Molecular Mechanisms and Tumor Biological Aspects of 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Establishment of the 5-Fluorouracil-Resistant HCT116 Cells
2.2. Anticancer Drug Response of the 5-FU-Resistant HCT116 Cells
2.3. Biological Features of the 5-FU-Resistant HCT116 Cells
2.4. Exome Sequencing Analysis of HCT116 Parent Cells and 5-FU-Resistant HCT116RF10 Cells
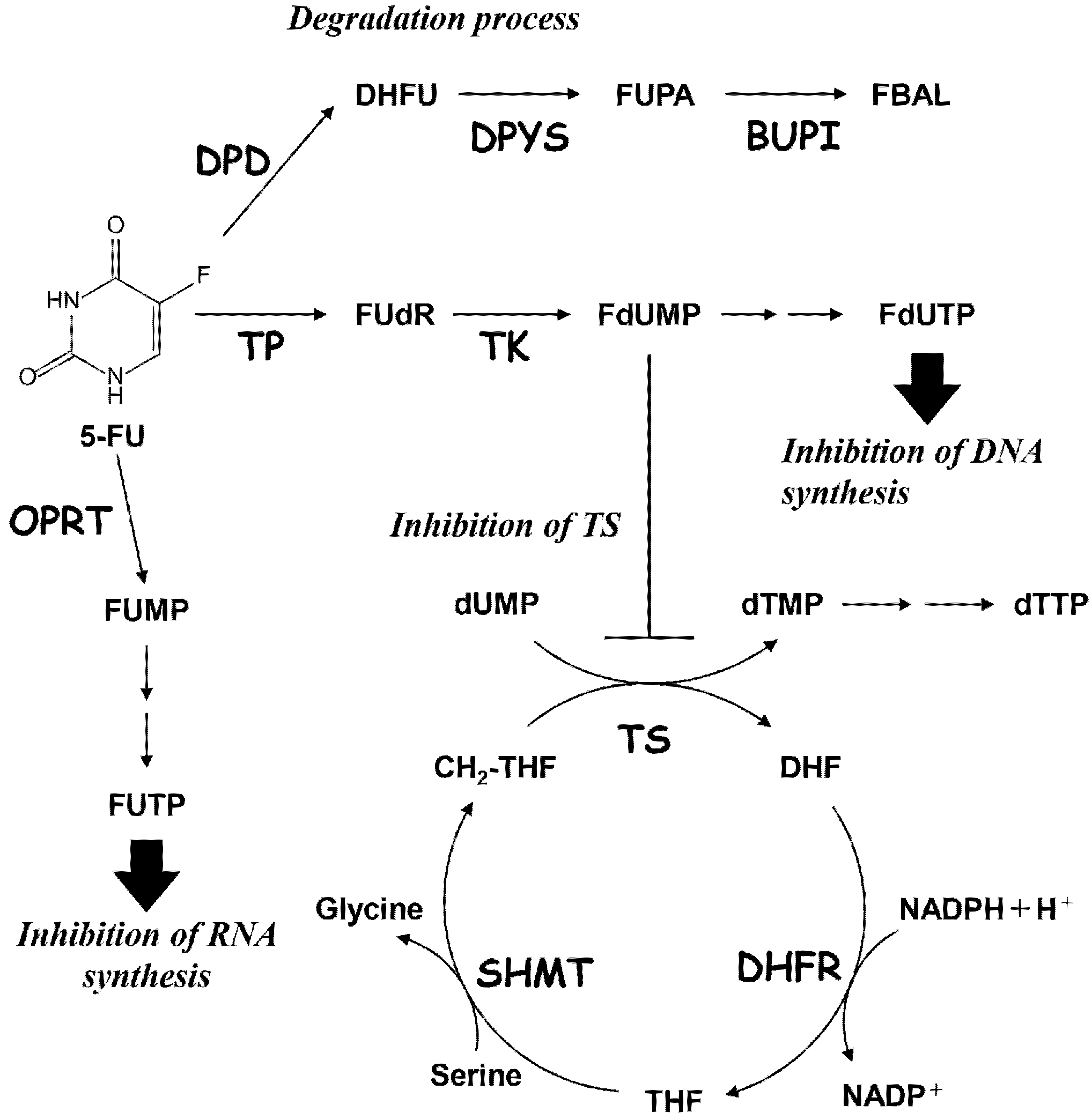
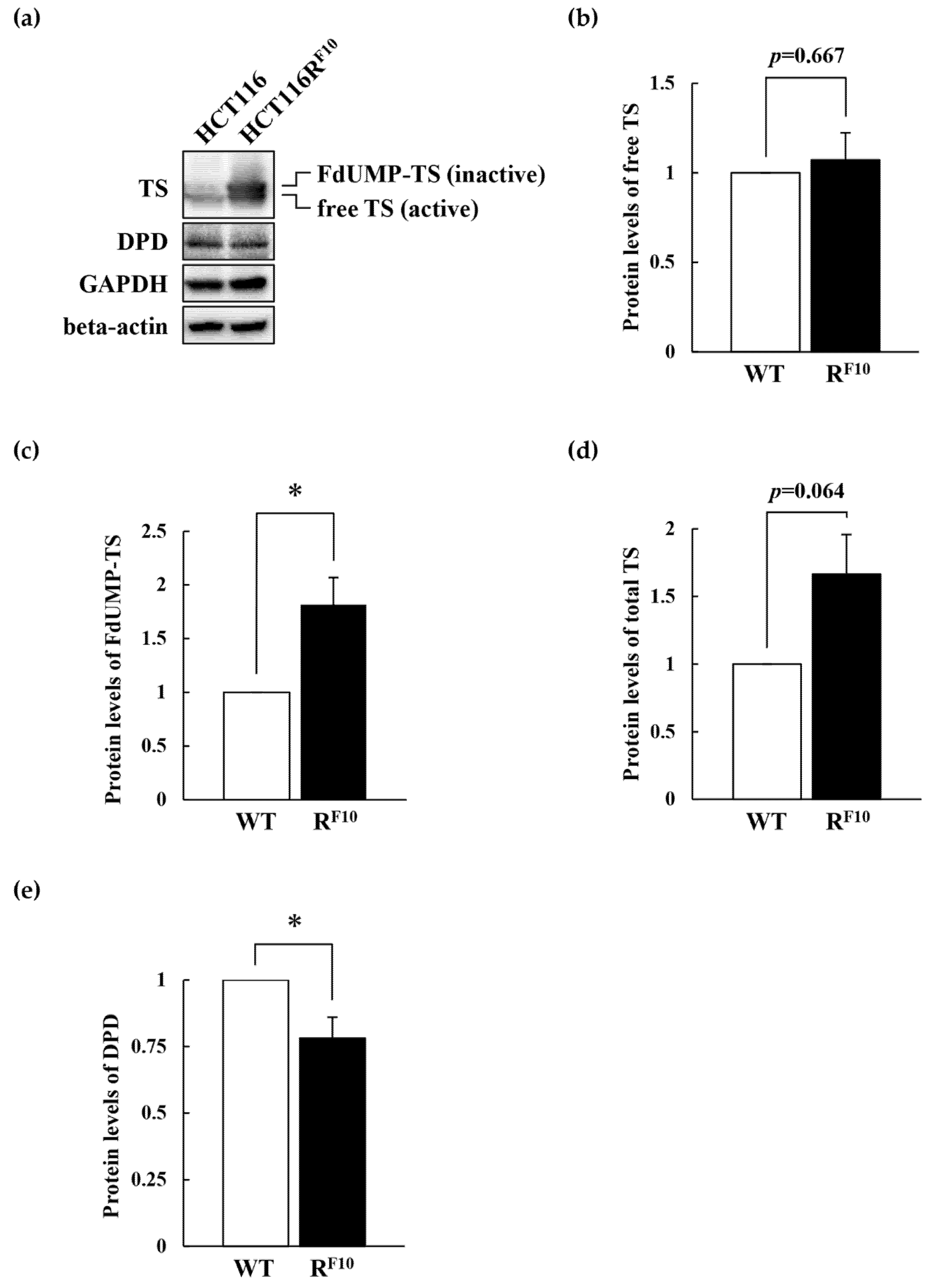
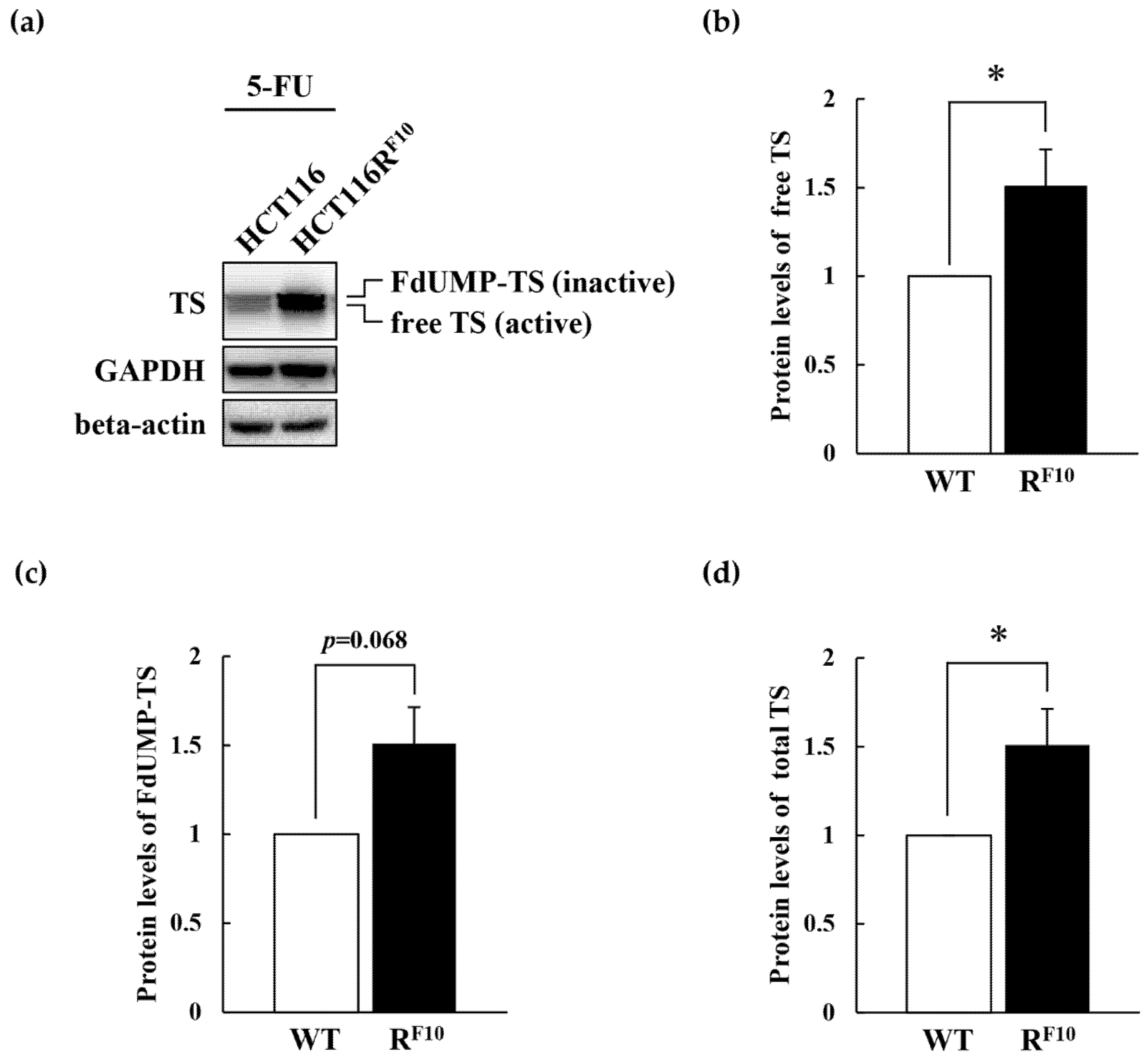
2.5. Regulation of TS and DPD in HCT116 Parent Cell and 5-FU-Resistant HCT116RF10 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Generation of the 5-FU-Resistant HCT116 Cell Line
4.4. Cell Viability by WST-8 Assay
4.5. Colony Formation Assay
4.6. Tumor Sphere Assay
4.7. Exome Sequencing Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Heidelberger, C. Fluorinated pyrimidines. Prog. Nucleic Acid. Res. Mol. Biol. 1965, 4, 1–50. [Google Scholar]
- Santi, D.V. Perspective on the design and biochemical pharmacology of inhibitors of thymidylate synthetase. J. Med. Chem. 1980, 23, 103–111. [Google Scholar] [CrossRef]
- Copur, S.; Aiba, K.; Drake, J.C.; Allegra, C.J.; Chu, E. Thymidylate synthase gene amplification in human colon cancer cell lines resistant to 5-fluorouracil. Biochem. Pharmacol. 1995, 49, 1419–1426. [Google Scholar] [CrossRef]
- Johnston, P.G.; Lenz, H.J.; Leichman, C.G.; Danenberg, K.D.; Allegra, C.J.; Danenberg, P.V.; Leichman, L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995, 55, 1407–1412. [Google Scholar]
