The Roles of MicroRNAs in Male Infertility
Abstract
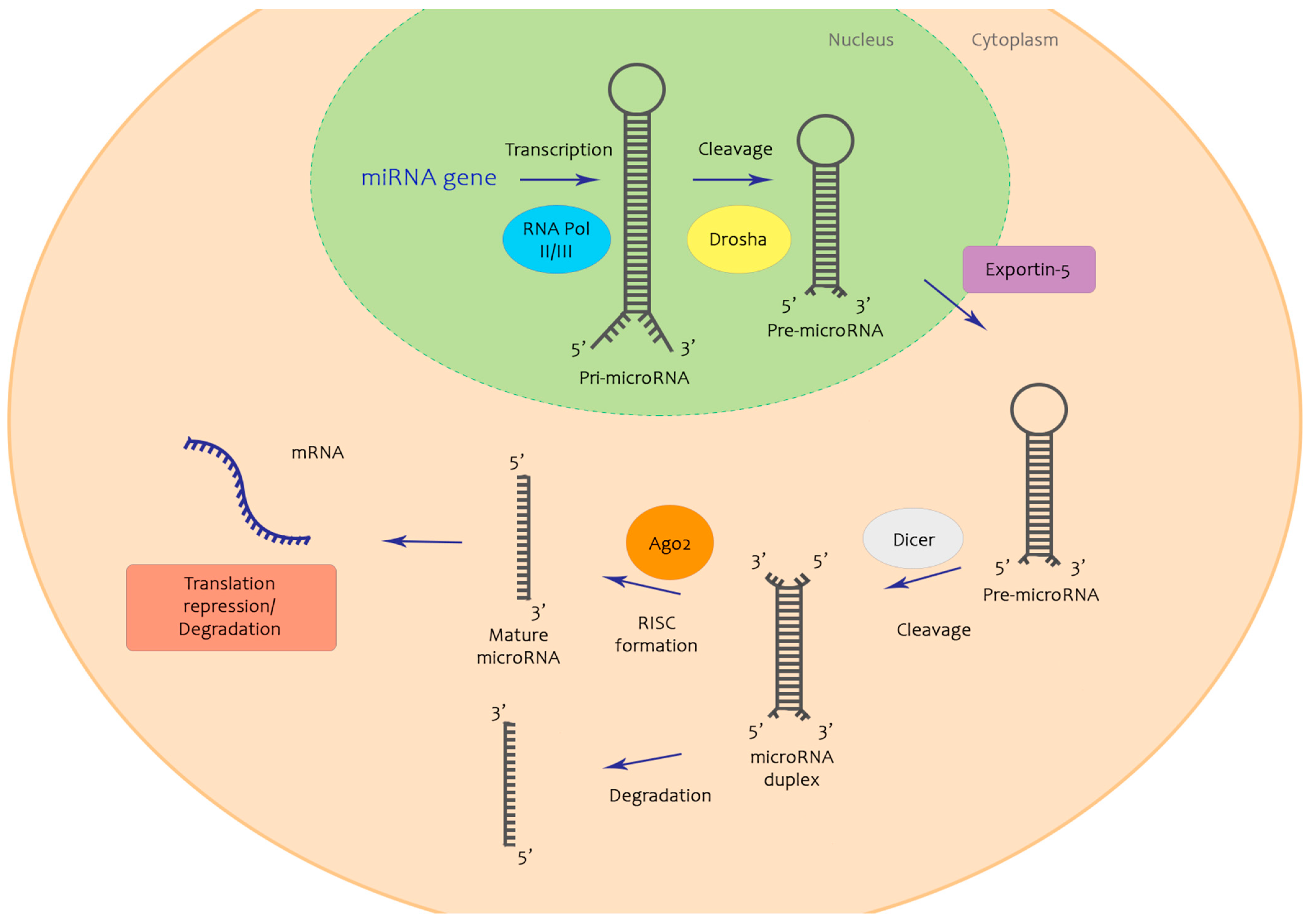
1. Introduction
2. MicroRNA Involvement in Spermatogenesis
| microRNA | Location | Action | Type of Sample | Study Population | References |
|---|---|---|---|---|---|
| miR-34c | SSCs, spermatocytes and round spermatids |
| Epididymal sperm [36], primary spermatocytes [37], adult mouse testis [38] | Mice | [36,37,38,43] |
| miR-449 |
| Epididymal sperm [36], primary spermatocytes [37], adult mouse testis [38] | [36,37,38,43] | ||
| miR-21 | SSCs |
| Germ-cell cultures | Mice | [44] |
| miR-146a | SSCs |
| Germ-cell cultures | Mice | [35] |
| miR-20 | SSCs |
| Germ-cell cultures | Mice | [45] |
| miR-106a | SSCs |
| Germ-cell cultures | Mice | [45] |
| miR-202-3p | SSCs |
| Germ-cell cultures | Mice | [39,46,47] |
| miR-202-5p | SSCs |
| Germ-cell cultures | Mice | [39] |
| miR-471 | Sertoli cells |
| Isolated Sertoli cells from mice testis | Mice | [40] |
| miR-463 | Sertoli cells |
| Isolated Sertoli cells from mice testis | Mice | [40] |
| miR-201 | Sertoli cells |
| Isolated Sertoli cells from mice testis | Mice | [40] |
3. The Implications of MicroRNA Dysregulation on Male Infertility
4. The Potential of MicroRNAs as Biomarkers
5. Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A. Guidelines on Male Infertility; European Association of Urology Guidelines: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bujan, L.; Daudin, M.; Charlet, J.-P.; Thonneau, P.; Mieusset, R. Increase in scrotal temperature in car drivers. Hum. Reprod. 2000, 15, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Sheynkin, Y.; Jung, M.; Yoo, P.; Schulsinger, D.; Komaroff, E. Increase in scrotal temperature in laptop computer users. Hum. Reprod. 2005, 20, 452–455. [Google Scholar] [CrossRef]
- Saalu, L. The Incriminating Role of Reactive Oxygen Species in Idiopathic Male Infertility: An Evidence Based Evaluation. Pak. J. Biol. Sci. 2010, 13, 413–422. [Google Scholar] [CrossRef]
- Khazaie, Y.; Esfahani, M.H.N. MicroRNA and Male Infertility: A Potential for Diagnosis. Int. J. Fertil. Steril. 2014, 8, 113–118. [Google Scholar]
- Bruscella, P.; Bottini, S.; Baudesson, C.; Pawlotsky, J.-M.; Feray, C.; Trabucchi, M. Viruses and miRNAs: More Friends than Foes. Front. Microbiol. 2017, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Claus, R.; Plass, C. Genome-Wide Epigenetic Regulation of miRNAs in Cancer. Cancer Res. 2013, 73, 473–477. [Google Scholar] [CrossRef]
- Grasso, M.; Piscopo, P.; Confaloni, A.; Denti, M.A. Circulating miRNAs as Biomarkers for Neurodegenerative Disorders. Molecules 2014, 19, 6891–6910. [Google Scholar] [CrossRef]
- Karnati, H.K.; Panigrahi, M.K.; Gutti, R.K.; Greig, N.H.; Tamargo, I.A. miRNAs: Key Players in Neurodegenerative Disorders and Epilepsy. J. Alzheimer’s Dis. 2015, 48, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Lv, M.; Li, Z.; Peng, D.; Tan, Y.; Wang, H.; Li, Q.; Li, F.; Liang, W. Semen-specific miRNAs: Suitable for the distinction of infertile semen in the body fluid identification? Forensic Sci. Int. Genet. 2018, 33, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.Z.; Guo, X.B.; Zhang, W.S.; Zhou, J.H.; Yang, C.; Bian, J.; Liu, C.D. Expressions of miR-525-3p and its target gene SEMG1 in the spermatozoa of patients with asthenozoospermia. Andrology 2019, 7, 220–227. [Google Scholar] [CrossRef]
