HDAC6-Selective Inhibitor Overcomes Bortezomib Resistance in Multiple Myeloma
Abstract
1. Introduction
2. Results
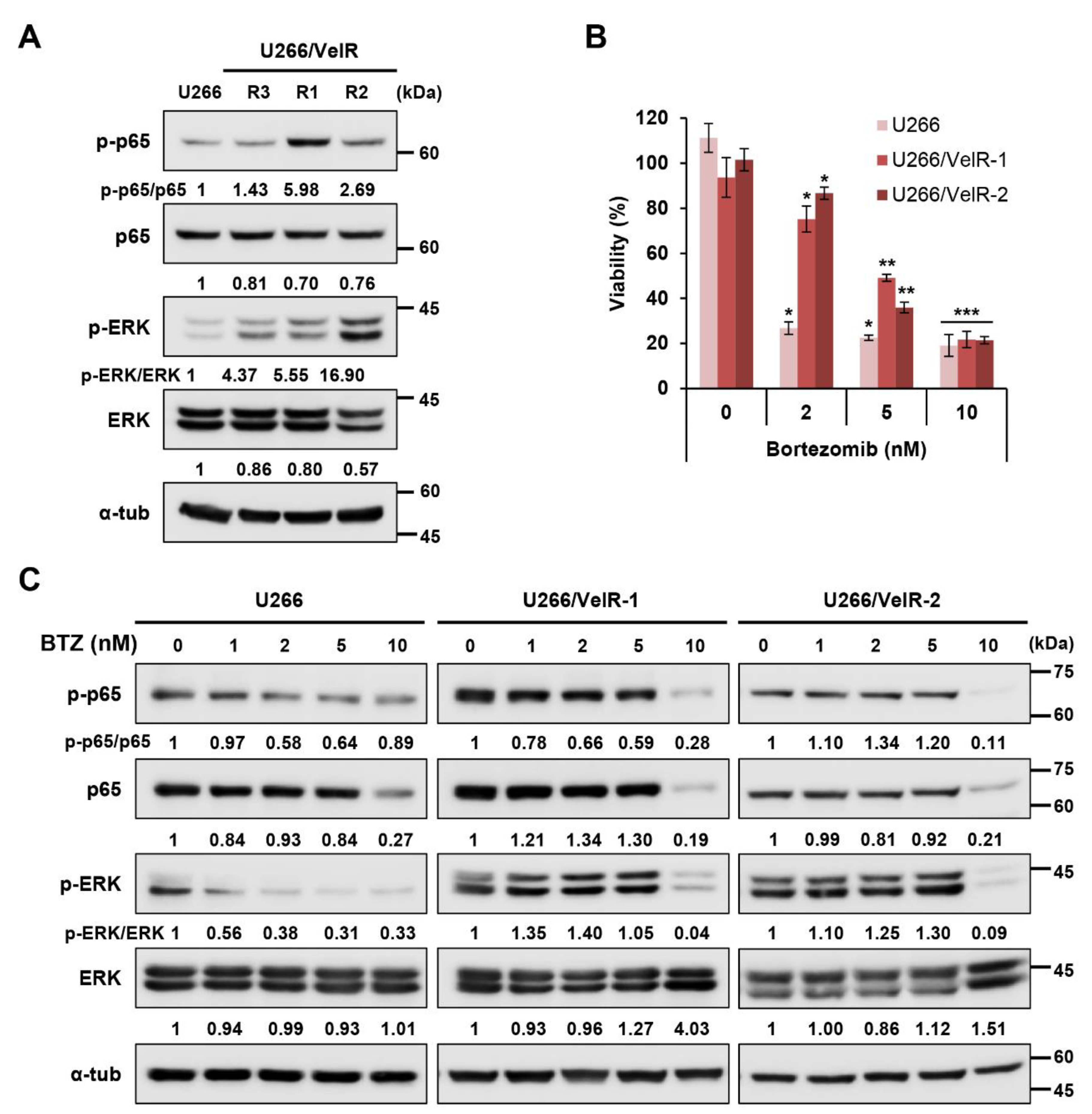
2.1. Establishment of BTZ-Resistant U266/VelR MM Cells
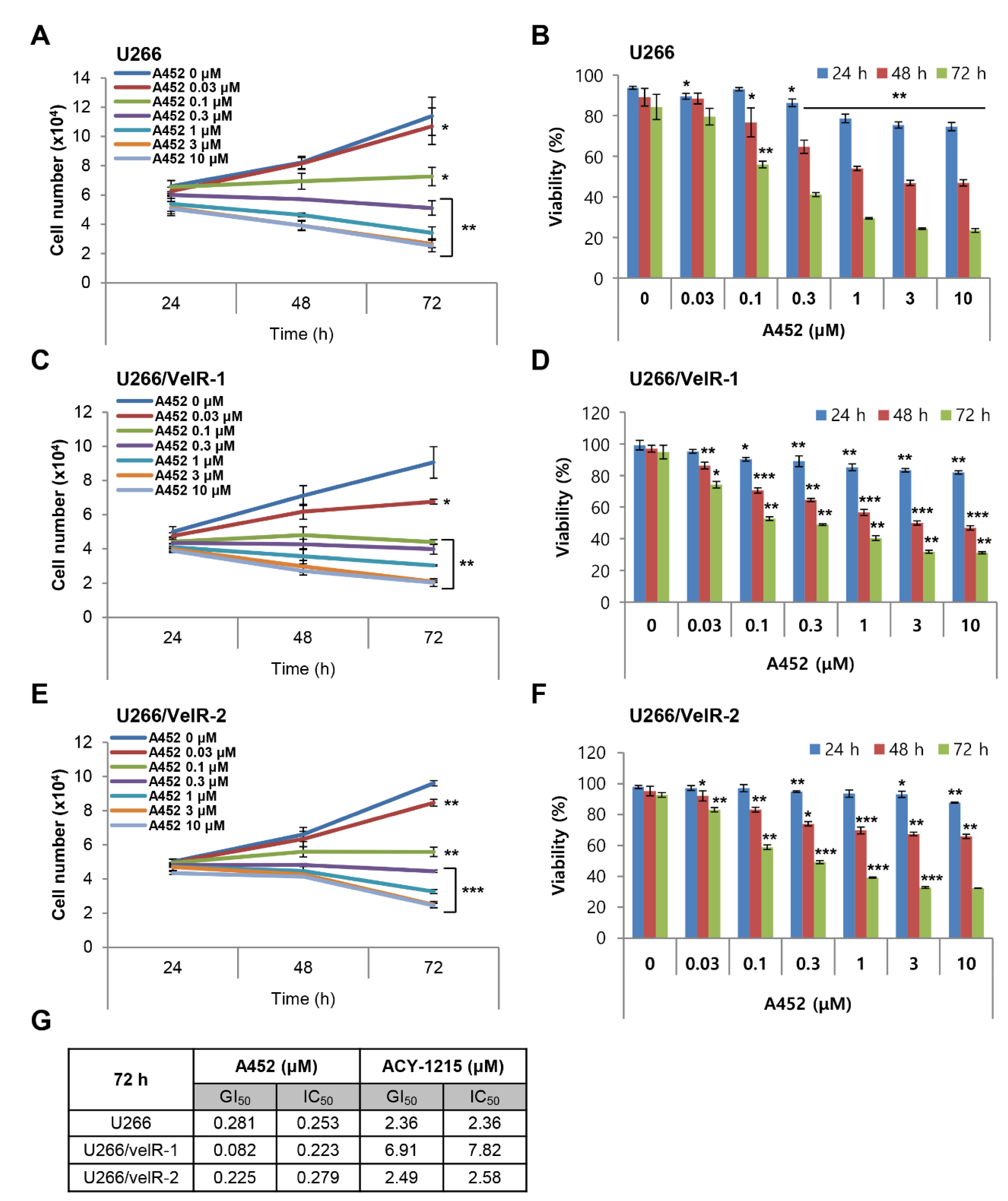
2.2. A452 is More Cytotoxic than ACY-1215 in Both BTZ-Sensitive and BTZ-Resistant U266 Cells
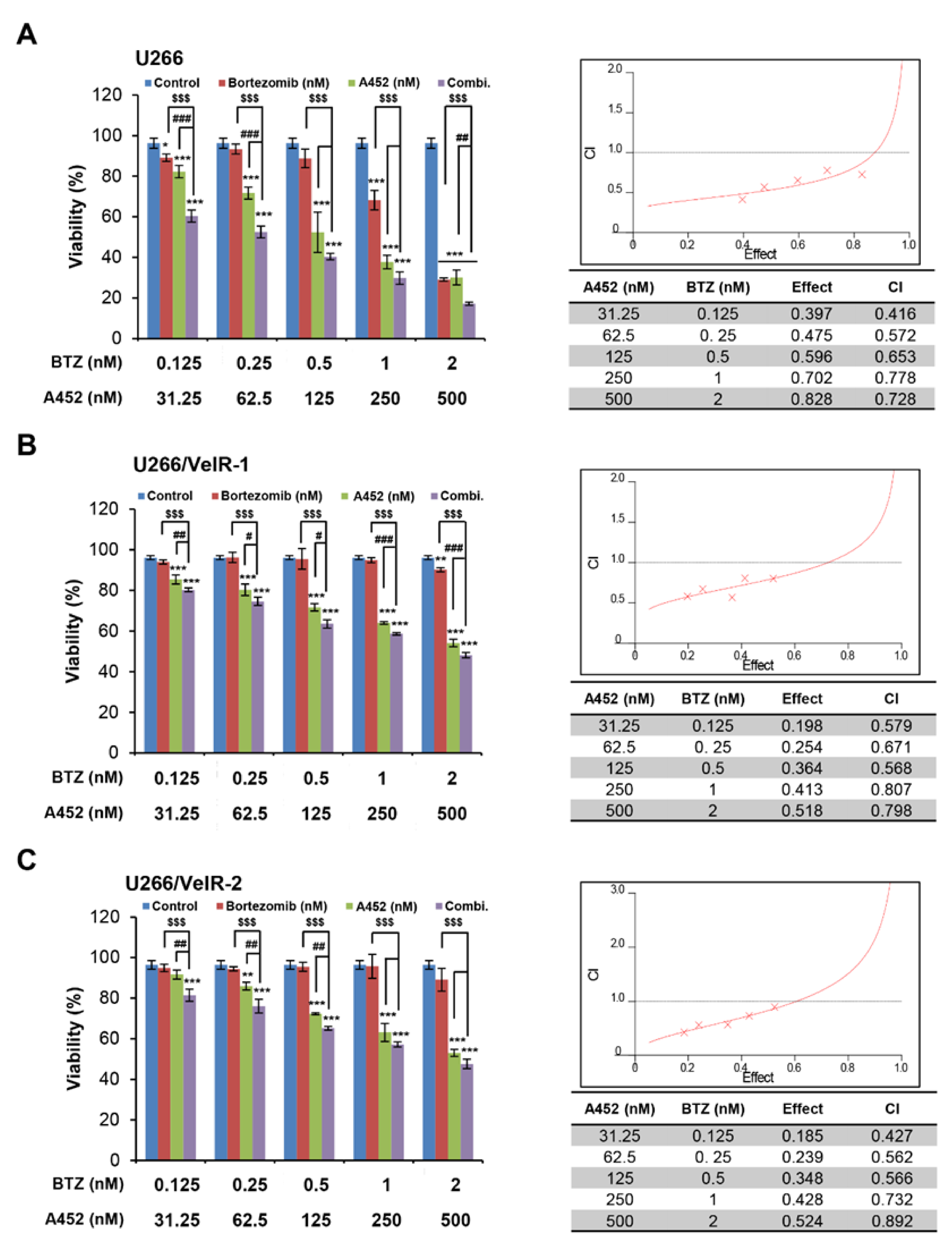
2.3. A452 in Combination with BTZ or CFZ Shows Synergistic Cytotoxicity in Both BTZ-Sensitive and BTZ-Resistant U266 Cells
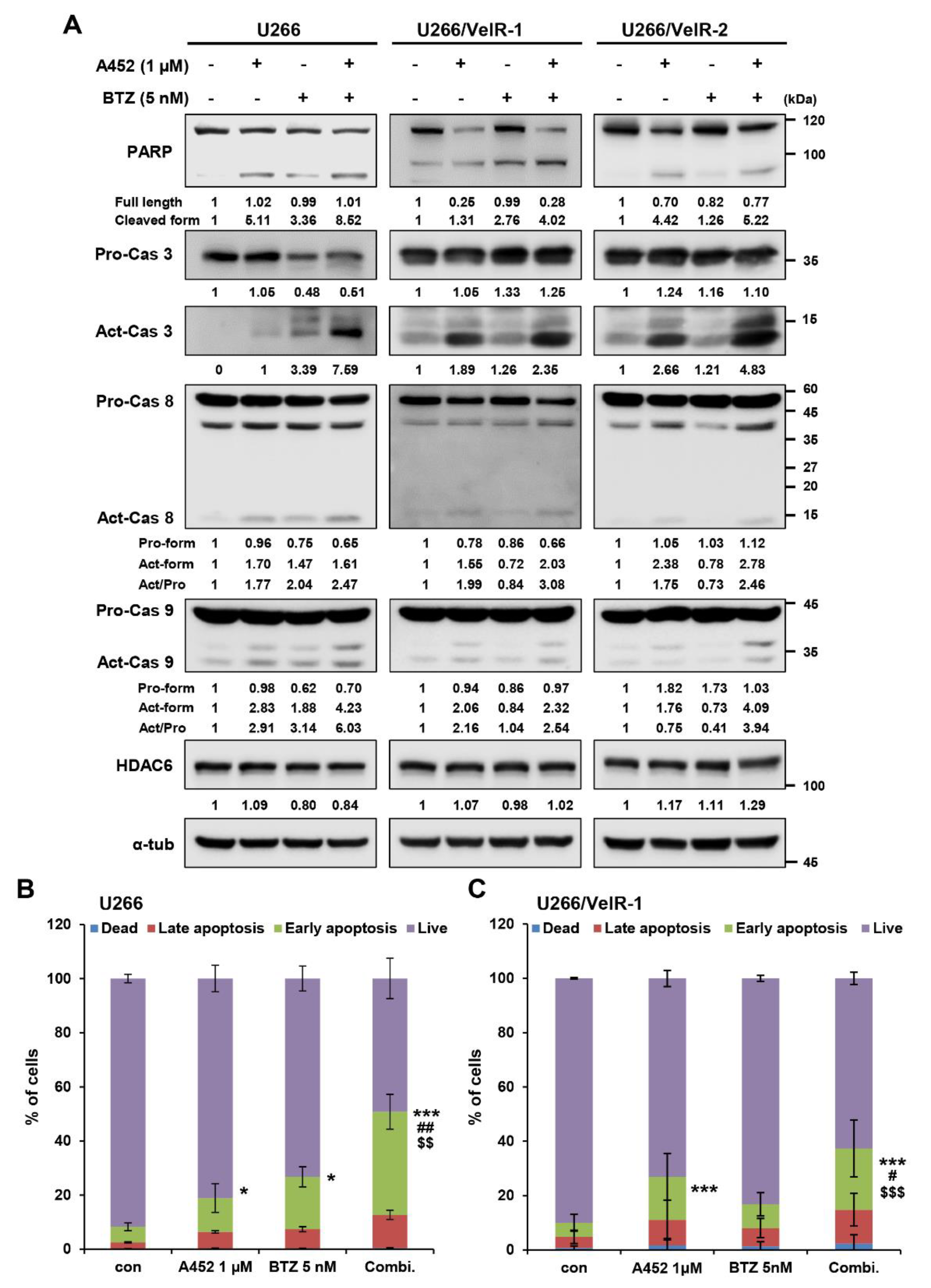
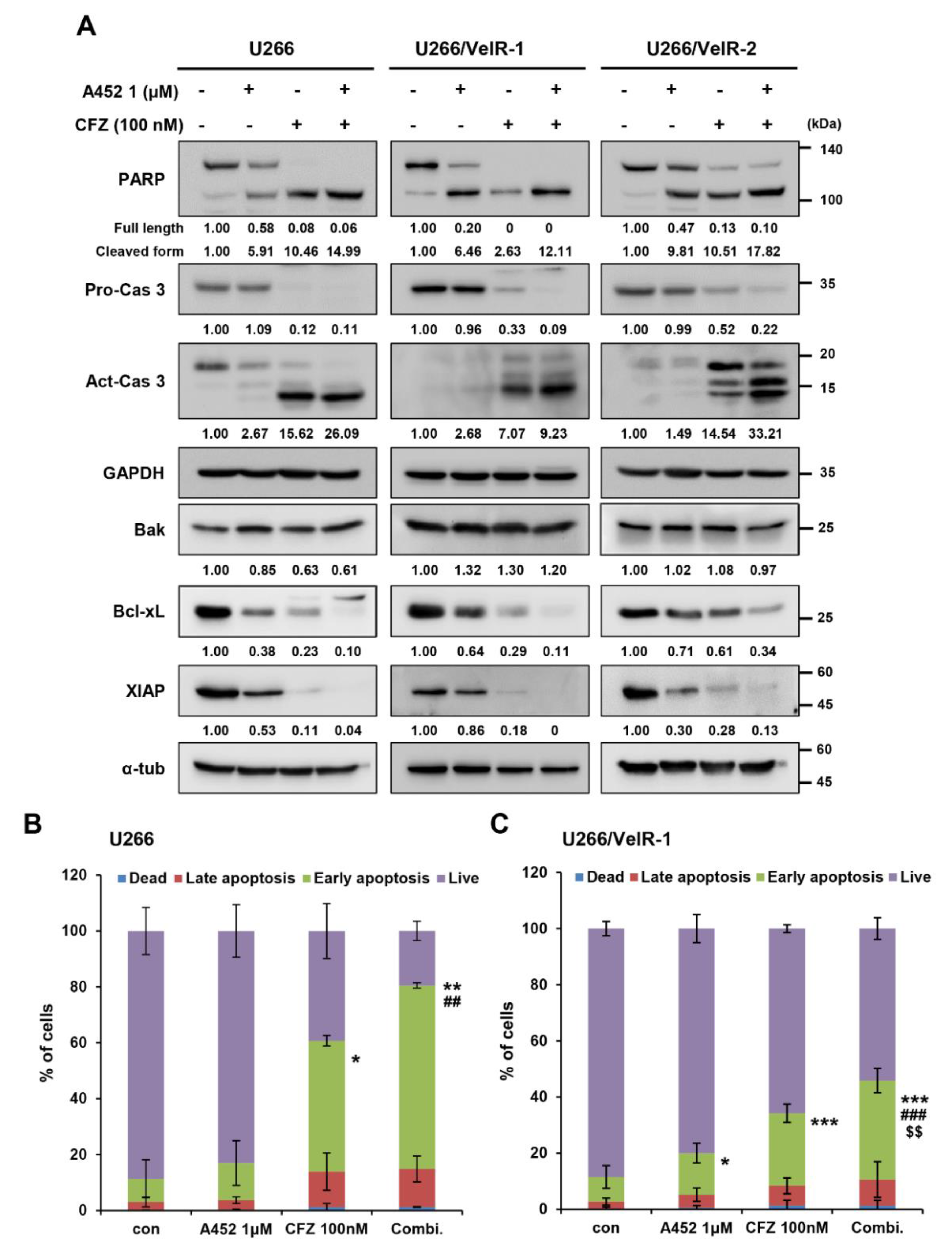
2.4. A452 in Combination with BTZ or CFZ Synergistically Leads to Apoptosis in BTZ-Sensitive and BTZ-Resistant U266 Cells
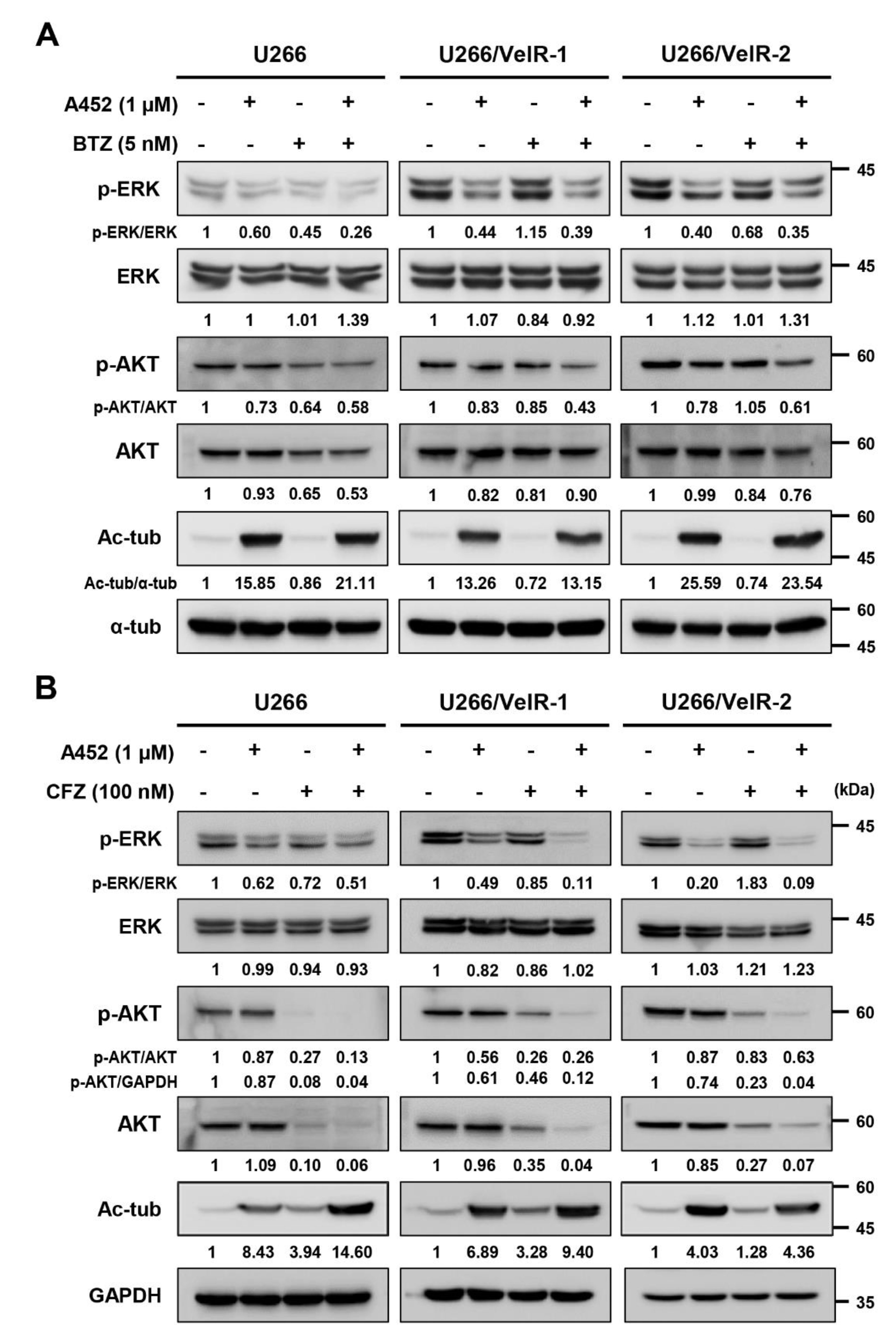
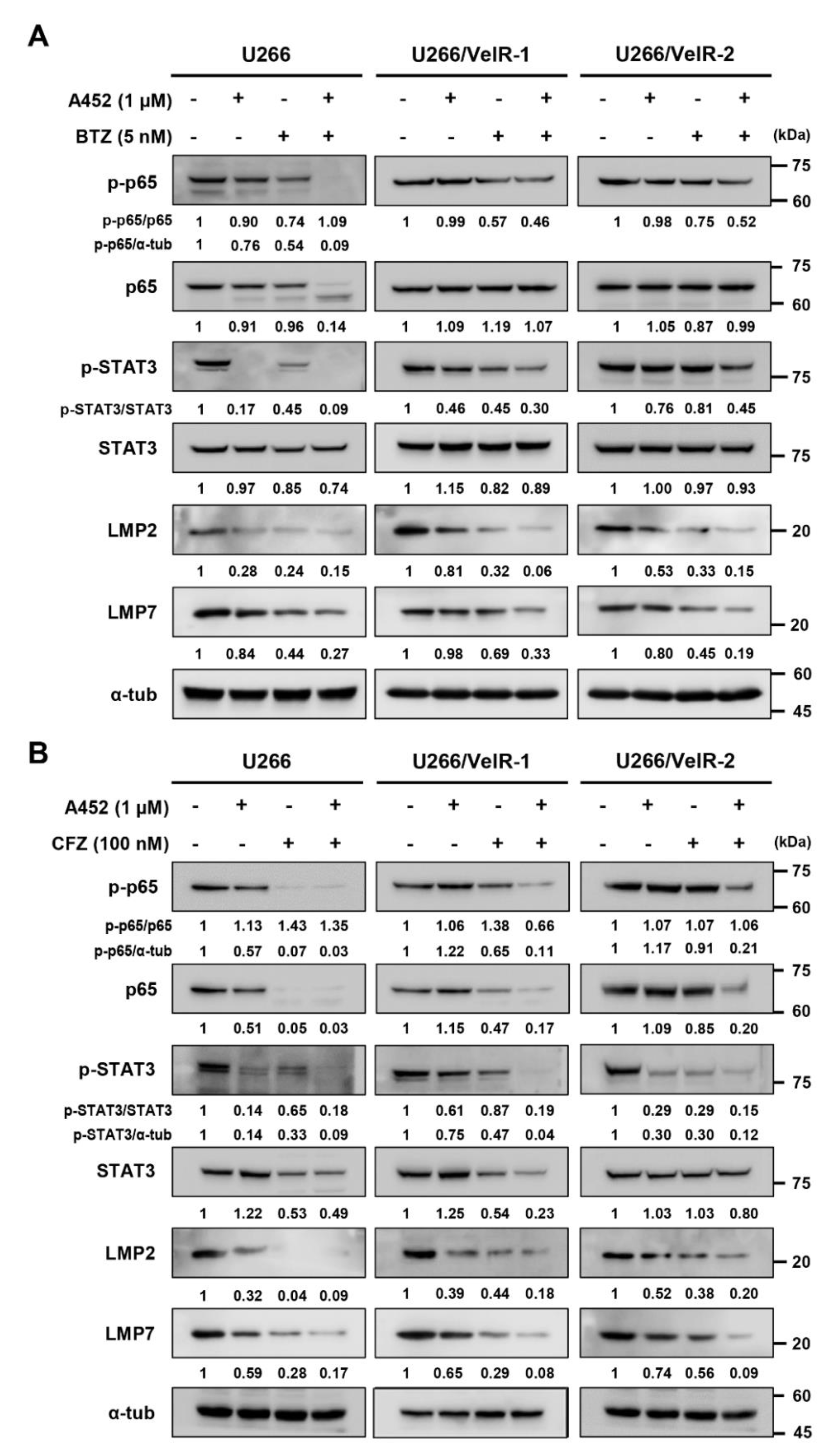
2.5. A452 in Combination with BTZ or CFZ Synergistically Inactivates the Cell Survival Signaling in BTZ-Sensitive and BTZ-Resistant U266 Cells
2.6. A452 Enhances MM Sensitivity to BTZ or CFZ in BTZ-Resistant U266 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. MM Cell Lines and Culture
4.3. MM Cell Lines and Culture
4.4. Cell Growth and Viability Assay
4.5. Growth Inhibitory and Viability Inhibitory Assays
4.6. Drug Combination Analysis
4.7. Apoptosis Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Bcl-xL | B-cell lymphoma-extra large protein |
| BTZ | Bortezomib |
| CFZ | Carfilzomib |
| CI | Combination index |
| DEX | Dexamethasone |
| ERK | Extracellular signal-regulated kinase |
| HDAC6 | Histone deacetylase 6 |
| HDACi | Histone deacetylase inhibitor |
| I-κB | NF-κB inhibitor |
| LMP | low-molecular-mass polypeptide |
| MM | Multiple myeloma |
| NF-κB | Nuclear factor-kappa B |
| PARP | Poly(ADP ribose) polymerase |
| PI | Proteasome inhibitor |
| STAT3 | Signal transducer and transcription |
| UPS | Ubiquitin-proteasome system |
References