- Wang, W.; Marsh, S.; Cassidy, J.; McLeod, H.L. Pharmacogenomic dissection of resistance to thymidylate synthase inhibitors. Cancer Res. 2001, 61, 5505–5510. [Google Scholar]
- Popat, S.; Matakidou, A.; Houlston, R.S. Thymidylate synthase expression and prognosis in colorectal cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2004, 22, 529–536. [Google Scholar] [CrossRef]
- Ishibiki, Y.; Kitajima, M.; Sakamoto, K.; Tomiki, Y.; Sakamoto, S.; Kamano, T. Intratumoural thymidylate synthase and dihydropyrimidine dehydrogenase activities are good predictors of 5-fluorouracil sensitivity in colorectal cancer. J. Int. Med. Res. 2003, 31, 181–187. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.; Koeller, D.M.; Johnston, P.G.; Zinn, S.; Allegra, C.J. Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon-gamma. Mol. Pharmacol. 1993, 43, 527–533. [Google Scholar] [PubMed]
- Drake, J.C.; Allegra, C.J.; Johnston, P.G. Immunological quantitation of thymidylate synthase-FdUMP-5,10-methylenetetrahydrofolate ternary complex with the monoclonal antibody TS 106. Anticancer Drugs 1993, 4, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Futamura, M.; Tanahashi, T.; Tanaka, Y.; Matsuhashi, N.; Yamaguchi, K.; Yoshida, K. 5FU resistance caused by reduced fluoro-deoxyuridine monophosphate and its reversal using deoxyuridine. Oncol. Lett. 2017, 14, 3162–3168. [Google Scholar] [CrossRef]