- Zhuang, X.; Li, Z.; Lin, H.; Gu, L.; Lin, Q.; Lu, Z.; Tzeng, C.-M. Integrated miRNA and mRNA expression profiling to identify mRNA targets of dysregulated miRNAs in non-obstructive azoospermia. Sci. Rep. 2015, 5, srep07922. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Carrell, D.T.; Jenkins, T.G.; Yeste, M. The role of miRNAs in male human reproduction: A systematic review. Andrology 2019, 8, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Guttman, M.; Rinn, J.L. Modular regulatory principles of large non-coding RNAs. Nature 2012, 482, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.-H.; Kim, Y.-K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B. R Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Kwak, P.B.; Tomari, Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 2012, 19, 145–151. [Google Scholar] [CrossRef]
- Chevillet, J.R. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014, 19, 6080–6105. [Google Scholar] [CrossRef]
- Kotaja, N. MicroRNAs and spermatogenesis. Fertil. Steril. 2014, 101, 1552–1562. [Google Scholar] [CrossRef]
- Neto, F.T.L.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.P.; Kotaja, N. Small RNAs in spermatogenesis. Mol. Cell. Endocrinol. 2014, 382, 498–508. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Hai, Y.; Hou, J.; Liu, Y.; Liu, Y.; Yang, H.; Li, Z.; He, Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 66–75. [Google Scholar] [CrossRef]
- Coviello, A.D.; Matsumoto, A.M.; Bremner, W.J.; Herbst, K.L.; Amory, J.K.; Anawalt, B.D.; Sutton, P.R.; Wright, W.W.; Brown, T.R.; Yan, X.; et al. Low-Dose Human Chorionic Gonadotropin Maintains Intratesticular Testosterone in Normal Men with Testosterone-Induced Gonadotropin Suppression. J. Clin. Endocrinol. Metab. 2005, 90, 2595–2602. [Google Scholar] [CrossRef]
- Matthiesson, K.L.; Stanton, P.G.; O’Donnell, L.; Meachem, S.; Amory, J.K.; Berger, R.; McLachlan, R. I Effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotro-pin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J. Clin. Endocrinol. Metab. 2005, 90, 5647–5655. [Google Scholar] [CrossRef]
- Bettegowda, A.; Wilkinson, M.F. Transcription and post-transcriptional regulation of spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1637–1651. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Huszar, J.M.; Payne, C.J. MicroRNA 146 (Mir146) Modulates Spermatogonial Differentiation by Retinoic Acid in Mice1. Biol. Reprod. 2013, 88, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-M.; Pang, R.T.K.; Chiu, P.C.N.; Wong, B.P.C.; Lao, K.; Lee, K.-F.; Yeung, W.S.B. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc. Natl. Acad. Sci. USA 2011, 109, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Tang, C.; Zhang, Y.; Wu, J.; Bao, J.; Zheng, H.; Xu, C.; Yan, W. mir-34b/c and mir-449a/b/c are required for spermatogenesis, but not for the first cleavage division in mice. Biol. Open 2015, 4, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhou, D.; Wei, C.; Luo, H.; Liu, J.; Fu, R.; Cui, S. MicroRNA-34c Enhances Murine Male Germ Cell Apoptosis through Targeting ATF1. PLoS ONE 2012, 7, e33861. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cai, T.; Zheng, C.; Lin, X.; Wang, G.; Liao, S.; Han, C. MicroRNA-202 maintains spermatogonial stem cells by inhibiting cell cycle regulators and RNA binding pro-teins. Nucleic Acids Res. 2017, 45, 4142–4157. [Google Scholar]