- Bilotti, E.; Gleason, C.L.; McNeill, A. Routine Health Maintenance in Patients Living with Multiple Myeloma. Clin. J. Oncol. Nurs. 2011, 15, 25–40. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple Myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Özkan, M.C.; Güneş, A.E.; Sahin, F.; Saydam, G. Quality of Life and Supportive Care in Multiple Myeloma. Turk. J. Hematol. 2013, 30, 234–246. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Moehler, T.; Goldschmidt, H. Therapy of Relapsed and Refractory Multiple Myeloma. Toxic. Assess. 2011, 183, 239–271. [Google Scholar] [CrossRef]
- Kane, R.C.; Bross, P.F.; Farrell, A.T.; Pazdur, R. Velcade ®: U.S. FDA Approval for the Treatment of Multiple Myeloma Progressing on Prior Therapy. Oncologist 2003, 8, 508–513. [Google Scholar] [CrossRef]
- Chauhan, D.; Catley, L.; Li, G.; Podar, K.; Hideshima, T.; Velankar, M.; Mitsiades, C.; Mitsiades, N.; Yasui, H.; Letai, A.; et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell 2005, 8, 407–419. [Google Scholar] [CrossRef]
- Groll, M.; Berkers, C.R.; Ploegh, H.L.; Ovaa, H. Crystal Structure of the Boronic Acid-Based Proteasome Inhibitor Bortezomib in Complex with the Yeast 20S Proteasome. Structure 2006, 14, 451–456. [Google Scholar] [CrossRef]
- Kubiczkova, L.; Pour, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. Proteasome inhibitors—Molecular basis and current perspectives in multiple myeloma. J. Cell. Mol. Med. 2014, 18, 947–961. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Hideshima, T.; Chauhan, D.; Richardson, P.G.; Mitsiades, C.S.; Mitsiades, N.; Hayashi, T.; Munshi, N.C.; Dang, L.; Castro, A.; Palombella, V.J.; et al. NF-κB as a Therapeutic Target in Multiple Myeloma. J. Biol. Chem. 2002, 277, 16639–16647. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Vassallo, B.; Carlisi, D.; Portanova, P.; Vento, R.; Tesoriere, G. JNK and AP-1 mediate apoptosis induced by bortezomib in HepG2 cells via FasL/caspase-8 and mitochondria-dependent pathways. Apoptosis 2006, 11, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, X.; Gao, G.; Dong, H. Caspase-2 functions upstream of mitochondria in endoplasmic reticulum stress-induced apoptosis by bortezomib in human myeloma cells. Mol. Cancer Ther. 2008, 7, 2298–2307. [Google Scholar] [CrossRef]
