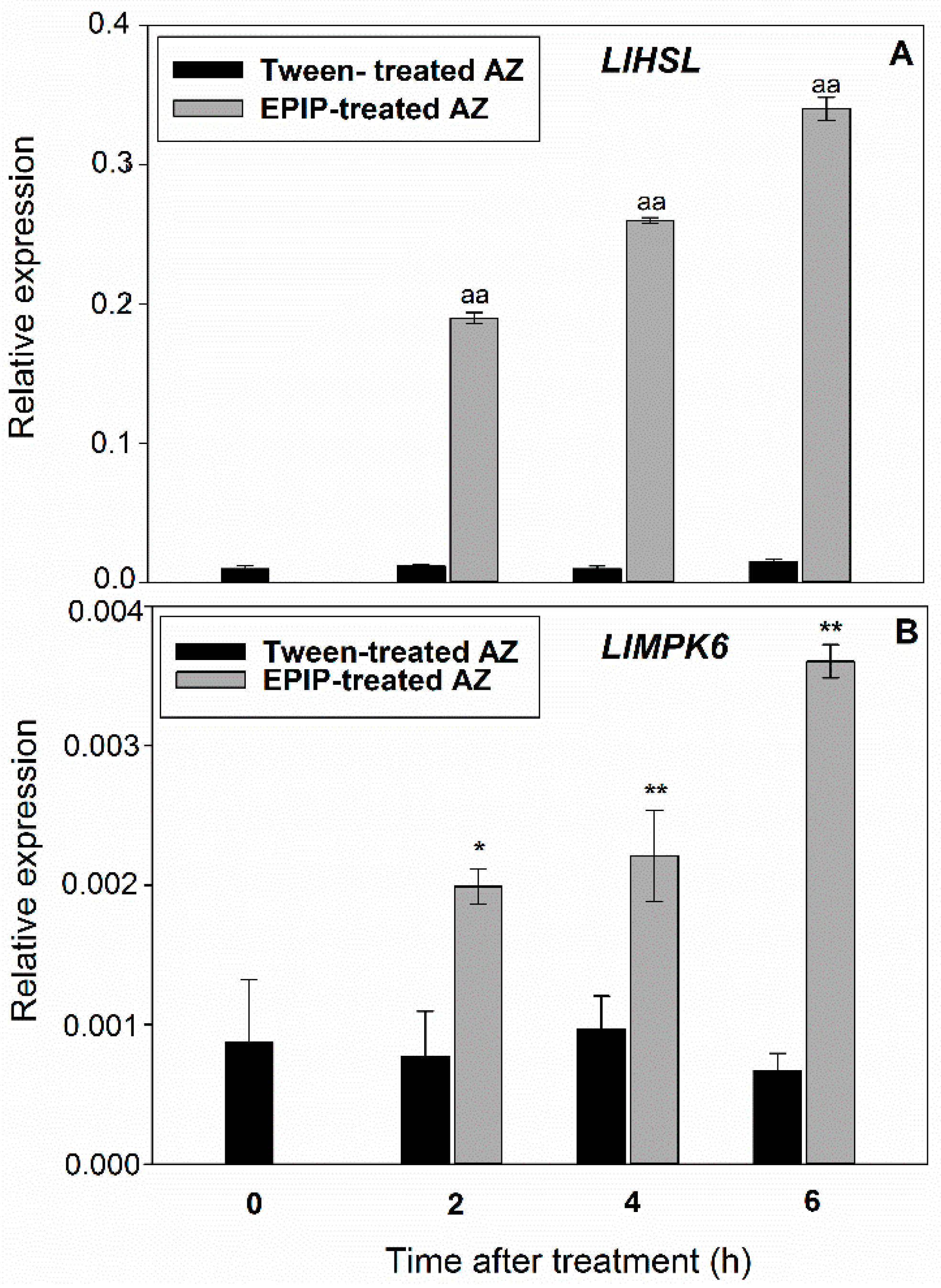
2.1. EPIP Peptide Induces Subsequent Components of a Pathway Responsible for Flower AZ Activation
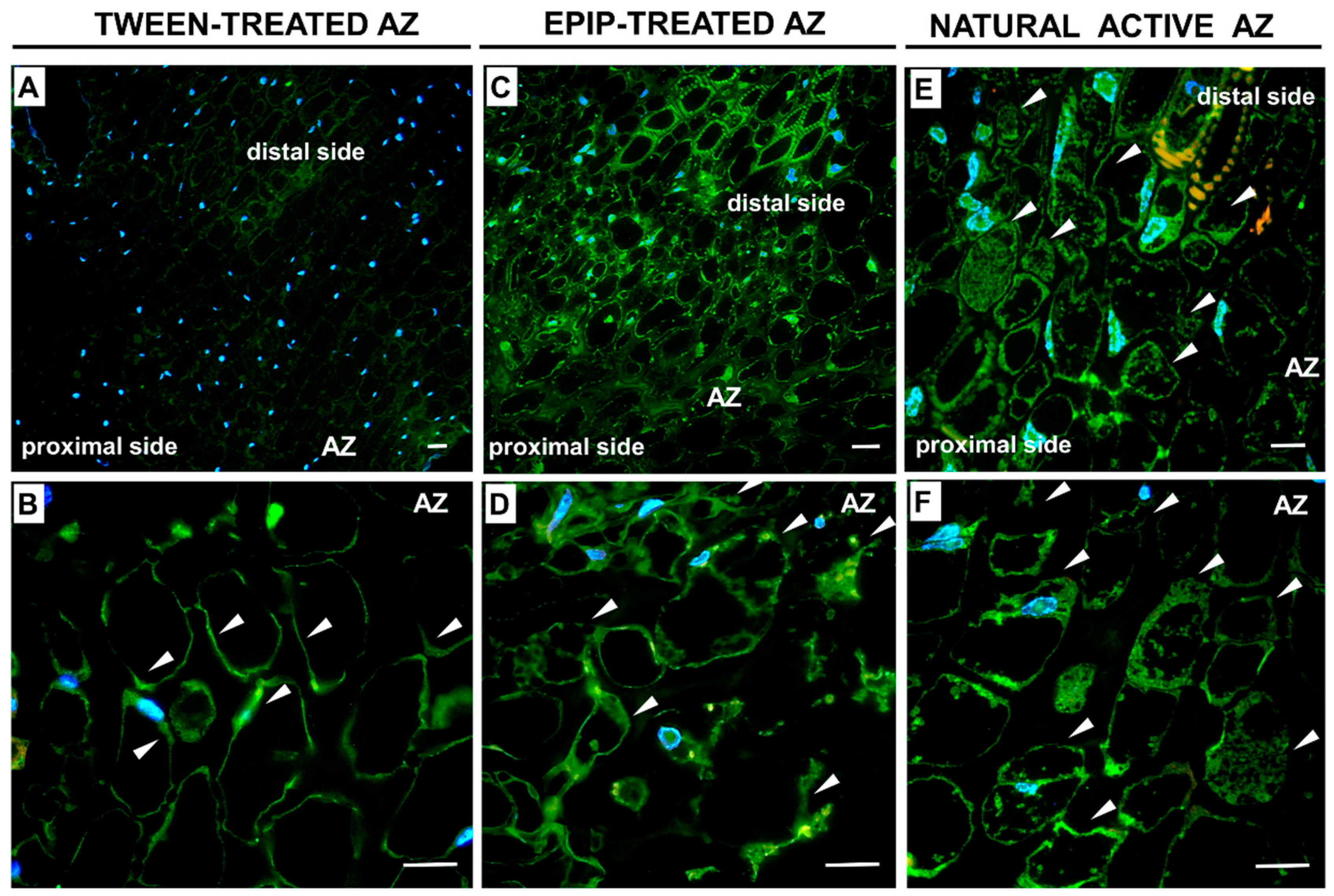
2.2. The Synthetic EPIP Peptide Treatment Results in Cellular Changes in the Floral AZ Similar to Those That Occur in the Naturally Active AZ
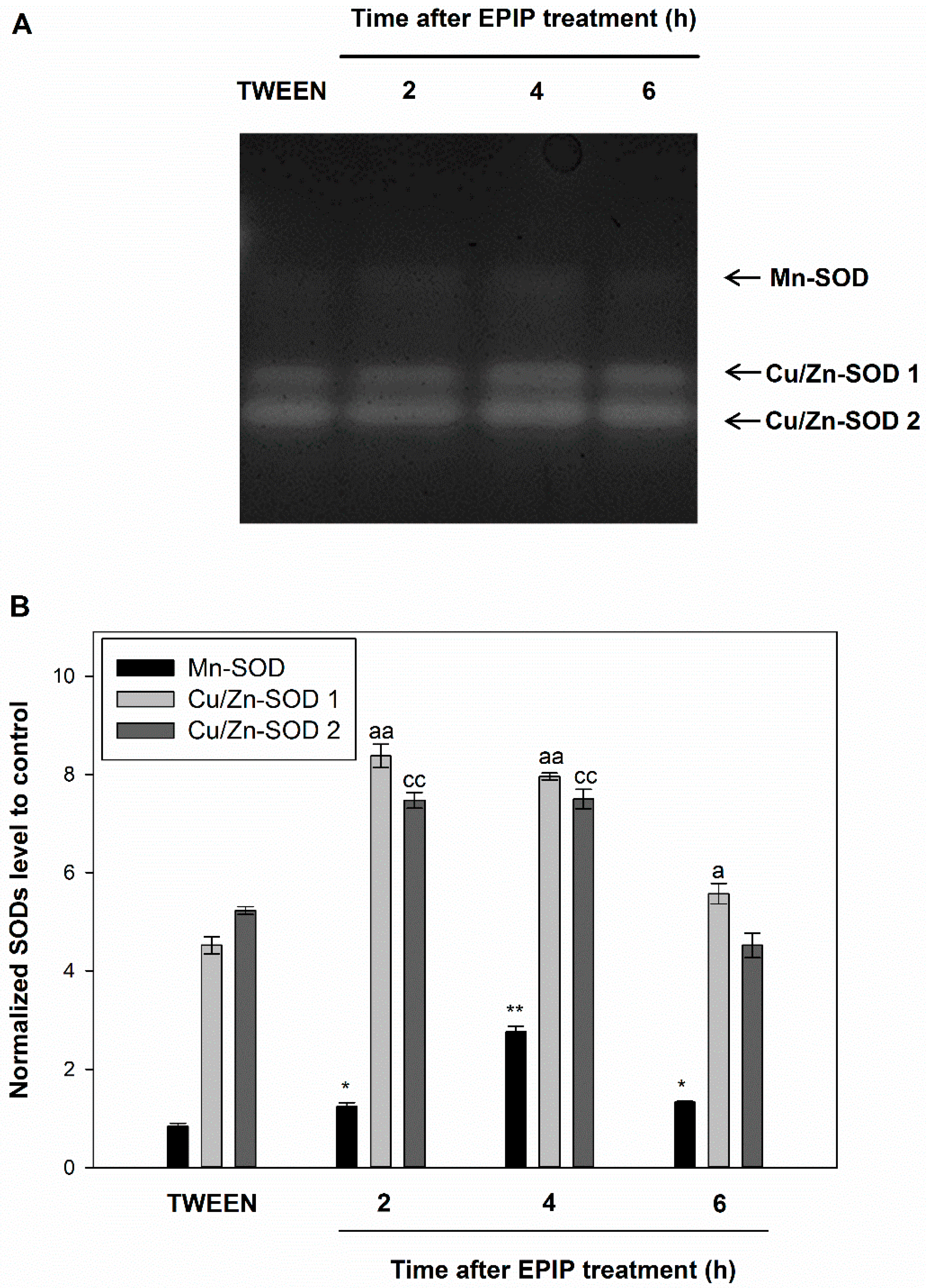
2.3. EPIP Peptide Influences the Redox Homeostasis in Flower AZ
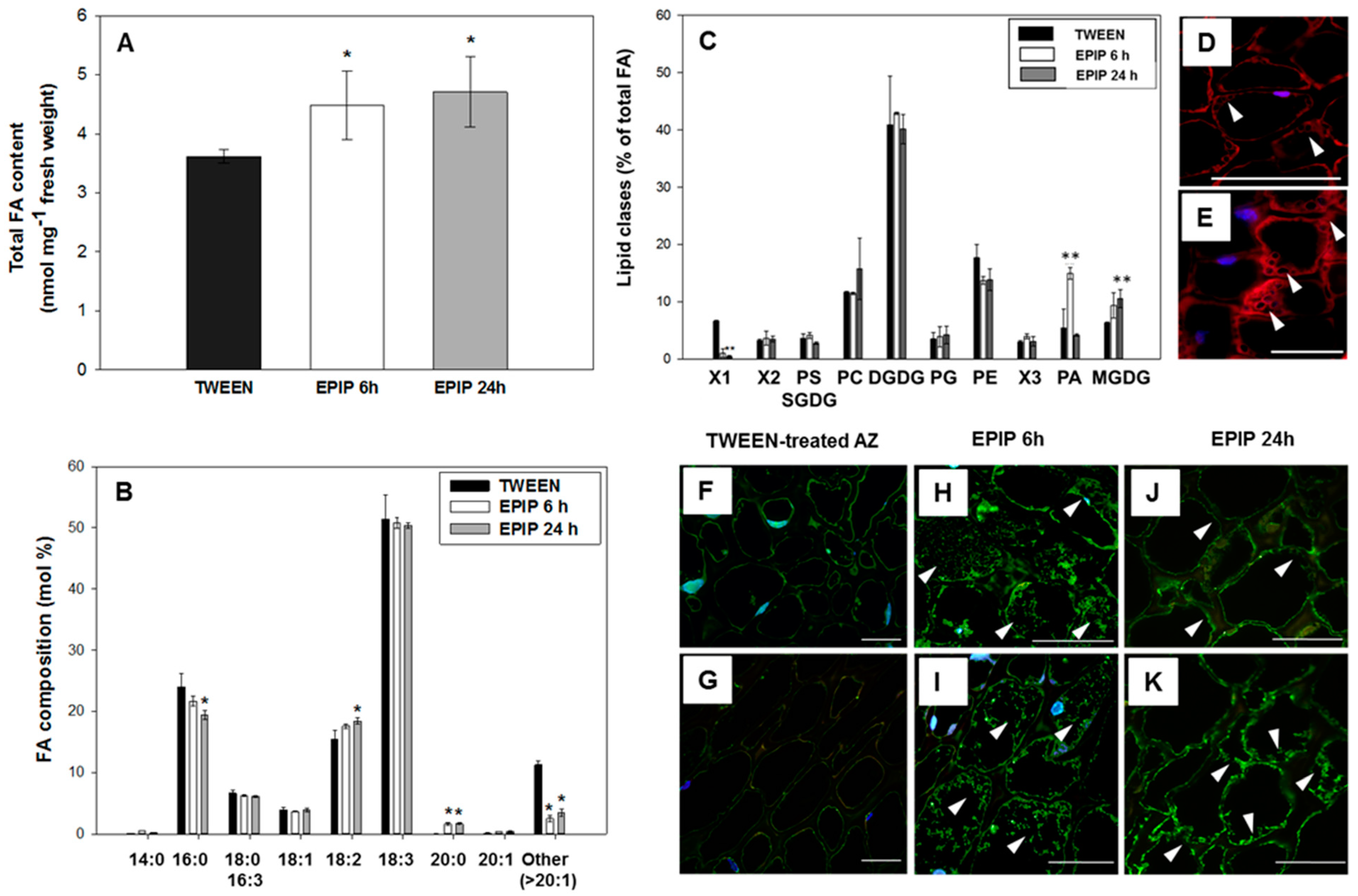
2.4. Exogenous EPIP Results in Enhanced Lipid Content and Changes Their Composition in AZ Cells
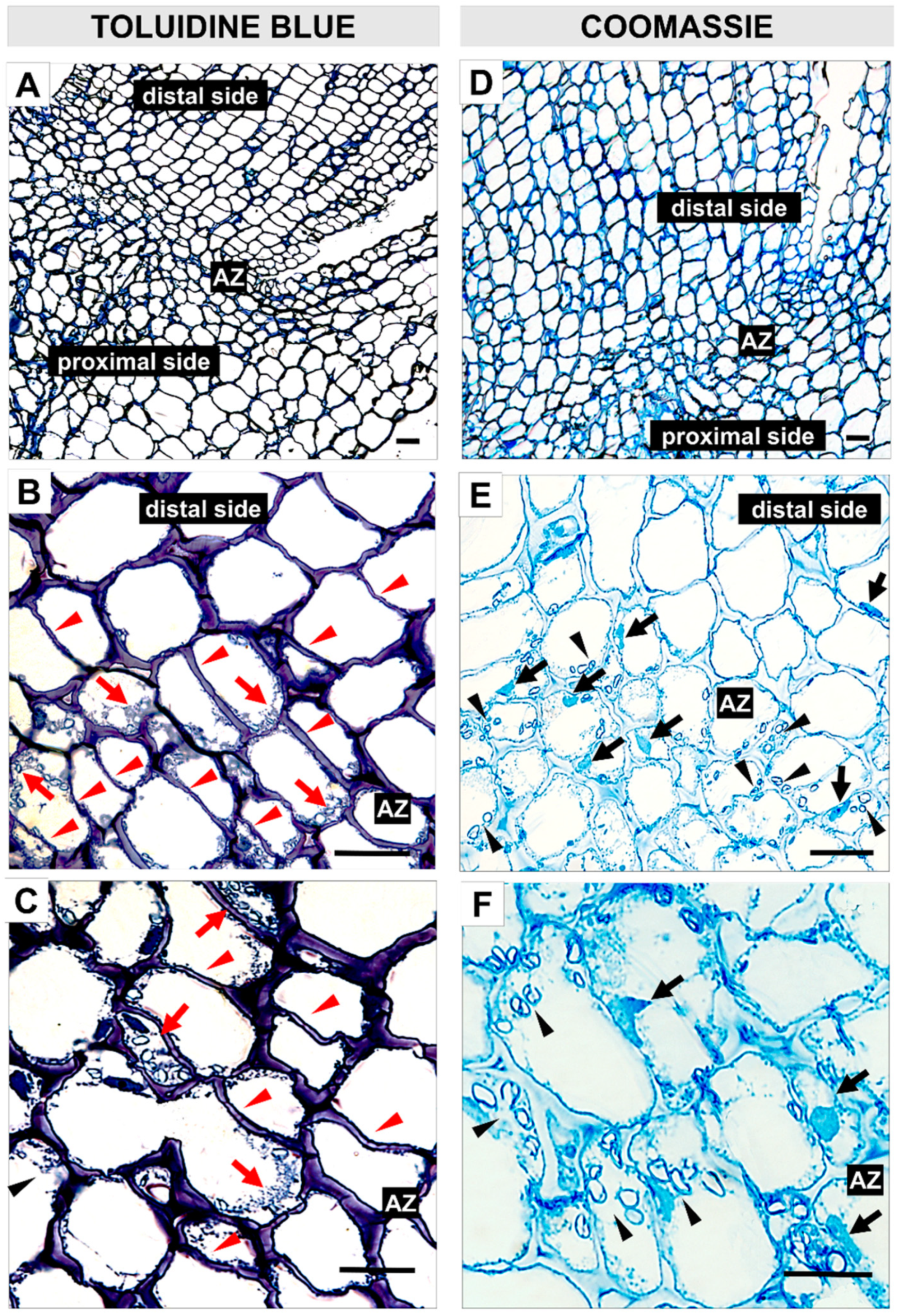
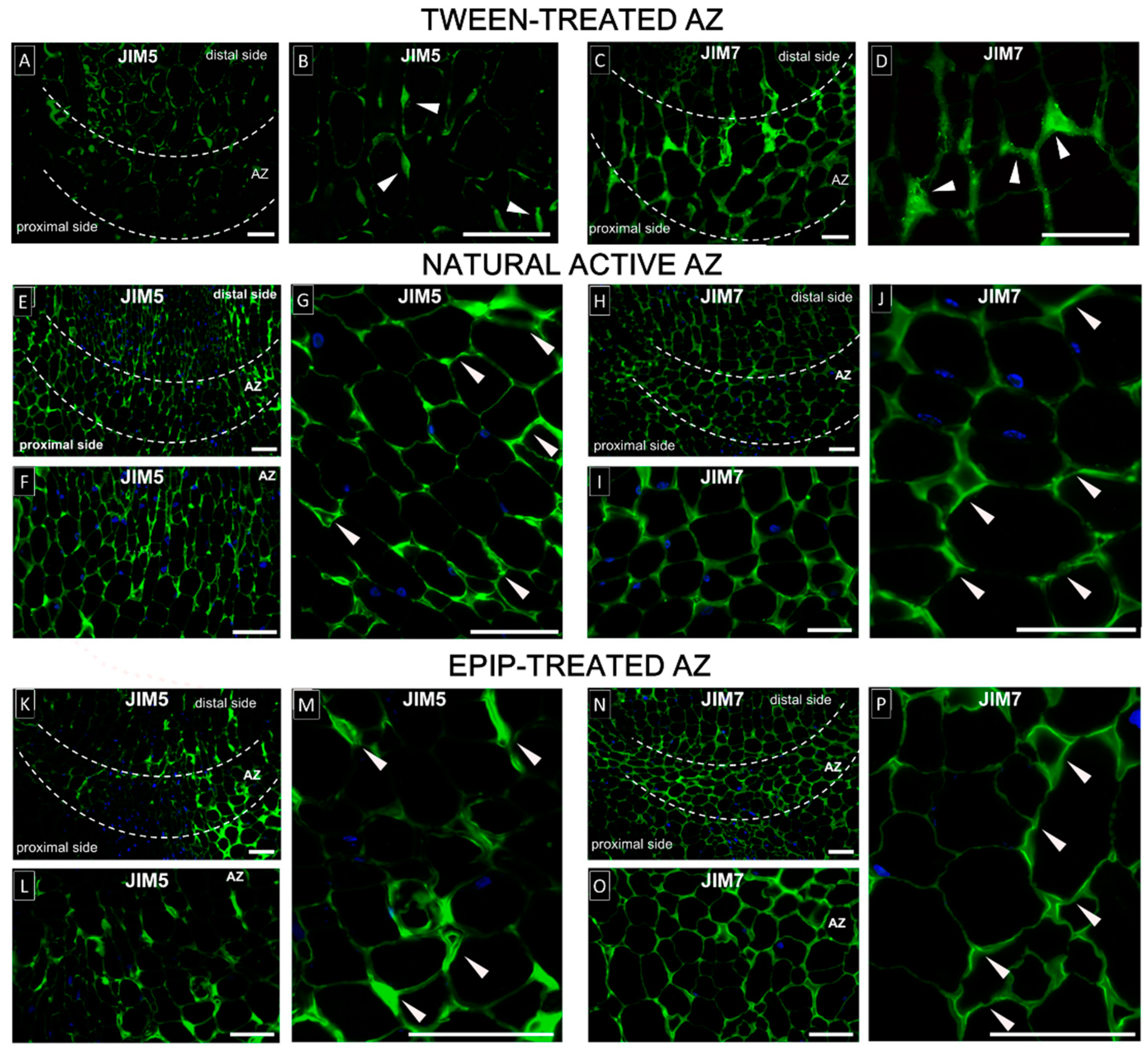
2.5. EPIP-Induced Flower Abscission Is Accompanied by Concomitant Modifications of Pectin Cell Wall Components
3. Discussion
3.1. The AZ Response Evoked by Exogenous EPIP Leads to Abscission Activation
3.2. The EPIP Treatment Leads to the Disruption of the Redox Balance and Modifies Lipids Composition
4. Material and Methods
4.1. Plant Material, Growth Conditions, and Treatments
4.2. RNA Extraction and RT-qPCR
4.3. Antioxidant Enzymes Activity Determination and H2O2 Measurements
4.4. Histological Assays
4.5. Immunocytological Assay of MPK6, CAT, JIM5, JIM7, and PLD
4.6. Lipid Profiling
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, J.A.; Whitelaw, C.A.; Gonzalez-Carranza, Z.H.; McManus, M.T. Cell Separation Processes in Plants: Models, Mechanisms and Manipulation. Ann. Bot. 2000, 86, 223–235. [Google Scholar] [CrossRef]
- Bar-Dror, T.; Dermastia, M.; Kladnik, A.; Znidaric, M.T.; Novak, M.P.; Meir, S.; Burd, S.; Philosoph-Hadas, S.; Ori, N.; Sonego, L.; et al. Programmed Cell Death Occurs Asymmetrically during Abscission in Tomato. Plant Cell 2011, 23, 4146–4163. [Google Scholar] [CrossRef]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating Mechanisms Underlying Organ Abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef]
- Tranbarger, T.J.; Tucker, M.L.; Roberts, J.A.; Meir, S. Editorial: Plant Organ Abscission: From Models to Crops. Front. Plant Sci. 2017, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.L.; Yang, R. IDA-like Gene Expression in Soybean and Tomato Leaf Abscission and Requirement for a Diffusible Stelar Abscission Signal. AoB Plants 2012, 2012, pls035. [Google Scholar] [CrossRef]
- Estornell, L.H.; Wildhagen, M.; Pérez-Amador, M.A.; Talón, M.; Tadeo, F.R.; Butenko, M.A. The IDA Peptide Controls Abscission in Arabidopsis and Citrus. Front. Plant Sci. 2015, 6, 1003. [Google Scholar] [CrossRef]
- Ying, P.; Li, C.; Liu, X.; Xia, R.; Zhao, M.; Li, J. Identification and Molecular Characterization of an IDA-like Gene from Litchi, LcIDL1, Whose Ectopic Expression Promotes Floral Organ Abscission in Arabidopsis. Sci. Rep. 2016, 6, 37135. [Google Scholar] [CrossRef] [PubMed]