- Panneerdoss, S.; Chang, Y.-F.; Buddavarapu, K.C.; Chen, H.-I.H.; Shetty, G.; Wang, H.; Chen, Y.; Kumar, T.R.; Rao, M.K. Androgen-Responsive MicroRNAs in Mouse Sertoli Cells. PLoS ONE 2012, 7, e41146. [Google Scholar] [CrossRef]
- Chen, S.-R.; Liu, Y.-X. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167. [Google Scholar] [CrossRef]
- Geng, X.-J.; Zhao, D.-M.; Mao, G.-H.; Tan, L. MicroRNA-150 regulates steroidogenesis of mouse testicular Leydig cells by targeting STAR. Reproduction 2017, 154, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bouhallier, F.; Allioli, N.; Lavial, F.; Chalmel, F.; Perrard, M.-H.; Durand, P.; Samarut, J.; Pain, B.; Rouault, J.-P. Role of miR-34c microRNA in the late steps of spermatogenesis. RNA 2010, 16, 720–731. [Google Scholar] [CrossRef]
- Niu, Z.; Goodyear, S.M.; Rao, S.; Wu, X.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12740–12745. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV Infection by Targeting MicroRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Guo, J.; Zhang, P.; Zeng, W. The roles of microRNAs in regulation of mammalian spermatogenesis. J. Anim. Sci. Biotechnol. 2017, 8, 35. [Google Scholar] [CrossRef]
- Bie, B.; Wang, Y.; Li, L.; Fang, H.; Liu, L.; Sun, J. Noncoding RNAs: Potential players in the self-renewal of mammalian spermatogonial stem cells. Mol. Reprod. Dev. 2018, 85, 720–728. [Google Scholar] [CrossRef]
- Khawar, M.B.; Mehmood, R.; Roohi, N. MicroRNAs: Recent insights towards their role in male infertility and reproductive cancers. Bosn. J. Basic Med. Sci. 2019, 19, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Björkgren, I.; Gylling, H.; Turunen, H.; Huhtaniemi, I.; Strauss, L.; Poutanen, M.; Sipilä, P. Imbalanced lipid homeostasis in the conditional Dicer1 knockout mouse epididymis causes instability of the sperm membrane. FASEB J. 2014, 29, 433–442. [Google Scholar] [CrossRef]
- Papaioannou, M.D.; Lagarrigue, M.; Vejnar, C.E.; Rolland, A.D.; Kühne, F.; Aubry, F.; Schaad, O.; Fort, A.; Descombes, P.; Neerman-Arbez, M.; et al. Loss of Dicer in Sertoli Cells Has a Major Impact on the Testicular Proteome of Mice. Mol. Cell. Proteom. 2011, 10, M900587-MCP200. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Romero, Y.; Warnefors, M.; Bilican, A.; Borel, C.; Smith, L.B.; Nef, S. Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the pro-gression of mammalian spermatogenesis and male fertility. PLoS ONE 2014, 9, e107023. [Google Scholar] [CrossRef] [PubMed]
- Romero, Y.; Meikar, O.; Papaioannou, M.D.; Conne, B.; Grey, C.; Weier, M.; Pralong, F.; de Massy, B.; Kaessmann, H.; Vassalli, J.-D.; et al. Dicer1 Depletion in Male Germ Cells Leads to Infertility Due to Cumulative Meiotic and Spermiogenic Defects. PLoS ONE 2011, 6, e25241. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, D.M.; Loveland, K.L.; McManus, M.T.; Moore, K.; Harfe, B.D. Dicer1 Is Required for Differentiation of the Mouse Male Germline1. Biol. Reprod. 2008, 79, 696–703. [Google Scholar] [CrossRef]