- Boyer, J.; McLean, E.G.; Aroori, S.; Wilson, P.; McCulla, A.; Carey, P.D.; Longley, D.B.; Johnston, P.G. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin. Cancer Res. 2004, 10, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Laskin, J.D.; Evans, R.M.; Slocum, H.K.; Burke, D.; Hakala, M.T. Basis for natural variation in sensitivity to 5-fluorouracil in mouse and human cells in culture. Cancer Res. 1979, 39, 383–390. [Google Scholar]
- Yoshioka, A.; Tanaka, S.; Hiraoka, O.; Koyama, Y.; Hirota, Y.; Ayusawa, D.; Seno, T.; Garrett, C.; Wataya, Y. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J. Biol. Chem. 1987, 262, 8235–8241. [Google Scholar] [CrossRef]
- Yamada, M.; Nakagawa, H.; Fukushima, M.; Shimizu, K.; Hayakawa, T.; Ikenaka, K. In vitro study on intrathecal use of 5-fluoro-2′-deoxyuridine (FdUrd) for meningeal dissemination of malignant brain tumors. J. Neurooncol. 1998, 37, 115–121. [Google Scholar] [CrossRef]
- Peters, G.J.; Backus, H.H.; Freemantle, S.; van Triest, B.; Codacci-Pisanelli, G.; van der Wilt, C.L.; Smid, K.; Lunec, J.; Calvert, A.H.; Marsh, S.; et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim. Biophys. Acta 2002, 1587, 194–205. [Google Scholar] [CrossRef]
- Chu, E.; Koeller, D.M.; Casey, J.L.; Drake, J.C.; Chabner, B.A.; Elwood, P.C.; Zinn, S.; Allegra, C.J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc. Natl. Acad. Sci. USA 1991, 88, 8977–8981. [Google Scholar] [CrossRef]