- Tranbarger, T.J.; Domonhedo, H.; Cazemajor, M.; Dubreuil, C.; Fischer, U.; Morcillo, F. The PIP Peptide of Inflorescence Deficient in Abscission Enhances Populus Leaf and Elaeis Guineensis Fruit Abscission. Plants 2019, 8, 143. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Frankowski, K.; Kućko, A.; Świdziński, M.; de Dios Alché, J.; Nowakowska, A.; Kopcewicz, J. The Influence of Abscisic Acid on the Ethylene Biosynthesis Pathway in the Functioning of the Flower Abscission Zone in Lupinus luteus. J. Plant Physiol. 2016, 206, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wilmowicz, E.; Kućko, A.; Ostrowski, M. Inflorescence Deficient in Abscission -like is an Abscission-associated and Phytohormone-regulated Gene in Flower Separation of Lupinus luteus. Plant Growth Regul. 2018, 85, 91–100. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Burchardt, S.; Przywieczerski, T. Molecular and Hormonal Aspects of Drought-triggered Flower Shedding in Yellow Lupine. IJMS 2019, 20, 3731. [Google Scholar] [CrossRef]
- Kućko, A.; Wilmowicz, E.; Ostrowski, M. Spatio-temporal IAA Gradient is Determined by Interactions with ET and Governs Flower Abscission. J. Plant Physiol. 2019, 23, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kućko, A.; Smoliński, D.J.; Wilmowicz, E.; Florkiewicz, A.; Alché, J. Spatio-temporal Localization of LlBOP Following Early Events of Floral Abscission in Yellow Lupine. Protoplasma 2019, 256, 1173–1183. [Google Scholar] [CrossRef]
- Butenko, M.A.; Patterson, S.E.; Grini, P.E.; Stenvik, G.E.; Amundsen, S.S.; Mandal, A.; Aalen, R.B. Inflorescence Deficient in Abscission Controls Floral Organ Abscission in Arabidopsis and Identifies a Novel Family of Putative Ligands in Plants. Plant Cell 2003, 15, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Stenvik, G.E.; Butenko, M.A.; Urbanowicz, B.R.; Rose, J.K.; Aalen, R.B. Overexpression of Inflorescence Deficient in Abscission Activates Cell Separation in Vestigial Abscission Zones in Arabidopsis. Plant Cell 2006, 18, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Stenvik, G.E.; Tandstad, N.M.; Guo, Y.; Shi, C.-L.; Kristiansen, W.; Holmgren, A.; Clark, S.E.; Aalen, R.B.; Butenko, M.A. The EPIP Peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is Sufficient to Induce Abscission in Arabidopsis through the Receptor-like Kinases HAESA and HAESA-LIKE2. Plant Cell 2008, 20, 1805–1817. [Google Scholar] [CrossRef]
- Aalen, R.B.; Wildhagen, M.; Stø, I.M.; Butenko, M.A. IDA: A Peptide Ligand Regulating Cell Separation Processes in Arabidopsis. J. Exp. Bot. 2013, 64, 5253–5261. [Google Scholar] [CrossRef]
- Vie, A.K.; Najafi, J.; Liu, B.; Winge, P.; Butenko, M.A.; Hornslien, K.S.; Kumpf, R.; Aalen, R.B.; Bones, A.M.; Brembu, T. The IDA/IDA-LIKE and PIP/PIP-LIKE Gene Families in Arabidopsis: Phylogenetic Relationship, Expression Patterns, and Transcriptional Effect of the PIPL3 Peptide. J. Exp. Bot. 2015, 66, 5351–5365. [Google Scholar] [CrossRef] [PubMed]