- Richardson, P.G.; Mitsiades, C.; Ghobrial, I.; Anderson, K. Beyond single-agent bortezomib: Combination regimens in relapsed multiple myeloma. Curr. Opin. Oncol. 2006, 18, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.S.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef]
- Oerlemans, R.; Franke, N.E.; Assaraf, Y.G.; Cloos, J.; Van Zantwijk, I.; Berkers, C.R.; Scheffer, G.L.; Debipersad, K.; Vojtekova, K.; Lemos, C.; et al. Molecular basis of bortezomib resistance: Proteasome subunit β5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood 2008, 112, 2489–2499. [Google Scholar] [CrossRef]
- Barrio, S.; Stühmer, T.; Da-Viá, M.; Barrio-Garcia, C.; Lehners, N.; Besse, A.; Cuenca, I.; Garitano-Trojaola, A.; Fink, S.; Leich, E.; et al. Spectrum and functional validation of PSMB5 mutations in multiple myeloma. Leukemia 2019, 33, 447–456. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Gregerson, D.S. Immunoproteasomes: Structure, function, and antigen presentation. Prog. Mol. Biol. Transl. Sci. 2012, 109, 75–112. [Google Scholar] [CrossRef]
- Miller, Z.; Lee, W.; Kim, K.B. The immunoproteasome as a therapeutic target for hematological malignancies. Curr. Cancer Drug Targets 2014, 14, 537–548. [Google Scholar] [CrossRef]
- Besse, A.; Besse, L.; Kraus, M.; Mendez-Lopez, M.; Bader, J.; Xin, B.-T.; De Bruin, G.; Maurits, E.; Overkleeft, H.S.; Driessen, C. Proteasome Inhibition in Multiple Myeloma: Head-to-Head Comparison of Currently Available Proteasome Inhibitors. Cell Chem. Biol. 2019, 26, 340–351.e3. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Baladandayuthapani, V.; Lin, H.; Mulligan, G.; Li, B.; Esseltine, D.