Loss of MT1-MMP in Alveolar Epithelial Cells Exacerbates Pulmonary Fibrosis
Abstract
1. Introduction
2. Results
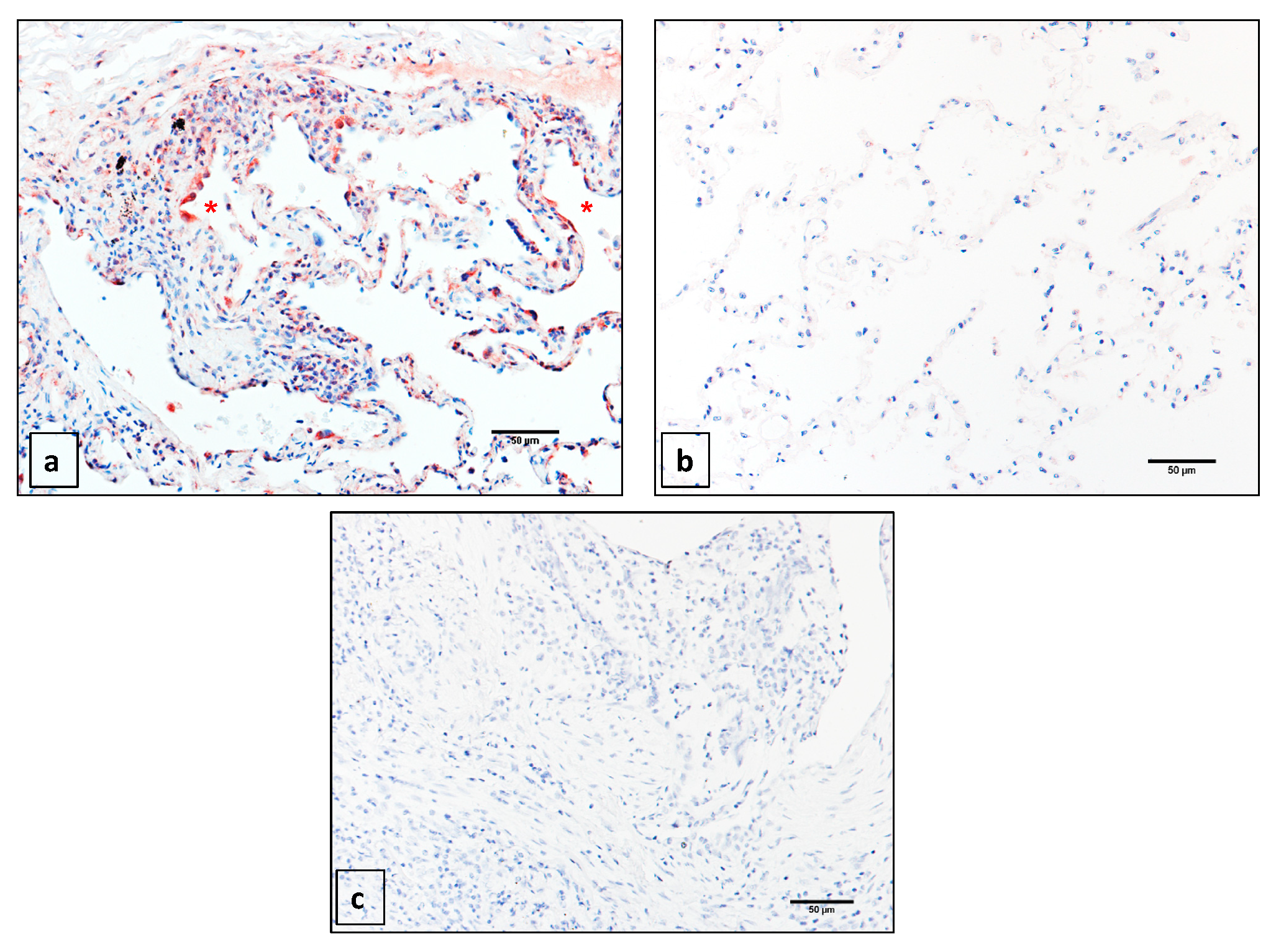
2.1. Matrix Metalloprotease 14 (MMP14) is Expressed Primarily in the Epithelium of Idiopathic Pulmonary Fibrosis (IPF) Patients
2.2. Generation of a Novel Conditional Mouse Model of Lung Epithelial MMP14-Specific Genetic Deletion
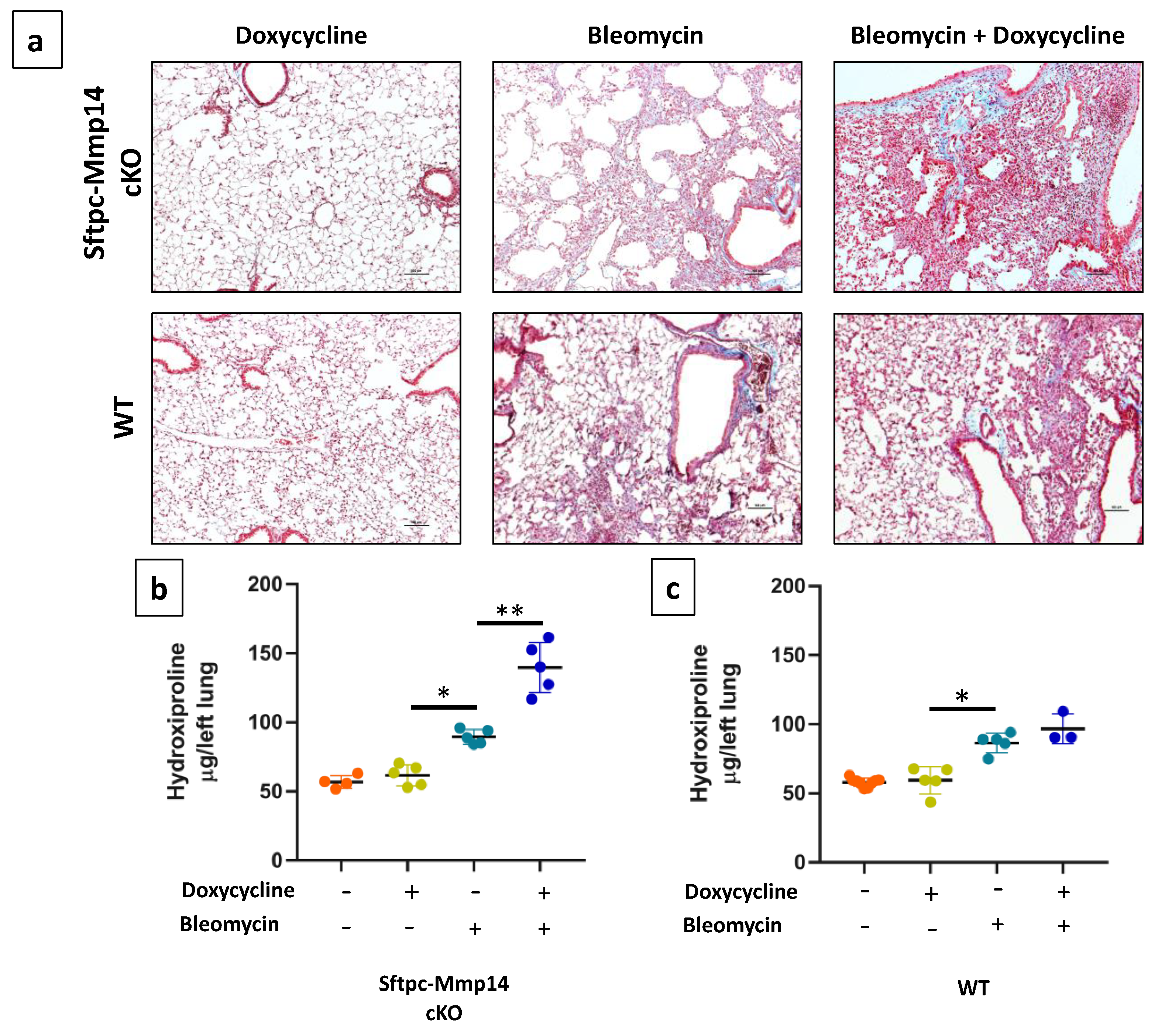
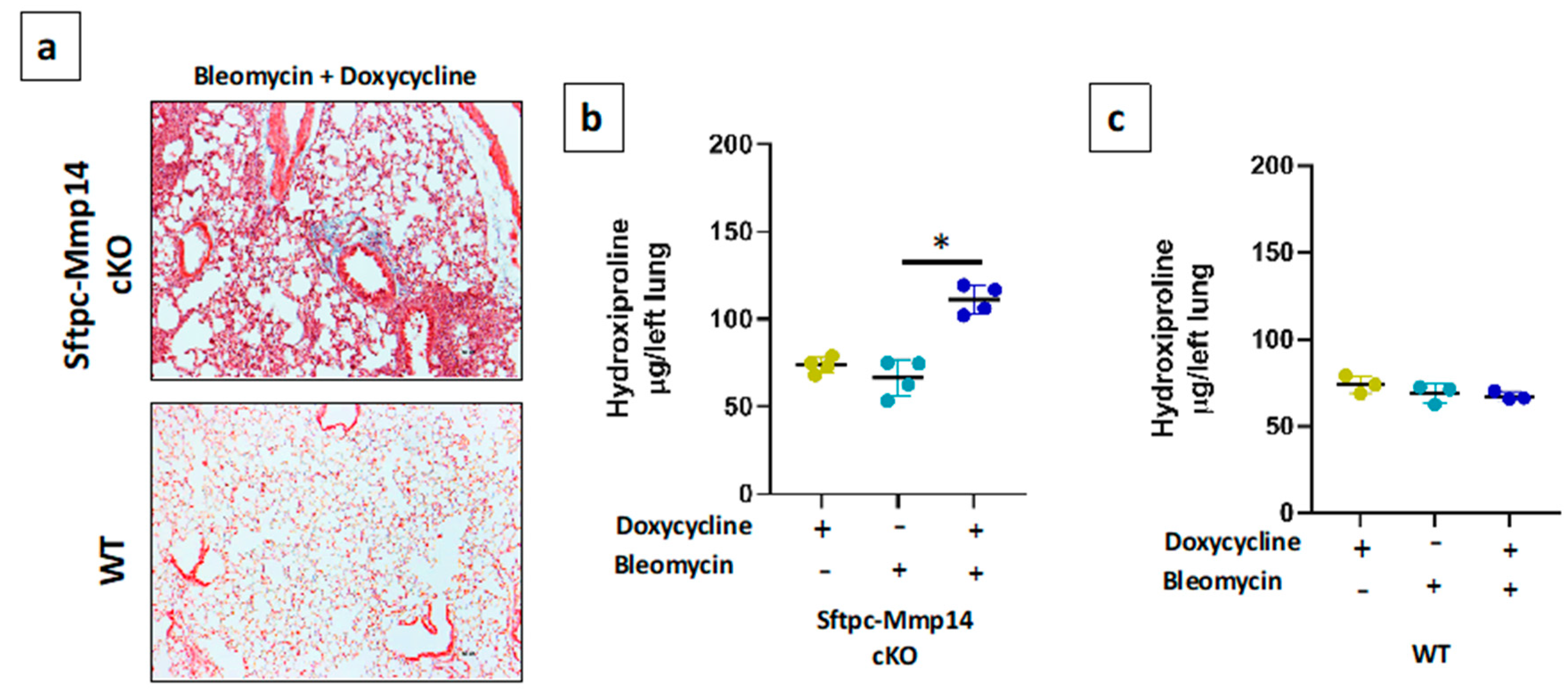
2.3. Epithelial Deletion of Mmp14 Exacerbates and Delays Resolution of Bleomycin-Induced Lung Fibrosis
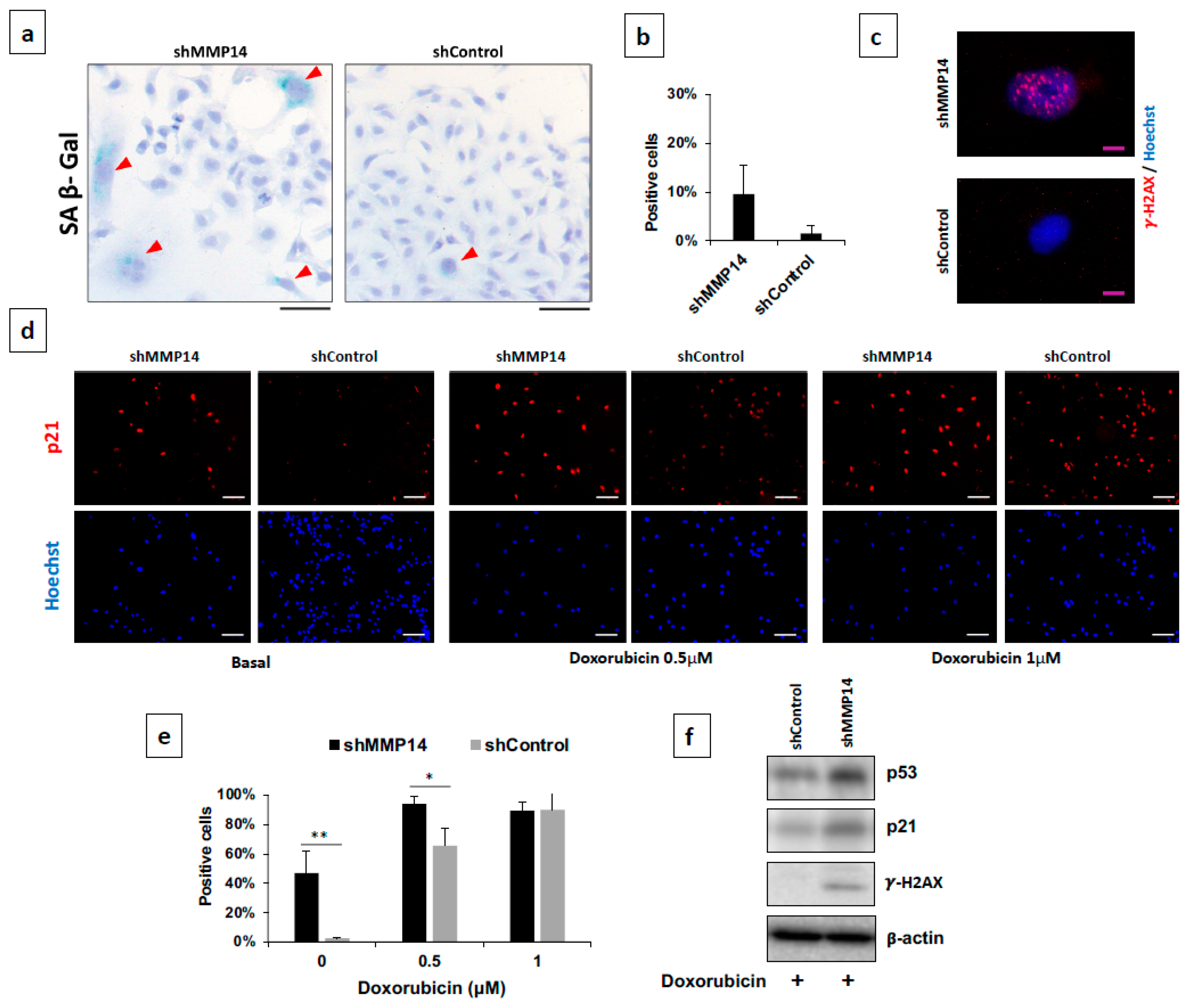
2.4. Epithelial MMP14 Deficiency Promotes Senescence In Vitro
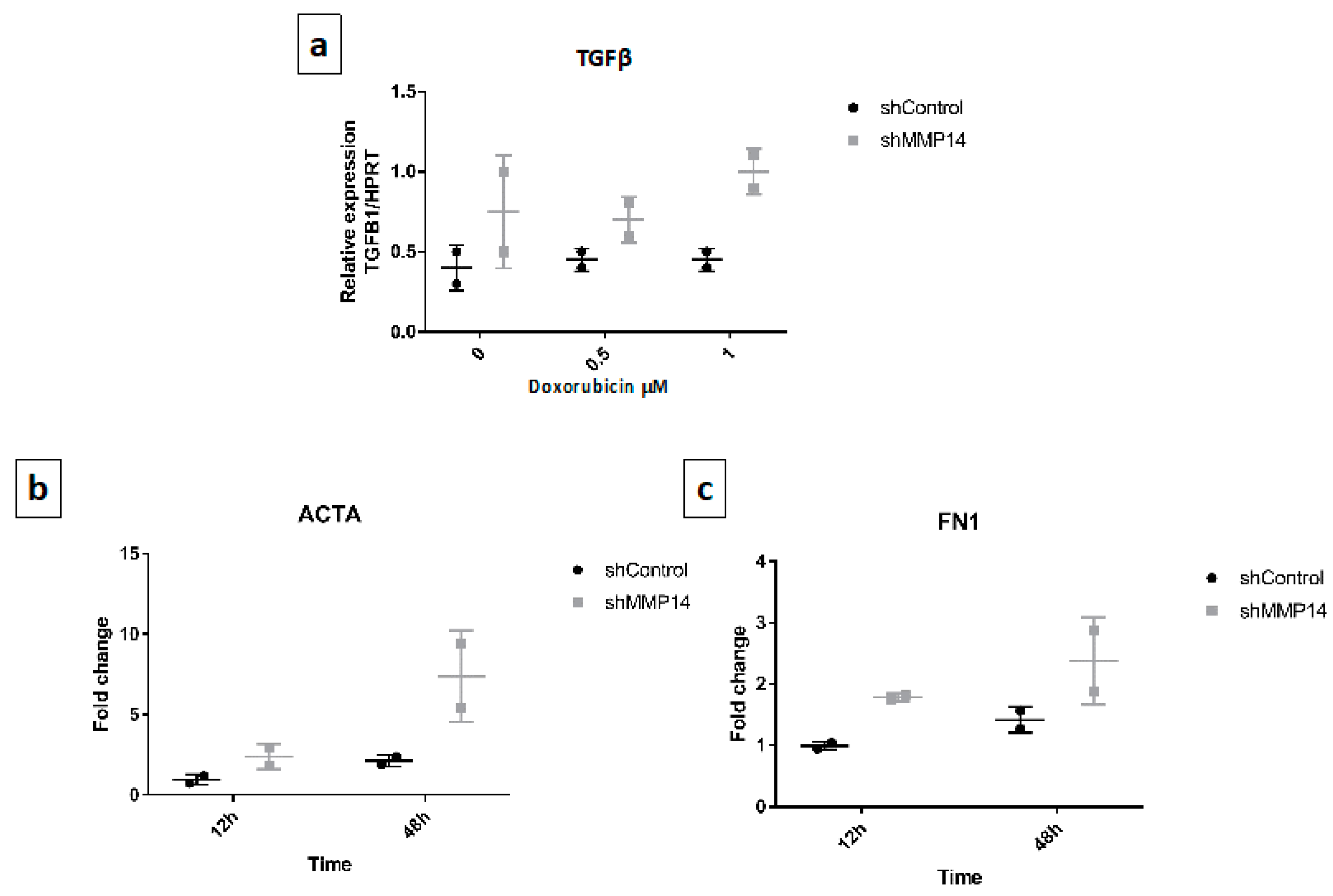
2.5. Senescent Epithelial Cells Overexpress Transforming Growth Factor-Beta (TGF-β) and Increase Profibrotic Markers in Fibroblasts
3. Discussion
4. Material and Methods
4.1. Human Samples
4.2. Immunohistochemistry (IHC)
4.3. Mice
4.4. Colorimetric Determination of Hydroxyproline
4.5. Epithelial Cells Isolation
4.6. Cell Culture
4.7. Cell Transfection
4.8. Western Blot
4.9. Growth Rate Assay
4.10. β-Galactosidase Assay
4.11. Real-Time Polymerase Chairn Reaction (PCR)
4.12. Immunofluorescence
