Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis
Abstract
1. Introduction
1.1. Breast Cancer Bone Metastasis
1.2. Interleukins
1.3. Role of Interleukins in Physiological Bone Remodeling
1.4. ILs and Breast Cancer Bone Metastasis
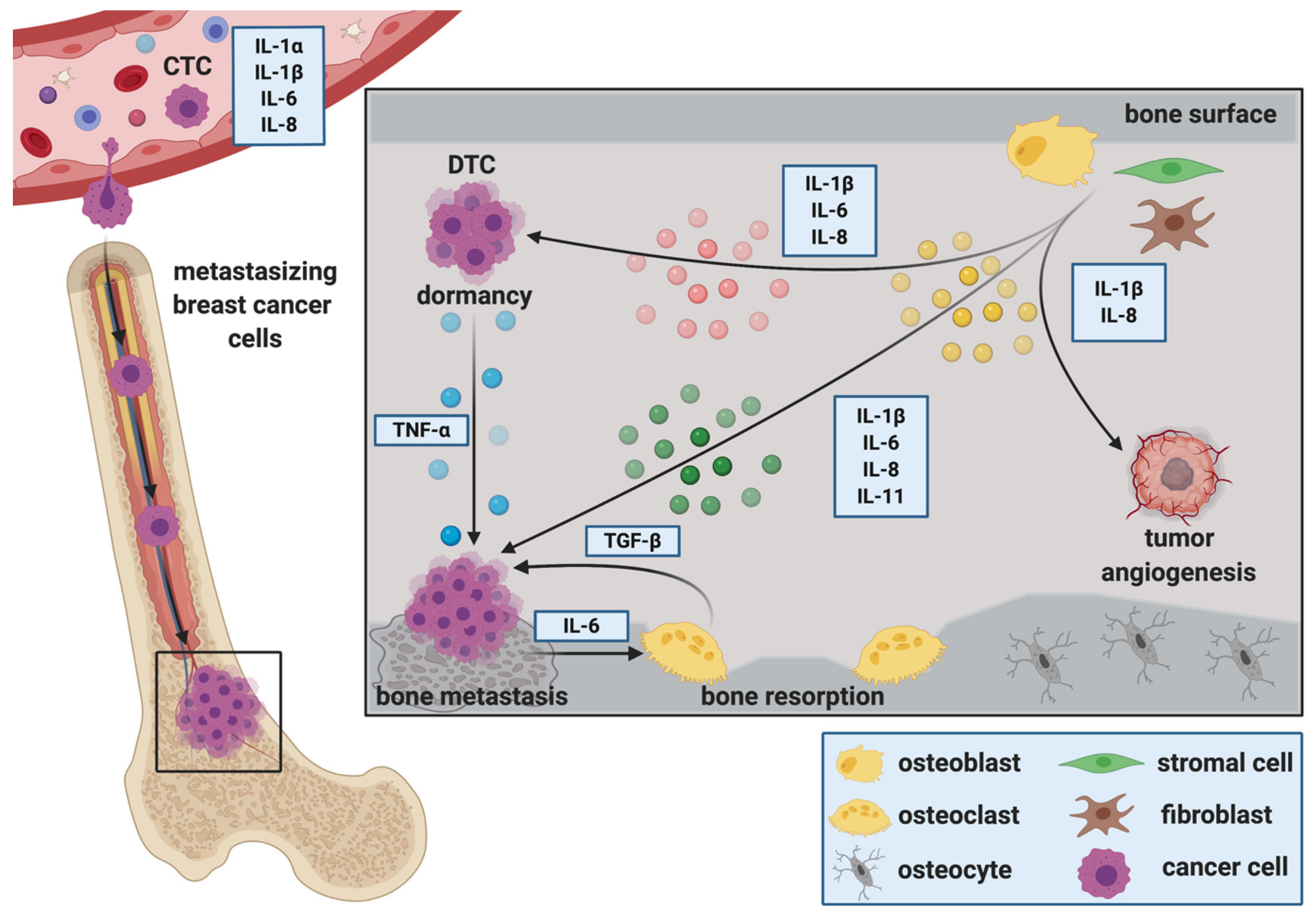
2. The Role of ILs during the Establishment and Progression of Bone Metastasis
2.1. Cancer Stem Cells, Circulating Tumor Cells and IL-Mediated Attraction to the Bone Metastatic Niche
2.2. The Role of ILs during the Extravasation of DTCs at the Metastatic Site
2.3. ILs during Migration, Invasion and Epithelial to Mesenchymal Transition (EMT) at the Metastatic Site
2.4. ILs as Regulators of DTC Dormancy at the Metastatic Site
2.5. ILs and Breast Cancer Colonization in Bone, Metastatic Outgrowth and the Creation of a Metastasis—Supporting Environment
2.5.1. IL-1ß
2.5.2. IL-6
2.5.3. IL-8
2.5.4. IL-11
3. Other ILs in Breast Cancer Bone Metastasis
4. Conclusions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Käkönen, S.M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Peyruchaud, O. Mechanisms of bone metastasis formation. J. Soc. Biol. 2007, 201, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited-The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Haider, M.T.; Smit, D.J.; Taipaleenmäki, H. The Endosteal Niche in Breast Cancer Bone Metastasis. Front. Oncol. 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Zarrer, J.; Haider, M.-T.; Smit, D.J.; Taipaleenmäki, H. Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment. Biomolecules 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.C.; Leal, A.C.; Gonçalves-Silva, T.; Mercadante, A.C.T.; Kestelman, F.; Chaves, S.B.; Azevedo, R.B.; Monteiro, J.P.; Bonomo, A. T Cells Induce Pre-Metastatic Osteolytic Disease and Help Bone Metastases Establishment in a Mouse Model of Metastatic Breast Cancer. PLoS ONE 2013, 8, e68171. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-X.; Evans, B.A.J.; Jiang, W.G. New Roles of Osteocytes in Proliferation, Migration and Invasion of Breast and Prostate Cancer Cells. Anticancer Res. 2016, 36, 1193–1201. [Google Scholar] [PubMed]
- Ma, Y.H.V.; Xu, L.; Mei, X.; Middleton, K.; You, L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell. Biochem. 2019, 120, 7590–7601. [Google Scholar] [CrossRef]
- Templeton, Z.S.; Lie, W.R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; Maloney, W.J.; Contag, C.H.; et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia (United States) 2015, 17, 849–861. [Google Scholar] [CrossRef]
- Allocca, G.; Hughes, R.; Wang, N.; Brown, H.K.; Ottewell, P.D.; Brown, N.J.; Holen, I. The bone metastasis niche in breast cancer-potential overlap with the haematopoietic stem cell niche in vivo. J. Bone Oncol. 2019, 17, 100244. [Google Scholar] [CrossRef]
- Dinarello, C.A. The history of fever, leukocytic pyrogen and interleukin-1. Temperature 2015, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P.; Stow, J.L. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Zheng, Y.; Chow, S.O.; Boernert, K.; Basel, D.; Mikuscheva, A.; Kim, S.; Fong-Yee, C.; Trivedi, T.; Buttgereit, F.; Sutherland, R.L.; et al. Direct crosstalk between cancer and osteoblast lineage cells fuels metastatic growth in bone via auto-amplification of IL-6 and RANKL signaling pathways. J. Bone Miner. Res. 2014, 29, 1938–1949. [Google Scholar] [CrossRef]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C.; Hirao, M.; Okamoto, M.; Koizumi, K.; Morimoto, T.; Yoshikawa, H.; Hashimoto, J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, B.; Cappariello, A.; Del Fattore, A.; Rucci, N.; De Benedetti, F.; Teti, A. C-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat. Commun. 