- Eikmans, M.; Anholts, J.D.H.; Blijleven, L.; Meuleman, T.; van Beelen, E.; van der Hoorn, M.-L.P.; Claas, F.H.J. Optimization of microRNA Acquirement from Seminal Plasma and Identification of Diminished Seminal microRNA-34b as Indicator of Low Semen Concentration. Int. J. Mol. Sci. 2020, 21, 4089. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, Z.; Hong, K.; Tang, W.-H.; Liu, D.-F.; Mao, J.-M.; Yang, Y.-Z.; Lin, H.-C.; Jiang, H. Altered microRNA profiles of testicular biopsies from patients with nonobstructive azoospermia. Asian J. Androl. 2020, 22, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Backes, C.; Fischer, U.; Leidinger, P.; Lubbad, A.M.; Keller, A.; Meese, E. Panel of five microRNAs as potential biomarkers for the diagnosis and assessment of male infertility. Fertil. Steril. 2014, 102, 989–997.e1. [Google Scholar] [CrossRef]
- Munoz, X.; Mata, A.; Bassas, L.; Larriba, S. Altered miRNA Signature of Developing Germ-cells in Infertile Patients Relates to the Severity of Sper-matogenic Failure and Persists in Spermatozoa. Sci. Rep. 2015, 5, 17991. [Google Scholar] [CrossRef]
- Cui, L.; Fang, L.; Shi, B.; Qiu, S.; Ye, Y. Spermatozoa micro ribonucleic acid–34c level is correlated with intracytoplasmic sperm injection outcomes. Fertil. Steril. 2015, 104, 312–317.e1. [Google Scholar] [CrossRef]
- Comazzetto, S.; di Giacomo, M.; Rasmussen, K.D.; Much, C.; Azzi, C.; Perlas, E.; Morgan, M.; O’Carroll, D. Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci. PLoS Genet. 2014, 10, e1004597. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, C.; Chen, X.; Yao, B.; Yang, C.; Zhu, C.; Li, L.; Wang, J.; Li, X.; Shao, Y.; et al. Altered Profile of Seminal Plasma MicroRNAs in the Molecular Diagnosis of Male Infertility. Clin. Chem. 2011, 57, 1722–1731. [Google Scholar] [CrossRef]
- Wu, W.; Hu, Z.; Qin, Y.; Dong, J.; Dai, J.; Lu, C.; Zhang, W.; Shen, H.; Xia, Y.; Wang, X. Seminal plasma microRNAs: Potential biomarkers for spermatogenesis status. Mol. Hum. Reprod. 2012, 18, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Gal, S.; Dunlop, H.M.; Pushkaran, B.; Liggins, A.P.; Pulford, K.; Banham, A.H.; Pezzella, F.; Boultwood, J.; Wainscoat, J.S.; et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br. J. Haematol. 2008, 141, 672–675. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 Reflect Myocardial Damage in Cardiovascular Disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Q.; You, W.; Chen, M.; Xia, J. MiRNAs as Biomarkers of Myocardial Infarction: A Meta-Analysis. PLoS ONE 2014, 9, e88566. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Jiang, Z.; Wu, F.; Ping, J.; Ming, L. Identification of microRNAs as diagnostic biomarkers for acute myocardial infarction in Asian populations: A systematic review and meta-analysis. Medicine 2017, 96, e7173. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Z.; Zhao, T.; Cao, W.; Zhang, L.; Li, H.; Wang, B. Plasma miR-1, miR-208, miR-499 as potential predictive biomarkers for acute myocardial infarction: An inde-pendent study of Han population. Exp. Gerontol. 2015, 72, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Lu, X.; Wang, T.; Li, S.; Kong, X.; Bo, C.; Li, J.; Wang, X.; Ma, H.; et al. MicroRNAs and nervous system diseases: Network insights and computational challenges. Brief. Bioinform. 2019, 21, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Vos, S.J.; Bos, I.; Bouwman, F.H.; Teunissen, C.E.; Scheltens, P. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Noebels, J. Pathway-driven discovery of epilepsy genes. Nat. Neurosci. 2015, 18, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Cardoso, A.L.; Mano, M.; de Lima, M.C.P. MicroRNAs in Glioblastoma: Role in Pathogenesis and Opportunities for Targeted Therapies. CNS Neurol. Disord. Drug Targets 2015, 14, 222–238. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Z.-M.; Guo, X.-M.; Su, D.-F.; Liu, X. An updated role of microRNA-124 in central nervous system disorders: A review. Front. Cell. Neurosci. 2015, 9, 193. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Tian, F.-F.; Yan, M.; Zhang, J.-M.; Liu, Q.; Lu, J.-Y.; Zhou, W.-B.; Yang, H.; Li, J. Delivery of an miR155 inhibitor by anti-CD20 single-chain antibody into B cells reduces the acetylcholine receptor-specific autoantibodies and ameliorates experimental autoimmune myasthenia gravis. Clin. Exp. Immunol. 2014, 176, 207–221. [Google Scholar] [CrossRef]
- Barbu, M.G.; Condrat, C.E.; Thompson, D.C.; Bugnar, O.L.; Cretoiu, D.; Toader, O.D.; Suciu, N.; Voinea, S.C. MicroRNA Involvement in Signaling Pathways During Viral Infection. Front. Cell Dev. Biol. 2020, 8, 143. [Google Scholar] [CrossRef]
- Thonneau, P.; Marchand, S.; Tallec, A.; Ferial, M.-L.; Ducot, B.; Lansac, J.; Lopes, P.; Tabaste, J.-M.; Spira, A. Incidence and main causes of infertility in a resident population (1 850 000) of three French regions (1988–1989). Hum. Reprod. 1991, 6, 811–816. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Backes, C.; Leidinger, P.; Keller, A.; Lubbad, A.M.; Hammadeh, M.; Meese, E. MicroRNA expression profiles in human testicular tissues of infertile men with different histopathologic patterns. Fertil. Steril. 2014, 101, 78–86.e2. [Google Scholar] [CrossRef] [PubMed]
- Malcher, A.; Rozwadowska, N.; Stokowy, T.; Kolanowski, T.; Jedrzejczak, P.; Zietkowiak, W.; Kurpisz, M. Potential biomarkers of nonobstructive azoospermia identified in microarray gene expression analysis. Fertil. Steril. 2013, 100, 1686–1694.e7. [Google Scholar] [CrossRef]
- Schrader, M.; Müller, M.; Heicappell, R.; Straub, B.; Miller, K. Quantification of human telomerase RNA (hTR) and human telomerase reverse transcriptase (hTERT) mRNA in testicular tissue of infertile patients. Asian J. Androl. 2001, 3, 263–270. [Google Scholar]
- Silber, S.J.; Nagy, Z.; Devroey, P.; Tournaye, H.; van Steirteghem, A.C. Distribution of spermatogenesis in the testicles of azoospermic men: The presence or absence of spermatids in the testes of men with germinal failure. Hum. Reprod. 1997, 12, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Schmitt, J.; Leidinger, P.; Keller, A.; Meese, E.; Backes, C. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil. Steril. 2013, 99, 1249–1255.e16. [Google Scholar] [CrossRef]
- Wu, W.; Qin, Y.; Li, Z.; Dong, J.; Dai, J.; Lu, C.; Guo, X.; Zhao, Y.; Zhu, Y.; Zhang, W.; et al. Genome-wide microRNA expression profiling in idiopathic non-obstructive azoospermia: Significant up-regulation of miR-141, miR-429 and miR-7-1-3p. Hum. Reprod. 2013, 28, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Zhang, X.; Tian, H.; Liang, N.; Wang, Y.; Liang, C.; Li, X.; Sun, F. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod. Biol. Endocrinol. 2009, 7, 13. [Google Scholar] [CrossRef]