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selman, M.; Te, K., Jr.; Pardo, A.; King, J.; Pardo, A. Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 2001, 134, 136–151. [Google Scholar] [CrossRef] [PubMed]
- King, T.E.; Pardo, A.; Selman, M.M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell. Signal. 2020, 66, 109482. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Kaminski, N.; Eugui, E.; Allard, J.; Yakhini, Z.; Ben-Dor, A. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc. Natl. Acad. Sci. USA 2002, 99. [Google Scholar] [CrossRef]
- Herrera, I.; Cisneros, J.; Maldonado, M.; Ramírez, R.; Ortiz-Quintero, B.; Anso, E. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. J. Biol. Chem. 2013, 288. [Google Scholar] [CrossRef]
- Yu, G.; Kovkarova-Naumovski, E.; Jara, P.; Parwani, A.; Kass, D.; Ruiz, V. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am. J. Respir. Crit. Care Med. 2012, 186. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Salgado-Aguayo, A.; Herrera, I.; Cabrera, S.; Ortíz-Quintero, B.; Staab-Weijnitz, C.A.; Eickelberg, O.; Ramírez, R.; Manicone, A.M.; Selman, M.; et al. Upregulation and nuclear location of MMP28 in alveolar epithelium of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 59, 77–86. [Google Scholar] [CrossRef]