- Chu, E.; Voeller, D.; Koeller, D.M.; Drake, J.C.; Takimoto, C.H.; Maley, G.F.; Maley, F.; Allegra, C.J. Identification of an RNA binding site for human thymidylate synthase. Proc. Natl. Acad. Sci. USA 1993, 90, 517–521. [Google Scholar] [CrossRef]
- Keyomarsi, K.; Samet, J.; Molnar, G.; Pardee, A.B. The thymidylate synthase inhibitor, ICI D1694, overcomes translational detainment of the enzyme. J. Biol. Chem. 1993, 268, 15142–15149. [Google Scholar] [CrossRef]
- Chu, E.; Allegra, C.J. The role of thymidylate synthase as an RNA binding protein. Bioessays 1996, 18, 191–198. [Google Scholar] [CrossRef]
- Kitchens, M.E.; Forsthoefel, A.M.; Barbour, K.W.; Spencer, H.T.; Berger, F.G. Mechanisms of acquired resistance to thymidylate synthase inhibitors: The role of enzyme stability. Mol. Pharmacol. 1999, 56, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, M.E.; Forsthoefel, A.M.; Rafique, Z.; Spencer, H.T.; Berger, F.G. Ligand-mediated induction of thymidylate synthase occurs by enzyme stabilization. Implications for autoregulation of translation. J. Biol. Chem. 1999, 274, 12544–12547. [Google Scholar] [CrossRef]
- Marverti, G.; Ligabue, A.; Paglietti, G.; Corona, P.; Piras, S.; Vitale, G.; Guerrieri, D.; Luciani, R.; Costi, M.P.; Frassineti, C.; et al. Collateral sensitivity to novel thymidylate synthase inhibitors correlates with folate cycle enzymes impairment in cisplatin-resistant human ovarian cancer cells. Eur. J. Pharmacol. 2009, 615, 17–26. [Google Scholar] [CrossRef]