-L.W.; Qing, Z.; Xu, J.; Hunziker, W.; Barlogie, B.; et al. Tight Junction Protein 1 Modulates Proteasome Capacity and Proteasome Inhibitor Sensitivity in Multiple Myeloma via EGFR/JAK1/STAT3 Signaling. Cancer Cell 2016, 29, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Malek, E.; Abdel-Malek, M.A.Y.; Jagannathan, S.; Vad, N.; Karns, R.; Jegga, A.G.; Broyl, A.; Van Duin, M.; Sonneveld, P.; Cottini, F.; et al. Pharmacogenomics and chemical library screens reveal a novel SCFSKP2 inhibitor that overcomes Bortezomib resistance in multiple myeloma. Leukemia 2016, 31, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Dimopoulos, M.A.; Lonial, S. Combined proteasome and histone deacetylase inhibition: A promising synergy for patients with relapsed/refractory multiple myeloma. Leuk. Res. 2010, 34, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Duvic, M.A.; Olsen, E.A.; Breneman, D.; Pacheco, T.R.; Parker, S.; Vonderheid, E.C.; Abuav, R.; Ricker, J.L.; Rizvi, S.; Chen, C.; et al. Evaluation of the Long-Term Tolerability and Clinical Benefit of Vorinostat in Patients with Advanced Cutaneous T-Cell Lymphoma. Clin. Lymphoma Myeloma 2009, 9, 412–416. [Google Scholar] [CrossRef]
- Sivaraj, D.; Green, M.M.; Gasparetto, C. Panobinostat for the management of multiple myeloma. Futur. Oncol. 2017, 13, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Badros, A.; Burger, A.M.; Philip, S.; Niesvizky, R.; Kolla, S.S.; Goloubeva, O.; Harris, C.; Zwiebel, J.; Wright, J.J.; Espinoza-Delgado, I.; et al. Phase I Study of Vorinostat in Combination with Bortezomib for Relapsed and Refractory Multiple Myeloma. Clin. Cancer Res. 2009, 15, 5250–5257. [Google Scholar] [CrossRef]
- Santo, L.; Hideshima, T.; Kung, A.L.; Tseng, J.-C.; Tamang, D.; Yang, M.; Jarpe, M.; Van Duzer, J.H.; Mazitschek, R.; Ogier, W.C.; et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood 2012, 119, 2579–2589. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Richardson, P.G.; Günther, A.; Sezer, O.; Siegel, D.; Bladé, J.; Leblanc, R.; Sutherland, H.; Sopala, M.; Mishra, K.K.; et al. Phase Ib Study of Panobinostat and Bortezomib in Relapsed or Relapsed and Refractory Multiple Myeloma. J. Clin. Oncol. 2013, 31, 3696–3703. [Google Scholar] [CrossRef]