- Vashisht, A.; Gahlay, G.K. Using miRNAs as diagnostic biomarkers for male infertility: Opportunities and challenges. Mol. Hum. Reprod. 2020, 26, 199–214. [Google Scholar] [CrossRef]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum MicroRNAs Are Promising Novel Biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [PubMed]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008, 19, 92–105. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, W.; Gao, Y.; Wang, H.; Liu, Z. Microarray analysis of microRNA expression patterns in the semen of infertile men with semen abnormalities. Mol. Med. Rep. 2012, 6, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, Z.; Peng, D.; Bai, X.; Liang, W. Expression difference of miR-10b and miR-135b between the fertile and infertile semen samples (p). Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e257–e259. [Google Scholar] [CrossRef]
- Mostafa, T.; Rashed, L.A.; Nabil, N.I.; Osman, I.; Mostafa, R.; Farag, M. Seminal miRNA Relationship with Apoptotic Markers and Oxidative Stress in Infertile Men with Varicocele. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, C.; Bobjer, J.; Rastkhani, H.; Dermitzaki, E.; Katrinaki, M.; Margioris, A.N.; Giwercman, Y.L. Serum miR-155 as a potential biomarker of male fertility. Hum. Reprod. 2015, 30, 853–860. [Google Scholar] [CrossRef]
- Trzybulska, D.; Bobjer, J.; Giwercman, A.; Tsatsanis, C. Serum microRNAs in male subfertility-biomarkers and a potential pathogenetic link to metabolic syn-drome. J. Assist. Reprod. Genet. 2017, 34, 1277–1282. [Google Scholar] [CrossRef]
- Qing, X.; Shi, J.; Dong, T.; Wu, C.; Hu, L.; Li, H. Dysregulation of an X-linked primate-specific epididymal microRNA cluster in unexplained asthenozoospermia. Oncotarget 2017, 8, 56839–56849. [Google Scholar] [CrossRef]
- Brunet Vega, A. microRNA expression profile in stage III colorectal cancer: Circulating miR-18a and miR-29a as prom-ising biomarkers. Oncol. Rep. 2013, 30, 320–326. [Google Scholar] [CrossRef]
- Witwer, K.W. Circulating MicroRNA Biomarker Studies: Pitfalls and Potential Solutions. Clin. Chem. 2015, 61, 56–63. [Google Scholar] [CrossRef]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef]
- Alberti, C.; Cochella, L. A framework for understanding the roles of miRNAs in animal development. Development 2017, 144, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Altuvia, Y. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005, 33, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Androsavich, J.R.; Sobczynski, D.J.; Liu, X.; Pandya, S.; Kaimal, V.; Owen, T.; Liu, K.; MacKenna, D.A.; Chau, B.N. Polysome shift assay for direct measurement of miRNA inhibition by anti-miRNA drugs. Nucleic Acids Res. 2016, 44, e13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, Z.; Jiang, J.; Kokkinaki, M.; Tang, L.; Zeng, W.; Gallicano, I.; Dym, M. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via tar-geting STAT3 and Ccnd1. Stem Cells 2013, 31, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Stenvang, J.; Petri, A.; Lindow, M.; Obad, S.; Kauppinen, S. Inhibition of microRNA function by antimiR oligonucleotides. Silence 2012, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Gabay-Ryan, M.; Baylot, V.; Lai, I.; Mosley, A.; Huang, X.; Felsher, D.W. Anti-miR-17 therapy delays tumorigenesis in MYC-driven hepatocellular carcinoma (HCC). Oncotarget 2017, 9, 5517–5528. [Google Scholar] [CrossRef]