- Washtien, W.L. Increased levels of thymidylate synthetase in cells exposed to 5-fluorouracil. Mol. Pharmacol. 1984, 25, 171–177. [Google Scholar] [PubMed]
- Mohsen, A.W.; Aull, J.L.; Payne, D.M.; Daron, H.H. Ligand-induced conformational changes of thymidylate synthase detected by limited proteolysis. Biochemistry 1995, 34, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Sato, A.; Uchiumi, F.; Tanuma, S.I. Cross resistance to diverse anticancer nicotinamide phosphoribosyltransferase inhibitors induced by FK866 treatment. Oncotarget 2018, 9, 16451–16461. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Sato, A.; Uchiumi, F.; Tanuma, S.I. Genomic and tumor biological aspects of the anticancer nicotinamide phosphoribosyltransferase inhibitor FK866 in resistant human colorectal cancer cells. Genomics 2019, 111, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Shindo, M.; Kobayashi, K.; Sato, A.; Yamamoto, Y.; Akasaki, Y.; Ichimura, K.; Tanuma, S.I. Anticancer effects of a non-narcotic opium alkaloid medicine, papaverine, in human glioblastoma cells. PLoS ONE 2019, 14, e0216358. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Sato, A.; Kawano, Y.; Aoyama, T.; Uchiumi, F.; Tanuma, S.I. Association of ABC Transporter With Resistance to FK866, a NAMPT Inhibitor, in Human Colorectal Cancer Cells. Anticancer Res. 2019, 39, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Satake, A.; Hiramoto, A.; Wataya, Y.; Kim, H.S. Protein expression profiles of necrosis and apoptosis induced