- Amengual, J.E.; Johannet, P.M.; Lombardo, M.; Zullo, K.M.; Hoehn, D.; Bhagat, G.; Scotto, L.; Jirau-Serrano, X.; Radeski, D.; Heinen, J.; et al. Dual Targeting of Protein Degradation Pathways with the Selective HDAC6 Inhibitor ACY-1215 and Bortezomib Is Synergistic in Lymphoma. Clin. Cancer Res. 2015, 21, 4663–4675. [Google Scholar] [CrossRef]
- Lee, N.H.; Kim, G.W.; Kwon, S.H. The HDAC6-selective inhibitor is effective against non-Hodgkin lymphoma and synergizes with ibrutinib in follicular lymphoma. Mol. Carcinog. 2019, 58, 944–956. [Google Scholar] [CrossRef]
- Mishima, Y.; Santo, L.; Loredana, S.; Cirstea, D.; Nemani, N.; Yee, A.J.; O’Donnell, E.; Selig, M.K.; Quayle, S.N.; Arastu-Kapur, S.; et al. Ricolinostat (ACY-1215) induced inhibition of aggresome formation accelerates carfilzomib-induced multiple myeloma cell death. Br. J. Haematol. 2015, 169, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Vogl, D.T.; Raje, N.; Jagannath, S.; Richardson, P.; Hari, P.; Orlowski, R.; Supko, J.G.; Tamang, D.; Yang, M.; Jones, S.S.; et al. Ricolinostat, the First Selective Histone Deacetylase 6 Inhibitor, in Combination with Bortezomib and Dexamethasone for Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2017, 23, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Lee, C.; Park, J.E.; Seo, J.J.; Cho, M.; Kang, J.S.; Kim, H.M.; Park, S.-K.; Lee, K.; Han, G. Structure and property based design, synthesis and biological evaluation of γ-lactam based HDAC inhibitors. Bioorgan. Med. Chem. Lett. 2011, 21, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bae, E.K.; Lee, C.; Choi, J.H.; Jung, W.J.; Ahn, K.S.; Yoon, S.S. Establishment and characterization of bortezomib-resistant U266 cell line: Constitutive activation of NF-kappaB-mediated cell signals and/or alterations of ubiquitylation-related genes reduce bortezomib-induced apoptosis. BMB Rep. 2014, 47, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.