- Bezan, A.; Gerger, A.; Pichler, M. MicroRNAs in testicular cancer: Implications for pathogenesis, diagnosis, prognosis and therapy. Anticancer Res. 2014, 34, 2709–2713. [Google Scholar]
- Lian, J. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carci-noma cell proliferation by targeting IRF1. Cell Death Dis. 2010, 1, e94. [Google Scholar] [CrossRef]
- McIver, S.; Roman, S.; Nixon, B.; McLaughlin, E. miRNA and mammalian male germ cells. Hum. Reprod. Updat. 2011, 18, 44–59. [Google Scholar] [CrossRef]
- Rounge, T.B.; Furu, K.; Skotheim, R.I.; Haugen, T.B.; Grotmol, T.; Enerly, E. Profiling of the small RNA populations in human testicular germ cell tumors shows global loss of piRNAs. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef]
- Palmer, R.D.; Murray, M.J.; Saini, H.K.; van Dongen, S.; Abreu-Goodger, C.; Muralidhar, B.; Pett, M.R.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. Malignant Germ Cell Tumors Display Common MicroRNA Profiles Resulting in Global Changes in Expression of Messenger RNA Targets. Cancer Res. 2010, 70, 2911–2923. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA Signatures in Human Cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.L.; Lobo, J.; Jerónimo, C.; Henrique, R. The epigenetics of testicular germ cell tumors: Looking for novel disease biomarkers. Epigenomics 2017, 9, 155–169. [Google Scholar] [CrossRef]
- Voorhoeve, P.M.; Le Sage, C.; Schrier, M.; Gillis, A.J.; Stoop, H.; Nagel, R.; Liu, Y.-P.; van Duijse, J.; Drost, J.; Griekspoor, A.; et al. A Genetic Screen Implicates miRNA-372 and miRNA-373 As Oncogenes in Testicular Germ Cell Tumors. Cell 2006, 124, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.M.; Stoop, H.J.; Hersmus, R.; Oosterhuis, J.W.; Sun, Y.; Chen, C.; Guenther, S.; Sherlock, J.; Veltman, I.; Baeten, J.; et al. High-throughput microRNAome analysis in human germ cell tumours. J. Pathol. 2007, 213, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, Y.; Coukos, G.; Zhang, L. Therapeutic MicroRNA Strategies in Human Cancer. AAPS J. 2009, 11, 747–757. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbu, M.G.; Thompson, D.C.; Suciu, N.; Voinea, S.C.; Cretoiu, D.; Predescu, D.V. The Roles of MicroRNAs in Male Infertility. Int. J. Mol. Sci. 2021, 22, 2910. https://doi.org/10.3390/ijms22062910
Barbu MG, Thompson DC, Suciu N, Voinea SC, Cretoiu D, Predescu DV. The Roles of MicroRNAs in Male Infertility. International Journal of Molecular Sciences. 2021; 22(6):2910. https://doi.org/10.3390/ijms22062910
Chicago/Turabian StyleBarbu, Madalina Gabriela, Dana Claudia Thompson, Nicolae Suciu, Silviu Cristian Voinea, Dragos Cretoiu, and Dragos Valentin Predescu. 2021. "The Roles of MicroRNAs in Male Infertility" International Journal of Molecular Sciences 22, no. 6: 2910. https://doi.org/10.3390/ijms22062910
APA StyleBarbu, M. G., Thompson, D. C., Suciu, N., Voinea, S. C., Cretoiu, D., & Predescu, D. V. (2021). The Roles of MicroRNAs in Male Infertility. International Journal of Molecular Sciences, 22(6), 2910. https://doi.org/10.3390/ijms22062910