- Butenko, M.A.; Stenvik, G.E.; Alm, V.; Saether, B.; Patterson, S.E.; Aalen, R.B. Ethylene-dependent and -independent Pathways Controlling Floral Abscission are Revealed to Converge Using Promoter: Reporter Gene Constructs in the Ida Abscission Mutant. J. Exp. Bot. 2006, 57, 3627–3637. [Google Scholar] [CrossRef]
- Butenko, M.A.; Shi, C.L.; Aalen, R.B. KNAT1, KNAT2 and KNAT6 Act Downstream in the IDA-HAE/HSL2 Signaling Pathway to Regulate Floral Organ Abscission. Plant. Signal. Behav. 2012, 7, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Tang, J.; Li, B.; de Oliveira, M.V.V.; Chai, J.; He, P.; Shan, L. Ligand-induced Receptor-like Kinase Complex Regulates Floral Organ Abscission in Arabidopsis. Cell Rep. 2016, 14, 1330–1338. [Google Scholar] [CrossRef]
- Butenko, M.A.; Wildhagen, M.; Albert, M.; Jehle, A.; Kalbacher, H.; Aalen, R.B.; Felix, G. Tools and Strategies to Match Peptide-ligand Receptor Pairs. Plant Cell 2014, 26, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.; Jinn, T.L.; Zhang, S.; Walker, J.C. Regulation of Floral Organ Abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 15629–15634. [Google Scholar] [CrossRef]
- Shi, C.L.; Stenvik, G.E.; Vie, A.K.; Bones, A.M.; Pautot, V.; Proveniers, M.; Aalen, R.B.; Butenko, M.A. Arabidopsis Class I KNOTTED-like Homeobox Proteins Act Downstream in the IDA-HAE/HSL2 Floral Abscission Signal. Plant Cell 2011, 2000, 1142. [Google Scholar]
- Eo, J.; Lee, B.Y. Anatomical and Histological Changes in the Fruit Abscission Zone of Water Dropwort (Oenanthe stolonifera DC.). Hort. Environ. Biotechnol. 2011, 52, 315–320. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, X.; Fan, Y.; Wang, H.; Li, J.; Huang, X. Burst of Reactive Oxygenspecies in Pedicel-mediated Fruit Abscission after Carbohydrate Supply was Cutoff in Longan (Dimocarpus longan). Front. Plant Sci. 2015, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Munemura, I.; Tomita, R.; Kobayashi, K. Involvement of Hydrogen Peroxide in Leaf Abscission Signaling, Revealed by Analysis with an in vitro Abscission System in Capsicum plants. Plant J. 2008, 56, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wang, G.; Li, Y.; Wang, B.; Zhang, P.; Peng, M. Reactive Oxygen Species Regulate Leaf Pulvinus Abscission Zone Cell Separation in Response to Water-deficit Stress in Cassava. Sci. Rep. 2016, 6, 21542. [Google Scholar] [CrossRef]
- Droillard, M.-J.; Bureau, D.; Paulin, A. Changes in Activities of Superoxide Dismutases during Aging of Petals of Cut Carnations (Dianthus caryophyllus). Physiol. Plant. 1989, 76, 149–154. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Stø, I.M.; Orr, R.J.S.; Fooyontphanich, K.; Jin, X.; Knutsen, J.M.B.; Fischer, U.; Tranbarger, T.J.; Nordal, I.; Aalen, R.B. Conservation of the Abscission Signaling Peptide IDA during Angiosperm Evolution: Withstanding Genome Duplications and Gain and Loss of the Receptors HAE/HSL2. Front. Plant Sci. 2015, 6, 931. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Brandt, B.; Wildhagen, M.; Hohmann, U.; Hothorn, L.A.; Butenko, M.A.; Hothorn, M. Mechanistic Insight into a Peptide Hormone Signaling Complex Mediating Floral Organ Abscission. eLife 2016, 5, e15075. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Kärkönen, A.; Kuchitsu, K. Reactive Oxygen Species in Cell Wall Metabolism and Development in Plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Lessire, R.; Cahoon, E.; Chapman, K.; Dyer, J.; Eastmond, P.; Heinz, E. Highlights of Recent Progress in Plant Lipid Research. Plant Physiol. Biochem. 2009, 47, 443–447. [Google Scholar] [CrossRef][Green Version]
- Lada, R.R.; MacDonald, M.T. Understanding the Physiology of Postharvest Needle Abscission in Balsam Fir. Front. Plant Sci. 2015, 6, 1069. [Google Scholar] [CrossRef]
- Alché, J.D. A concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.; MacDonald, M.T. Potential Roles of Fatty Acids and Lipids in Postharvest Needle Abscission Physiology. AJPS 2019, 10, 1069–1089. [Google Scholar] [CrossRef]
- Nakano, T.; Fujisawa, M.; Shima, Y.; Ito, Y. Expression Profiling of Tomato Pre-abscission Pedicels Provides Insights into Abscission Zone Properties Including Competence to Respond to Abscission Signals. BMC Plant Biol. 2013, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.R.; Frost, E.; Vidi, P.A.; Kessler, F.; Staehelin, L.A. Plastoglobules are Lipoprotein Subcompartments of the Chloroplast That are Permanently Coupled to Thylakoid Membranes and Contain Biosynthetic Enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid Remodelling: Unravelling the Response to Cold Stress in Arabidopsis and Its Extremophile Relative Eutremasalsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.R.; Browse, J.; Jaworski, J.; Ohlrogge, J. Lipids. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; ASPB: Rockville, MD, USA, 2000; Chapter 10; pp. 456–526. [Google Scholar]
- Benning, C.; Xu, C.; Awai, K. Non-vesicular and Vesicular Lipid Trafficking Involving Plastids. Curr. Opin. Plant Biol. 2006, 9, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Harholt, J.; Suttangkakul, A.; Vibe Scheller, H. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving Views of Pectin Biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-induced Changes in Cell Wall Mechanics Underlie Organ Initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Limberg, G.; Bucholt, H.C.; van Alebeek, G.-J.; Benen, J.; Christensen, T.M.I.E.; Visser, J.; Voragen, A.; Mikkelsen, J.D.; Knox, J.P. Analysis of Pectic Epitopes Recognised by Hybridoma and Phage Display Monoclonal Antibodies Using Defined Oligosaccharides, Polysaccharides and Enzymatic Degradation. Carbohydrate Res. 2000, 327, 309–320. [Google Scholar] [CrossRef]
- Clausen, M.H.; Willats, W.G.; Knox, J.P. Synthetic Methyl Hexagalacturonate Hapten Inhibitors of Anti-homogalacturonan Monoclonal Antibodies LM7, JIM5 and JIM7. Carbohydr Res. 2003, 338, 1797–1800. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in Abscission Signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Nakagami, H.; Pitzschke, A.; Hirt, H. Emerging MAP Kinase Pathways in Plant Stress Signalling. Trends Plant Sci. 2005, 10, 339–346. [Google Scholar] [CrossRef]