by 5-fluoro-2′-deoxyuridine in mouse cancer cells. J. Proteome Res. 2010, 9, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Nakama, K.; Watanabe, H.; Satake, A.; Yamamoto, A.; Omi, T.; Hiramoto, A.; Masutani, M.; Wataya, Y.; Kim, H.S. Role of activating transcription factor 3 protein ATF3 in necrosis and apoptosis induced by 5-fluoro-2′-deoxyuridine. FEBS J. 2014, 281, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
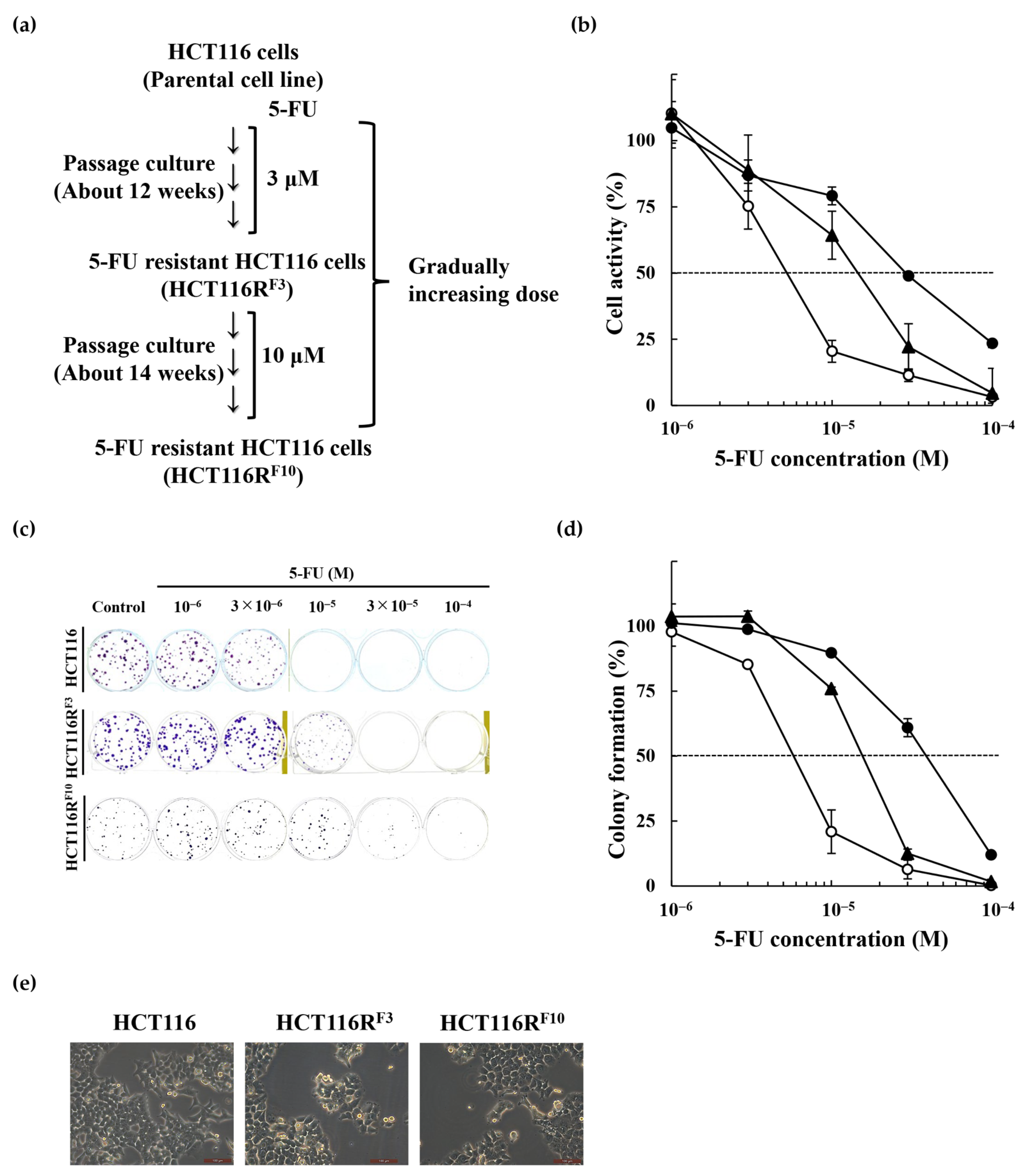
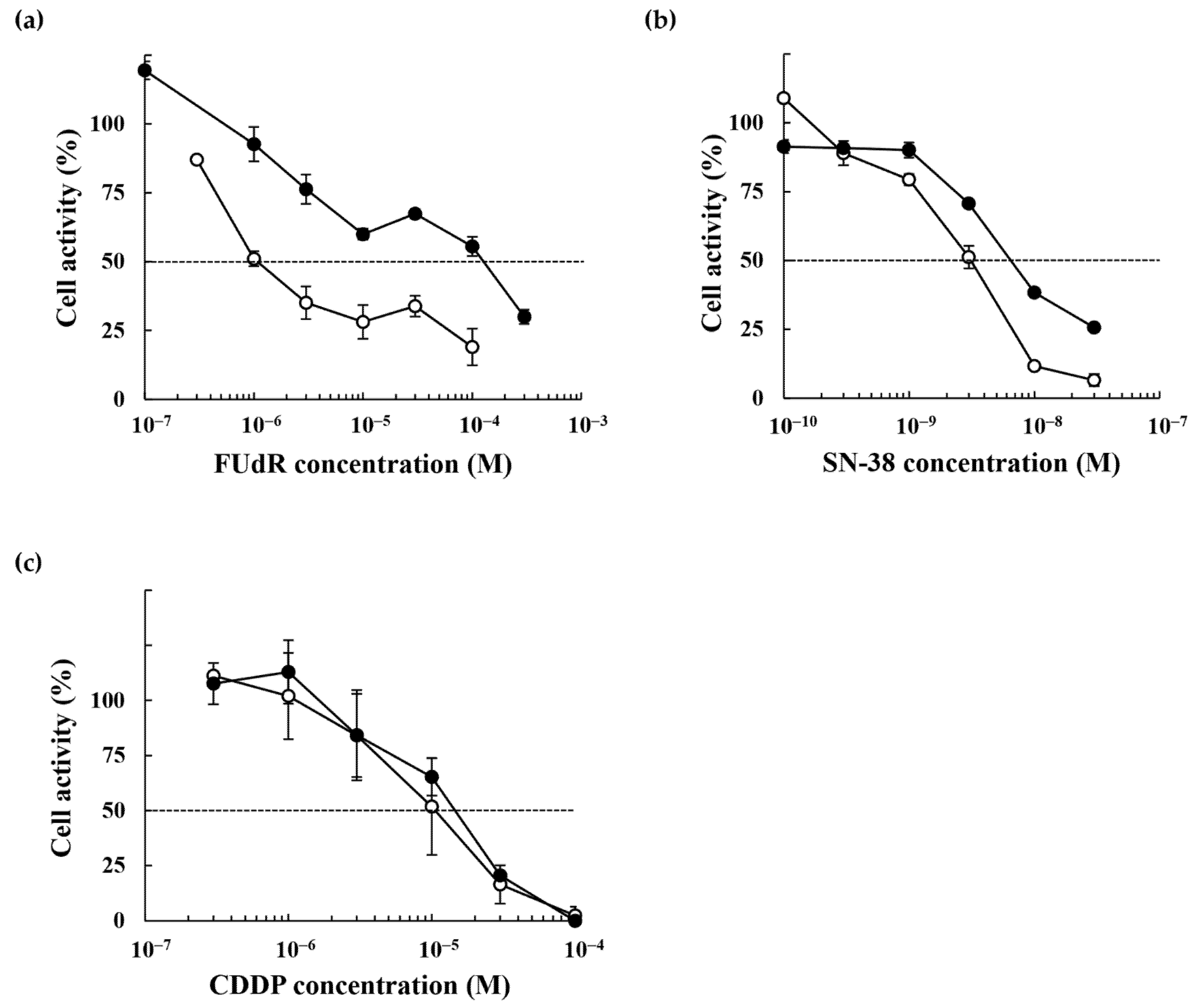
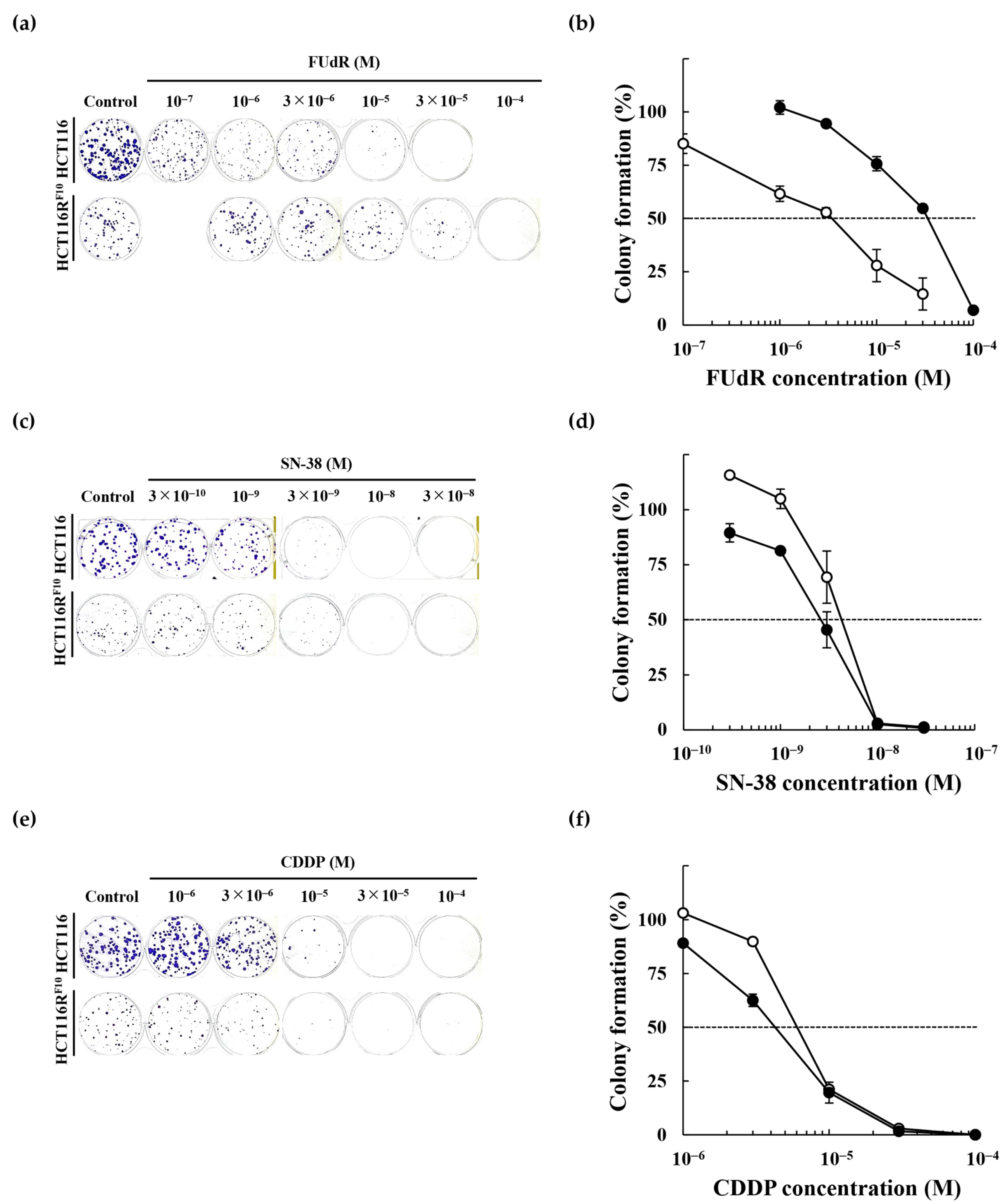
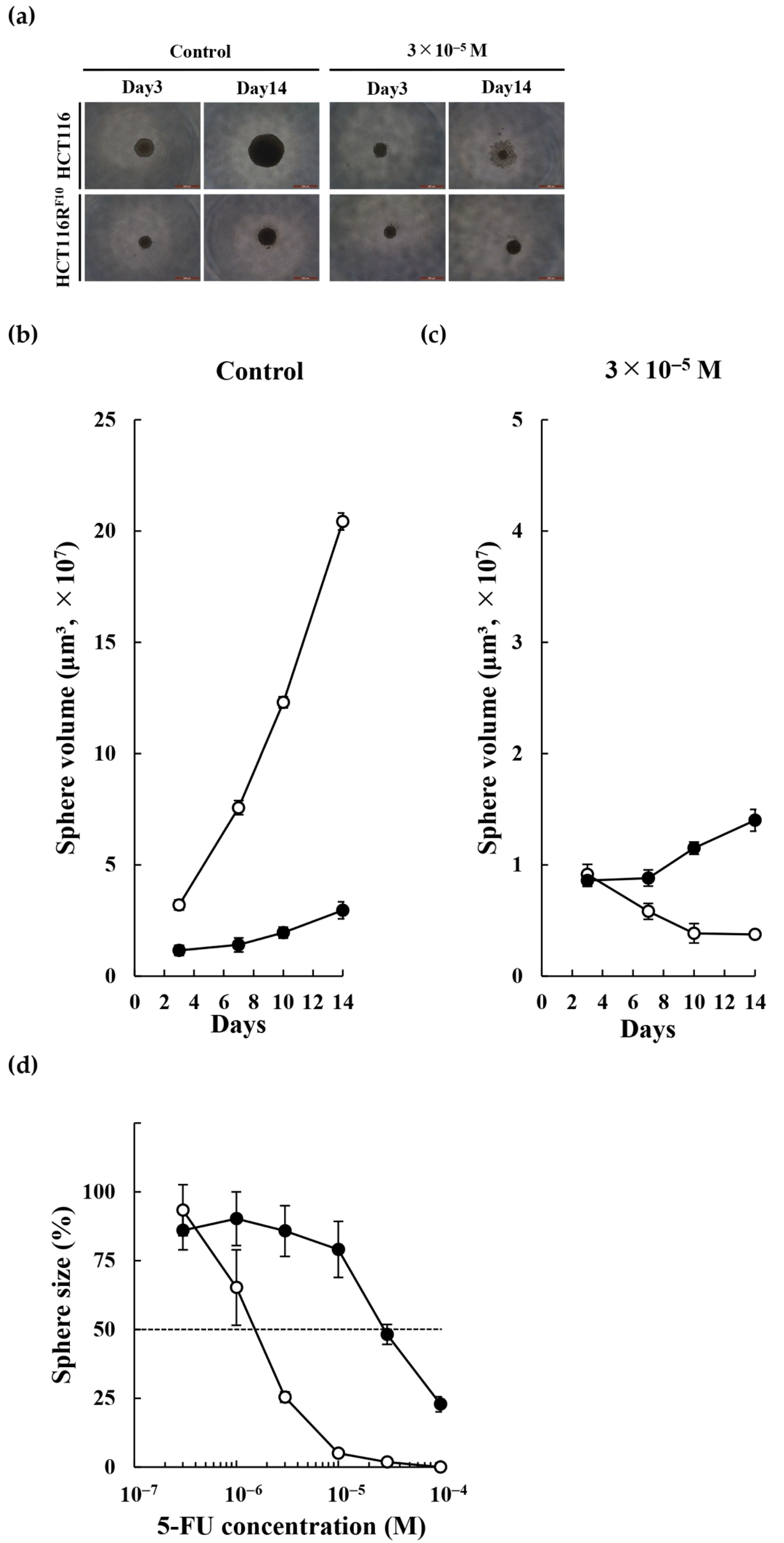
| Cell line | EC50 (M) | RI | ||
|---|---|---|---|---|
| WST-8 Assay | CFA | WST-8 Assay | CFA | |
| HCT116 | 5.1 × 10−6 | 5.5 × 10−6 | 1 | 1 |
| HCT116RF3 | 1.5 × 10−5 | 1.6 × 10−5 | 2.9 | 2.9 |
| HCT116RF10 | 2.9 × 10−5 | 3.8 × 10−5 | 5.7 | 6.9 |
| EC50 (M, WST-8) | EC50 (M, CFA) | |||||
|---|---|---|---|---|---|---|
| HCT116 | HCT116RF10 | RI | HCT116 | HCT116RF10 | RI | |
| FUdR | 1.5 × 10−6 | 1.2 × 10−4 | 80.0 | 3.4 × 10−6 | 3.3 × 10−5 | 9.7 |
| SN-38 | 3.1 × 10−9 | 6.6 × 10−9 | 2.1 | 4.2 × 10−9 | 3.0 × 10−9 | 0.7 |
| CDDP | 1.0 × 10−5 | 1.4 × 10−5 | 1.4 | 5.2 × 10−6 | 4.5 × 10−6 | 0.9 |
| Gene Symbol | HCT116 | HCT116RF10 |
|---|---|---|
| DPYD | wt mt(c.2908-58G>C)het mt(.2907+55C>T)hom wt wt mt(c.2442+77_2442+78delAA)het mt(c.2059-94G>T)het mt(c.1740+40A>G)hom mt(c.1740+39C>T)het mt(c.1627A>G)het mt(c.234-123G>C)het mt(c.40-461delT)het mt(c.-113T>C)het | mt(c.2999A>T)het mt(c.2908-58G>C)het mt(.2907+55C>T)hom mt(c.2623-59T>G)het mt(c.2442+78delA)het wt mt(c.2059-94G>T)het mt(c.1740+40A>G)hom mt(c.1740+39C>T)het mt(c.1627A>G)het mt(c.234-123G>C)het wt wt |