- Yamashita, C.M.; Dolgonos, L.; Zemans, R.L.; Young, S.K.; Robertson, J.; Briones, N. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am. J. Pathol. 2011, 179. [Google Scholar] [CrossRef]
- García-Prieto, E.; González-López, A.; Cabrera, S.; Astudillo, A.; Gutiérrez-Fernández, A.; Fanjul-Fernandez, M. Resistance to bleomycin-induced lung fibrosis in MMP-8 deficient mice is mediated by interleukin-10. PLoS ONE 2010, 5, e13242. [Google Scholar] [CrossRef]
- Cabrera, S.; Maciel, M.; Hernández-Barrientos, D.; Calyeca, J.; Gaxiola, M.; Selman, M.; Pardo, A. Delayed resolution of bleomycin-induced pulmonary fibrosis in absence of MMP13 (collagenase 3). Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L961–L976. [Google Scholar] [CrossRef]
- Sato, H.; Takino, T.; Okada, Y.; Cao, J.; Shinagawa, A.; Yamamoto, E.; Seiki, M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370, 61–65. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Cabrera, S.; Selman, M.; Lonzano-Bolaños, A.; Konishi, K.; Richards, T.J.; Kaminski, N.; Pardo, A. Gene expression profiles reveal molecular mechanisms involved in the progression and resolution of bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, 593–601. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, J.; Ramirez, R.; Sampieri, C.L.; Nuttall, R.K.; Edwards, D.R.; Selman, M.; Pardo, A. Membrane type-matrix metalloproteinases in idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2006, 23, 13–21. [Google Scholar]
- Holmbeck, K.; Bianco, P.; Caterina, J.; Yamada, S.; Kromer, M.; Kuznetsov, S.A. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99. [Google Scholar] [CrossRef]
- Xu, Y.; Mizuno, T.; Sridharan, A.; Du, Y.; Guo, M.; Tang, J.; Wikenheiser-Brokamp, K.A.; Perl, A.-K.T.; Funari, V.A.; Gokey, J.J.; et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 2017, 1. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Königshoff, M.; Kramer, M.; Balsara, N.; Wilhelm, J.; Amarie, O.V.; Jahn, A.; Rose, F.; Fink, L.; Seeger, W.; Schaefer, L.; et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Investig. 2009, 119, 772–787. [Google Scholar] [CrossRef]
- Zigrino, P.; Brinckmann, J.; Niehoff, A.; Lu, Y.; Giebeler, N.; Eckes, B.; Kadler, K.E.; Mauch, C. Fibroblast-Derived MMP-14 Regulates Collagen Homeostasis in Adult Skin. J. Investig. Dermatol. 2016, 136, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Sabeh, F.; Ota, I.; Holmbeck, K.; Birkedal-Hansen, H.; Soloway, P.; Balbin, M.; Lopez-Otin, C.; Shapiro, S.; Inada, M.; Krane, S.; et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004, 167, 769–781. [Google Scholar] [CrossRef]