- Cai, S.; Lashbrook, C.C. Stamen Abscission Zone Transcriptome Profiling Reveals New Candidates for Abscission Control: Enhanced Retention of Floral Organs in Transgenic Plants Overexpressing Arabidopsis ZINC FINGER PROTEIN2. Plant Physiol. 2008, 146, 1305–1321. [Google Scholar] [CrossRef]
- Kumpf, R.P.; Shi, C.-L.; Larrieu, A.; Sto, I.M.; Butenko, M.A.; Peret, B.; Riiser, E.S.; Bennett, M.J.; Aalen, R.B. Floral Organ Abscission Peptide IDA and Its HAE/HSL2 Receptors Control Cell Separation during Lateral Root Emergence. Proc. Natl. Acad. Sci. USA 2013, 110, 5235–5240. [Google Scholar] [CrossRef]
- Niederhuth, C.; Patharkar, O.R.; Walker, J. Transcriptional Profiling of the Arabidopsis Abscission Mutant hae hsl2 by RNA-Seq. BMC Genom. 2013, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, F.M.; Racusen, R.H. Microsurgery Reveals Regional Capabilities for Pattern Re-establishment in Somatic Carrot Embryos. Dev. Biol. 1990, 141, 211–219. [Google Scholar] [CrossRef]
- Jensen, T.E.; Valdovinos, J.G. Fine Structure of Abscission Zones, I. Abscission Zones of the Pedicels of Tobacco and Tomato Flowers at Anthesis. Planta 1967, 77, 298–318. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Schindler, C.B.; Tucker, G.A. Ethylene-promoted Tomato Flower Abscission and the Possible Involvement of an Inhibitor. Planta 1984, 160, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Stead, A.D. Abscission of Flowers and Floral Parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Lee, Y.; Derbyshire, P.; Knox, J.P.; Hvoslef-Eide, A.K. Sequential Cell Wall Transformations in Response to the Induction of a Pedicel Abscission Event in Euphorbia pulcherrima (poinsettia). Plant J. 2008, 54, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Ruperti, B. The Yes and No of the Ethylene Involvement in Abscission. Plants 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.W.; Valdovinos, J.G.; Jensen, T.E. Peroxidases in Tobacco Abscission Zone Tissue. II. Time-course of Peroxidase Activity during Ethylene-induced Abscission. Plant Physiol. 1974, 54, 192–196. [Google Scholar] [CrossRef][Green Version]
- Evensen, K.B.; Page, A.M.; Stead, A.D. Anatomy of Ethylene Induced Petal Abscission in Pelargonium x hortorum. Ann. Bot. 1993, 71, 559–566. [Google Scholar] [CrossRef]
- Meir, S.; Philosoph-Hadas, S.; Sundaresan, S.; Selvaraj, K.S.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Microarray Analysis of the Abscission-related Transcriptome in the Tomato Flower Abscission Zone in Response to Auxin Depletion. Plant Physiol. 2010, 154, 1929–1956. [Google Scholar] [CrossRef]
- Wang, R.; Shi, C.; Wang, X.; Li, R.; Meng, Y.; Cheng, L.; Qi, M.; Xu, T.; Li, T. Tomato SlIDA has a Critical Role in Tomato Fertilization by Modifying ROS Homeostasis. Plant J. 2020, 103, 2100–2118. [Google Scholar] [CrossRef] [PubMed]