2012, 3, 630. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Tatro, J.M.; Lowe, R.; Goldberg, V.M.; Greenfield, E.M. Comparison of the roles of IL-1, IL-6, and TNFα in cell culture and murine models of aseptic loosening. Bone 2007, 40, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Storci, G.; Tavolari, S.; Guarnieri, T.; Giovannini, C.; Taffurelli, M.; Ceccarelli, C.; Santini, D.; Paterini, P.; Marcu, K.B.; et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J. Clin. Investig. 2007, 117, 3988–4002. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Vosshenrich, C.A.J.; Di Santo, J.P. Interleukin signaling. Curr. Biol. 2002, 12, R760–R763. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. The bone remodeling compartment: A circulatory function for bone lining cells. J. Bone Miner. Res. 2001, 16, 1583–1585. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Kurihara, N.; Civin, C.; Roodman, G.D. Osteotropic factor responsiveness of highly purified populations of early and late precursors for human multinucleated cells expressing the osteoclast phenotype. J. Bone Miner. Res. 1991, 6, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Girasole, G.; Passeri, G.; Jilka, R.L.; Manolagas, S.C. Interleukin-11: A new cytokine critical for osteoclast development. J. Clin. Investig. 1994, 93, 1516–1524. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Li, Y.; Bäckesjö, C.M.; Haldosén, L.A.; Lindgren, U. IL-6 receptor expression and IL-6 effects change during osteoblast differentiation. Cytokine 2008, 43, 165–173. [Google Scholar] [CrossRef]
- Lui, E.L.H.; Ao, C.K.L.; Li, L.; Khong, M.L.; Tanner, J.A. Inorganic polyphosphate triggers upregulation of interleukin 11 in human osteoblast-like SaOS-2 cells. Biochem. Biophys. Res. Commun. 2016, 479, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Silfverswärd, C.J.; Frost, A.; Brändström, H.; Nilsson, O.; Ljunggren, Ö. Interleukin-4 and interleukin-13 potentiate interleukin-1 induced secretion of interleukin-6 in human osteoblast-like cells. J. Orthop. Res. 2004, 22, 1058–1062. [Google Scholar] [CrossRef]
- Malysheva, K.; de Rooij, K.; Löwik, C.W.G.M.; Baeten, D.L.; Rose-John, S.; Stoika, R.; Korchynskyi, O. Interleukin 6/Wnt interactions in rheumatoid arthritis: Interleukin 6 inhibits Wnt signaling in synovial fibroblasts and osteoblasts. Croat. Med. J. 2016, 57, 89–98. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Ichinose, S.; Akiyama, M.; Kasahara, Y.; Tachikawa, N.; Nakahama, K.I. Involvement of CX3CL1 in the Migration of Osteoclast Precursors Across Osteoblast Layer Stimulated by Interleukin-1ß. J. Cell. Physiol. 2017, 232, 1739–1745. [Google Scholar] [CrossRef]
- Liu, X.H.; Kirschenbaum, A.; Yao, S.; Levine, A.C. Cross-talk between the interleukin-6 and prostaglandin E2 signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-κB (RANK) ligand/RANK system. Endocrinology 2005, 146, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ma, Q.; Ding, N.; Luo, F.; Bai, Y.; Kang, F.; Gong, X.; Dong, R.; Dai, J.; Dai, Q.; et al. IL-11 is essential in promoting osteolysis in breast cancer bone metastasis via RANKL-independent activation of osteoclastogenesis. Cell Death Dis. 2019, 10, 353. [Google Scholar] [CrossRef]