| DPYS | mt(c.1444-145C>T)hom mt(c.951-113T>C)hom mt(c.424-62G>T)hom mt(c.265-58T>C)hom mt(c.216C>T)hom mt(c.-1T>C)hom | mt(c.1444-145C>T)hom mt(c.951-113T>C)hom mt(c.424-62G>T)hom mt(c.265-58T>C)hom mt(c.216C>T)hom mt(c.-1T>C)hom |
| BUPI | n.d. | n.d. |
| TP | n.d. | n.d. |
| TK1 | mt(c.393+168C>T)het mt(c.393+1G>A)het mt(c.225A>G)het mt(c.98+97_98+101delCCCCT)het mt(c.33T>C)het | wt wt mt(c.225A>G)het mt(c.98+97_98+101delCCCCT)het mt(c.33T>C)het |
| TK2 | mt(c.619-53A>G)het mt(c.619-63C>G)het mt(c.156+836G>A)het mt(c.156+742G>A)het mt(c.125-116G>A)het mt(c.-30C>G)het mt(c.-38A>G)het | mt(c.619-53A>G)het mt(c.619-63C>G)het mt(c.156+836G>A)het mt(c.156+742G>A)het mt(c.125-116G>A)het mt(c.-30C>G)het mt(c.-38A>G)het |
| TYMS | mt(c.97T>C)het mt(c.280-43G>A)hom mt(c.454+50T>C)hom wt wt mt(c.454+200_454+202delTTT)hom mt(c.556+123_556+126delATTG)hom mt(c.*19C>T)hom mt(c.*89A>G)het | mt(c.97T>C)het mt(c.280-43G>A)hom mt(c.454+50T>C)hom mt(c.454+197_454+202delTTTTTT)het mt(c.454+199_454+202delTTTT)het wt mt(c.556+123_556+126delATTG)hom mt(c.*19C>T)hom mt(c.*89A>G)het |
| DHFR1 | wt wt mt(c.-204T>C)het | mt(c.137-25T>G)het mt(c.137-43T>C)het mt(c.-204T>C)het |
| DHFR2 | mt(c.247C>G)hom | mt(c.247C>G)hom |