- Kephart, S.R. Starch Gel Electrophoresis of Plant Isoenzymes: A Comparative Analysis of Techniques. Am. J. Bot. 1990, 77, 693–712. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Burstein, C.; Biton, I.; Ben Sasson, S.; Sadeh, A.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Mugira, Y.; Schneider, D.; et al. Ethephon Induced Oxidative Stress in the Olive Leaf Abscission Zone Enables Development of a Selective Abscission Compound. BMC Plant Biol. 2017, 17, 87. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate Peroxidase - A Hydrogen Peroxide-scavenging Enzyme in Plants. PHYSIOL. Plant. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Chen, G.X.; Asada, K. Ascorbate Peroxidase in Tea Leaves: Occurrence of Two Isozymes and the Differences in Their Enzymatic and Molecular Properties. Plant Cell Physiol. 1989, 30, 987–998. [Google Scholar]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of Oxidative Cross-linking of Cell Wall Structural Proteins in Plant Disease Resistance. Plant Cell 1994, 6, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, P. Hydroxyl Radical-induced Cell-wall Loosening in vitro and in vivo: Implications for the Control of Elongation Growth. Plant J. 2001, 28, 679–688. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, T.H.; Lee, J.; Jeon, S.Y.; Lee, J.H.; Lee, M.K.; Chen, H.; Yun, J.; Oh, S.Y.; Wen, X.; et al. A Lignin Molecular Brace Controls Precision Processing of Cell Walls Critical for Surface Integrity in Arabidopsis. Cell 2018, 173, 1468–1480. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Salleh, F.M.; Mariotti, L.; Spadafora, N.D.; Price, A.M.; Picciarelli, P.; Wagstaff, C.; Lombardi, L.; Rogers, H. Interaction of Plant Growth Regulators and Reactive Oxygen Species to Regulate Petal Senescence in Wallflowers (Erysimum linifolium). BMC Plant Biol. 2016, 16, 77. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Neunzig, J.; Lee, Y.; Philippar, K. Plant Membrane-protein Mediated Intracellular Traffic of Fatty Acids and Acyl Lipids. Curr. Opin. Plant Biol. 2017, 40, 138–146. [Google Scholar] [CrossRef]
- Wang, X. Regulatory Functions of Phospholipase D and Phosphatidic Acid in Plant Growth, Development, and Stress Responses. Plant Physiol. 2005, 139, 566–573. [Google Scholar] [CrossRef]
- Sun, J.; Cardoza, V.; Mitchell, D.M.; Bright, L.; Oldroyd, G.; Harris, J.M. Crosstalk between Jasmonic Acid, Ethylene and Nod Factor Signaling Allows Integration of Diverse Inputs for Regulation of Nodulation. Plant J. 2006, 46, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Saito, K. Roles of Lipids as Signaling Molecules and Mitigators during Stress Response in Plants. Plant J. 2014, 79, 584–596. [Google Scholar] [CrossRef]