-W.; Shin, D.-H.; Lee, D.H.; Choi, J.; Han, G.; Lee, K.Y.; Kwon, S.H. HDAC6 deacetylates p53 at lysines 381/382 and differentially coordinates p53-induced apoptosis. Cancer Lett. 2017, 391, 162–171. [Google Scholar] [CrossRef]
- Ryu, H.-W.; Won, H.-R.; Lee, D.H.; Kwon, S.H. HDAC6 regulates sensitivity to cell death in response to stress and post-stress recovery. Cell Stress Chaperon 2017, 22, 253–261. [Google Scholar] [CrossRef]
- Won, H.-R.; Ryu, H.-W.; Shin, D.-H.; Yeon, S.-K.; Lee, D.H.; Kwon, S.H. A452, an HDAC6-selective inhibitor, synergistically enhances the anticancer activity of chemotherapeutic agents in colorectal cancer cells. Mol. Carcinog. 2018, 57, 1383–1395. [Google Scholar] [CrossRef]
- Won, H.; Lee, D.H.; Yeon, S.; Ryu, H.; Kim, G.W.; Kwon, S.H. HDAC6-selective inhibitor synergistically enhances the anticancer activity of immunomodulatory drugs in multiple myeloma. Int. J. Oncol. 2019, 55, 499–512. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.J.; Chen, Q.; Voorhees, P.M.; Strader, J.S.; Shenk, K.D.; Sun, C.M.; Demo, S.D.; Bennett, M.K.; Van Leeuwen, F.W.B.; Chanan-Khan, A.A.; et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 2007, 110, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, C.S.; Hayden, P.J.; Anderson, K.C.; Richardson, P.G. From the bench to the bedside: Emerging new treatments in multiple myeloma. Best Pr. Res. Clin. Haematol. 2007, 20, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.L.; White, L.C.; Kelly, A.; Beck, S.; Trowsdale, J.; Ting, J.P. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J. Exp. Med. 1995, 181, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Dalton, W.S. Drug resistance and drug development in multiple myeloma. Semin. Oncol. 2002, 29, 21–25. [Google Scholar] [CrossRef]