- Gutierrez-Fernandez, A.; Soria-Valles, C.; Osorio, F.G.; Gutierrez-Abril, J.; Garabaya, C.; Aguirre, A.; Fueyo, A.; Fernandez-Garcia, M.S.; Puente, X.S.; Lopez-Otin, C. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 2015, 34, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.L.; Rojas, M.; Pardo, A.; Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017, 16, 755–772. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Jee, H.J.; Um, J.-H.; Kim, Y.M.; Bae, S.S.; Yun, J. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell 2017, 16, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Ruiz, V.; Cabrera, S.; Segura, L.; Ramírez, R.; Barrios, R.; Pardo, A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am. J. Physiol. Cell. Mol. Physiol. 2000, 279, L562–L574. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Brands, J.; Voltz, L.; Fiedler, K.; Mays, B.; St Croix, C.; Sembrat, J.; Mallampalli, R.K.; Rojas, M.; Mora, A.L. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell 2018, 17, e12720. [Google Scholar] [CrossRef]
- Pardo, A.; Barrios, R.; Gaxiola, M.; Segura-Valdez, L.; Carrillo, G.; Estrada, A.; Mejía, M.; Selman, M. Increase of lung neutrophils and upregulation of neutrophil gelatinase B and collagenase in hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2000, 161, 1698–1704. [Google Scholar] [CrossRef]
- Cabrera, S.; Maciel, M.; Herrera, I.; Nava, T.; Vergara, F.; Gaxiola, M.; López-Otín, C.; Selman, M.; Pardo, A. Essential role for the ATG4B protease and autophagy in bleomycin-induced pulmonary fibrosis. Autophagy 2015, 11, 670–684. [Google Scholar] [CrossRef]
- Negreros, M.; Hagood, J.S.; Espinoza, C.R.; Balderas-Martínez, Y.I.; Selman, M.; Pardo, A. Transforming growth factor beta 1 induces methylation changes in lung fibroblasts. PLoS ONE 2019, 14, e0223512. [Google Scholar] [CrossRef] [PubMed]


| Gene | Direction | Sequence (5′ --> 3′) |
|---|---|---|
| COL1A1 | Forward | GAGGGCCAAGACGAAGACATC |
| Reverse | CAGATCACGTCATCGCACAAC | |
| FnEDA | Forward | CTGGTTCAGACTGCAGTAACC |
| Reverse | CAGGGAATAGCTCATGGATTCC | |
| ACTA2 | Forward | AAAAGACAGCTACGTGGGTGA |
| Reverse | GCCATGTTCTATCGGGTACTTC | |
| TGFB1 | Forward | GGCCAGATCCTGTCCAAGC |
| Reverse | GTGGGTTTCCACCATTAGCAC | |
| HPRT | Forward | AAGGACCCCACGAAGTGTTG |
| Reverse | GGCTTTGTATTTTGCTTTTCCA | |
| SDH | Forward | CAAACAGGAACCCGAGGTTTT |