- Benning, C. Mechanisms of Lipid Transport Involved in Organelle Biogenesis in Plant Cells. Ann. Rev. Cell Dev. Biol. 2009, 25, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Härtel, H.; Dormann, P.; Benning, C. DGD1-independent Biosynthesis of Extraplastidic Galactolipids after Phosphate Deprivation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2000, 97, 10649–10654. [Google Scholar] [CrossRef] [PubMed]
- Torres-Franklin, M.L.; Gigon, A.; de Melo, D.F.; Zuily-Fodil, Y.; Pham-Thi, A.T. Drought Stress and Rehydration Affect the Balance between MGDG and DGDG Synthesis in Cowpea Leaves. Physiol. Plant 2007, 131, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lev, S. Nonvesicular Lipid Transfer from the Endoplasmic Reticulum. Cold Spring. Harb. Perspect. Biol. 2012, 4, a013300. [Google Scholar] [CrossRef]
- Uemura, M.; Tominaga, Y.; Nakagawara, C.; Shigematsu, S.; Minami, A.; Kawamura, U.E.; Nakamura, S. Abscission of Azolla Branches Induced by Ethylene and Sodium Azide. Plant Cell Physiol. 2000, 41, 1365–1372. [Google Scholar]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic Acid: An Evolutionarily Conserved Signaling Molecule Modulates Plant Stress Signaling Networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef] [PubMed]
- Bostock, R.M.; Kuc, J.A.; Laine, R.A. Eicosapentaenoic and Arachidonic Acids from Phyfophfhora infesfans Elicit Fungitoxic Sesquiterpenes in the Potato. Science 1981, 212, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.I.; Wang, H.; Lincoln, J.-E.; Lulai, E.C.; Gilchrist, D.G.; Bostock, R.M. Hydroperoxides of Fatty Acids Induce Programmed Cell Death in Tomato Protoplasts. Physiol. Mol. Plant Pathol. 2001, 59, 277–286. [Google Scholar] [CrossRef]
- Garcia-Pineda, E.; Castro-Mercado, E.; Lozoya-Gloria, E. Gene Expression and Enzyme Activity of Pepper (Capsicum annuum L.) Ascorbate Oxidase during Elicitor and Wounding Stress. Plant Sci. 2004, 166, 237–243. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The Lipoxygenase Pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Merelo, P.; Agustí, J.; Arbona, V.; Costa, M.L.; Estornell, L.H.; Gómez-Cadenas, A.; Coimbra, S.; Gómez, M.D.; Pérez-Amador, M.A.; Domingo, C.; et al. Cell Wall Remodeling in Abscission Zone Cells during Ethylene-promoted Fruit Abscission in Citrus. Front. Plant Sci. 2017, 8, 126. [Google Scholar]
- Daher, F.B.; Braybrook, S.A. How to Let Go: Pectin and Plant Cell Adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef]
- Bowling, A.J.; Vaughn, K.C. Leaf Abscission in Impatiens (Balsaminaceae) is Due to Loss of Highly De-esterified Homogalacturonans in the Middle Lamellae. Am. J. Bot. 2011, 98, 619–629. [Google Scholar] [CrossRef]
- Iwai, H.; Terao, A.; Satoh, S. Changes in Distribution of Cell Wall Polysaccharides in Floral and Fruit Abscission Zones during Fruit Development in Tomato (Solanum lycopersicum). J. Plant Res. 2013, 126, 427–437. [Google Scholar] [CrossRef]