- Mateos, M.-V. Management of treatment-related adverse events in patients with multiple myeloma. Cancer Treat. Rev. 2010, 36, S24–S32. [Google Scholar] [CrossRef]
- DasMahapatra, G.; Patel, H.; Friedberg, J.; Quayle, S.N.; Jones, S.S.; Grant, S. In Vitro and In Vivo Interactions between the HDAC6 Inhibitor Ricolinostat (ACY1215) and the Irreversible Proteasome Inhibitor Carfilzomib in Non-Hodgkin Lymphoma Cells. Mol. Cancer Ther. 2014, 13, 2886–2897. [Google Scholar] [CrossRef]
- Madrid, L.V.; Mayo, M.W.; Reuther, J.Y.; Baldwin, A.S., Jr. Akt Stimulates the Transactivation Potential of the RelA/p65 Subunit of NF-κB through Utilization of the IκB Kinase and Activation of the Mitogen-activated Protein Kinase p38. J. Biol. Chem. 2001, 276, 18934–18940. [Google Scholar] [CrossRef]
- Chen, L.-F.; Williams, S.A.; Mu, Y.; Nakano, H.; Duerr, J.M.; Buckbinder, L.; Greene, W.C. NF-κB RelA Phosphorylation Regulates RelA Acetylation. Mol. Cell. Biol. 2005, 25, 7966–7975. [Google Scholar] [CrossRef]
- Iaconelli, J.; LaLonde, J.; Watmuff, B.; Liu, B.; Mazitschek, R.; Haggarty, S.J.; Karmacharya, R. Lysine Deacetylation by HDAC6 Regulates the Kinase Activity of AKT in Human Neural Progenitor Cells. ACS Chem. Biol. 2017, 12, 2139–2148. [Google Scholar] [CrossRef]
- Busse, A.; Kraus, M.; Rietz, A.; Scheibenbogen, C.; Driessen, C.; Blau, I.W.; Thiel, E.; Keilholz, U.; Na, I.-K. Sensitivity of tumor cells to proteasome inhibitors is associated with expression levels and composition of proteasome subunits. Cancer 2008, 112, 659–670. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, R.; Peng, H.; Liu, H.; Wen, L.; Wu, T.; Yi, H.; Li, A.; Zhang, Z. miR-451 suppresses the NF-kappaB-mediated proinflammatory molecules expression through inhibiting LMP7 in diabetic nephropathy. Mol. Cell. Endocrinol. 2016, 433, 75–86. [Google Scholar] [CrossRef]