| Reverse | CAGCTTGGTAACACATGCTGTAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Placido, L.; Romero, Y.; Maldonado, M.; Toscano-Marquez, F.; Ramírez, R.; Calyeca, J.; Mora, A.L.; Selman, M.; Pardo, A. Loss of MT1-MMP in Alveolar Epithelial Cells Exacerbates Pulmonary Fibrosis. Int. J. Mol. Sci. 2021, 22, 2923. https://doi.org/10.3390/ijms22062923
Placido L, Romero Y, Maldonado M, Toscano-Marquez F, Ramírez R, Calyeca J, Mora AL, Selman M, Pardo A. Loss of MT1-MMP in Alveolar Epithelial Cells Exacerbates Pulmonary Fibrosis. International Journal of Molecular Sciences. 2021; 22(6):2923. https://doi.org/10.3390/ijms22062923
Chicago/Turabian StylePlacido, Luis, Yair Romero, Mariel Maldonado, Fernanda Toscano-Marquez, Remedios Ramírez, Jazmín Calyeca, Ana L. Mora, Moisés Selman, and Annie Pardo. 2021. "Loss of MT1-MMP in Alveolar Epithelial Cells Exacerbates Pulmonary Fibrosis" International Journal of Molecular Sciences 22, no. 6: 2923. https://doi.org/10.3390/ijms22062923
APA StylePlacido, L., Romero, Y., Maldonado, M., Toscano-Marquez, F., Ramírez, R., Calyeca, J., Mora, A. L., Selman, M., & Pardo, A. (2021). Loss of MT1-MMP in Alveolar Epithelial Cells Exacerbates Pulmonary Fibrosis. International Journal of Molecular Sciences, 22(6), 2923. https://doi.org/10.3390/ijms22062923