- Roongsattham, P.; Morcillo, F.; Fooyontphanich, K.; Jantasuriyarat, C.; Tragoonrung, S.; Amblard, P.; Collin, M.; Mouille, G.; Verdeil, J.L.; Tranbarger, T.J. Cellular and Pectin Dynamics during Abscission Zone Development and Ripe Fruit Abscission of the Monocot Oil Palm. Front. Plant Sci. 2016, 7, 540. [Google Scholar] [CrossRef]
- Frankowski, K.; Wilmowicz, E.; Kućko, A.; Zienkiewicz, A.; Zienkiewicz, K.; Kopcewicz, J. Profiling the BLADE-ON-PETIOLE Gene Expression in the Abscission Zone of Generative Organs in Lupinus luteus. Acta Physiol. Plant. 2015, 37, 220. [Google Scholar] [CrossRef]
- Tukaj, Z.; Pokora, W. Individual and Combined Effect of Anthracene, Cadmium, and Chloridazone on Growth and Activity of SOD Izoformes in Three Scenedesmus Species. Ecotoxicol. Environ. Saf. 2006, 65, 323–331. [Google Scholar]
- Kućko, A.; Wilmowicz, E.; Pokora, W.; Alché, J.D. Disruption of the Auxin Gradient in the Abscission Zone Area Evokes Asymmetrical Changes Leading to Flower Separation in Yellow Lupine. Int. J. Mol. Sci. 2020, 21, 3815. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 2, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assays Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Slazak, B.; Kapusta, M.; Malik, S.; Bohdanowicz, J.; Kuta, E.; Malec, P.; Göransson, U. Immunolocalization of Cyclotides in Plant Cells, Tissues and Organ Supports Their Role in Host Defense. Planta 2016, 244, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Siloto, R.M.; Findlay, K.; Lopez-Villalobos, A.; Yeung, E.C.; Nykiforuk, C.L.; Moloney, M.M. The Accumulation of Oleosins Determines the Size of Seed Oilbodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmowicz, E.; Kućko, A.; Pokora, W.; Kapusta, M.; Jasieniecka-Gazarkiewicz, K.; Tranbarger, T.J.; Wolska, M.; Panek, K. EPIP-Evoked Modifications of Redox, Lipid, and Pectin Homeostasis in the Abscission Zone of Lupine Flowers. Int. J. Mol. Sci. 2021, 22, 3001. https://doi.org/10.3390/ijms22063001
Wilmowicz E, Kućko A, Pokora W, Kapusta M, Jasieniecka-Gazarkiewicz K, Tranbarger TJ, Wolska M, Panek K. EPIP-Evoked Modifications of Redox, Lipid, and Pectin Homeostasis in the Abscission Zone of Lupine Flowers. International Journal of Molecular Sciences. 2021; 22(6):3001. https://doi.org/10.3390/ijms22063001
Chicago/Turabian StyleWilmowicz, Emilia, Agata Kućko, Wojciech Pokora, Małgorzata Kapusta, Katarzyna Jasieniecka-Gazarkiewicz, Timothy John Tranbarger, Magdalena Wolska, and Katarzyna Panek. 2021. "EPIP-Evoked Modifications of Redox, Lipid, and Pectin Homeostasis in the Abscission Zone of Lupine Flowers" International Journal of Molecular Sciences 22, no. 6: 3001. https://doi.org/10.3390/ijms22063001
APA StyleWilmowicz, E., Kućko, A., Pokora, W., Kapusta, M., Jasieniecka-Gazarkiewicz, K., Tranbarger, T. J., Wolska, M., & Panek, K. (2021). EPIP-Evoked Modifications of Redox, Lipid, and Pectin Homeostasis in the Abscission Zone of Lupine Flowers. International Journal of Molecular Sciences, 22(6), 3001. https://doi.org/10.3390/ijms22063001