- Soriano, G.P.; Besse, L.; Li, N.; Kraus, M.; Besse, A.; Meeuwenoord, N.; Bader, J.; Everts, B.; Dulk, H.D.; Overkleeft, H.S.; et al. Proteasome inhibitor-adapted myeloma cells are largely independent from proteasome activity and show complex proteomic changes, in particular in redox and energy metabolism. Leukemia 2016, 30, 2198–2207. [Google Scholar] [CrossRef]
- Gutman, D.A.; Morales, A.A.; Boise, L.H. Acquisition of a multidrug-resistant phenotype with a proteasome inhibitor in multiple myeloma. Leukemia 2009, 23, 2181–2183. [Google Scholar] [CrossRef]
- Cheng, F.; Lienlaf, M.; Wang, H.-W.; Perez-Villarroel, P.; Lee, C.; Woan, K.; Rock-Klotz, J.; Sahakian, E.; Woods, D.; Pinilla-Ibarz, J.; et al. A Novel Role for Histone Deacetylase 6 in the Regulation of the Tolerogenic STAT3/IL-10 Pathway in APCs. J. Immunol. 2014, 193, 2850–2862. [Google Scholar] [CrossRef]
- Kwon, S.; Zhang, Y.; Matthias, P. The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response. Genes Dev. 2007, 21, 3381–3394. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.W.; Yeon, S.-K.; Kim, G.W.; Lee, D.H.; Jeon, Y.H.; Yoo, J.; Kim, S.Y.; Kwon, S.H. HDAC6-Selective Inhibitor Overcomes Bortezomib Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2021, 22, 1341. https://doi.org/10.3390/ijms22031341
Lee SW, Yeon S-K, Kim GW, Lee DH, Jeon YH, Yoo J, Kim SY, Kwon SH. HDAC6-Selective Inhibitor Overcomes Bortezomib Resistance in Multiple Myeloma. International Journal of Molecular Sciences. 2021; 22(3):1341. https://doi.org/10.3390/ijms22031341
Chicago/Turabian StyleLee, Sang Wu, Soo-Keun Yeon, Go Woon Kim, Dong Hoon Lee, Yu Hyun Jeon, Jung Yoo, So Yeon Kim, and So Hee Kwon. 2021. "HDAC6-Selective Inhibitor Overcomes Bortezomib Resistance in Multiple Myeloma" International Journal of Molecular Sciences 22, no. 3: 1341. https://doi.org/10.3390/ijms22031341
APA StyleLee, S. W., Yeon, S.-K., Kim, G. W., Lee, D. H., Jeon, Y. H., Yoo, J., Kim, S. Y., & Kwon, S. H. (2021). HDAC6-Selective Inhibitor Overcomes Bortezomib Resistance in Multiple Myeloma. International Journal of Molecular Sciences, 22(3), 1341. https://doi.org/10.3390/ijms22031341