| SHMT1 | mt(c.*66C>T)het mt(c.*47C>G)het mt(c.1420C>T)het mt(c.1171+59A>G)het mt(c.1054+141C>T)het mt(c.815-23C>T)het mt(c.601+174C>T)het mt(c.601+173G>A)het mt(c.243-256A>G)het mt(c.-19-101T>C)hom | mt(c.*66C>T)het mt(c.*47C>G)het mt(c.1420C>T)het mt(c.1171+59A>G)het mt(c.1054+141C>T)het mt(c.815-23C>T)het mt(c.601+174C>T)het mt(c.601+173G>A)het mt(c.243-256A>G)het mt(c.-19-101T>C)hom |
| SHMT2 | mt(c.595-6G>A)het mt(c.717+14dupG)het mt(c.1279+30G>A)het | mt(c.595-6G>A)het mt(c.717+14dupG)het mt(c.1279+30G>A)het |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurasaka, C.; Ogino, Y.; Sato, A. Molecular Mechanisms and Tumor Biological Aspects of 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 2916. https://doi.org/10.3390/ijms22062916
Kurasaka C, Ogino Y, Sato A. Molecular Mechanisms and Tumor Biological Aspects of 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells. International Journal of Molecular Sciences. 2021; 22(6):2916. https://doi.org/10.3390/ijms22062916
Chicago/Turabian StyleKurasaka, Chinatsu, Yoko Ogino, and Akira Sato. 2021. "Molecular Mechanisms and Tumor Biological Aspects of 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells" International Journal of Molecular Sciences 22, no. 6: 2916. https://doi.org/10.3390/ijms22062916
APA StyleKurasaka, C., Ogino, Y., & Sato, A. (2021). Molecular Mechanisms and Tumor Biological Aspects of 5-Fluorouracil Resistance in HCT116 Human Colorectal Cancer Cells. International Journal of Molecular Sciences, 22(6), 2916. https://doi.org/10.3390/ijms22062916