- Lin, F.H.; Chang, J.B.; McGuire, M.H.; Yee, J.A.; Brigman, B.E. Biphasic effects of interleukin-1β on osteoblast differentiation in vitro. J. Orthop. Res. 2010, 28, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Nakayama, Y.; Takai, H.; Ogata, Y. Effects of interleukin-11 on the expression of human bone sialoprotein gene. J. Bone Miner. Metab. 2015, 33, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y.; Miyaura, C.; Jin, C.H.; Akatsu, T.; Abe, E.; Nakamura, Y.; Yamaguchi, A.; Yoshiki, S.; Matsuda, T.; Hirano, T. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 1990, 145, 3297–3303. [Google Scholar] [PubMed]
- Franchimont, N.; Rydziel, S.; Canalis, E. Transforming growth factor-β increases interleukin-6 transcripts in osteoblasts. Bone 2000, 26, 249–253. [Google Scholar] [CrossRef]
- Srirangan, S.; Choy, E.H. The role of Interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef]
- Nakamura, I.; Jimi, E. Regulation of Osteoclast Differentiation and Function by Interleukin-1. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2006; Volume 74, pp. 357–370. ISBN 0127098747. [Google Scholar] [CrossRef]
- Salamanna, F.; Borsari, V.; Brogini, S.; Torricelli, P.; Cepollaro, S.; Cadossi, M.; Fini, M. A Human 3D In Vitro Model to Assess the Relationship Between Osteoporosis and Dissemination to Bone of Breast Cancer Tumor Cells. J. Cell. Physiol. 2017, 232, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Clines, G.A.; Guise, T.A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 2008, 10, 1. [Google Scholar] [CrossRef]
- Ren, L.; Wang, X.; Dong, Z.; Liu, J.; Zhang, S. Bone metastasis from breast cancer involves elevated IL-11 expression and the gp130/STAT3 pathway. Med. Oncol. 2013, 30, 634. [Google Scholar] [CrossRef]
- Kamalakar, A.; Bendre, M.S.; Washam, C.L.; Fowler, T.W.; Carver, A.; Dilley, J.D.; Bracey, J.W.; Akel, N.S.; Margulies, A.G.; Skinner, R.A.; et al. Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone 2014, 61, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J.; Adachi, I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999, 19, 1427–1432. [Google Scholar]
- Salgado, R.; Junius, S.; Benoy, I.; Van Dam, P.; Vermeulen, P.; Van Marck, E.; Huget, P.; Dirix, L.Y. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int. J. Cancer 2003, 103, 642–646. [Google Scholar] [CrossRef]
- Tulotta, C.; Lefley, D.V.; Freeman, K.; Gregory, W.M.; Hanby, A.M.; Heath, P.R.; Nutter, F.; Mark Wilkinson, J.; Spicer-Hadlington, A.R.; Liu, X.; et al. Endogenous production of IL1B by breast cancer cells drives metastasis and colonization of the bone microenvironment. Clin. Cancer Res. 2019, 25, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Nutter, F.; Holen, I.; Brown, H.K.; Cross, S.S.; Alyson Evans, C.; Walker, M.; Coleman, R.E.; Westbrook, J.A.; Selby, P.J.; Brown, J.E.; et al. Different molecular profiles are associated with breast cancer cell homing compared with colonisation of bone: Evidence using a novel bone-seeking cell line. Endocr. Relat. Cancer 2014, 21, 327–341. [Google Scholar] [CrossRef]
- De Larco, J.E.; Wuertz, B.R.K.; Rosner, K.A.; Erickson, S.A.; Gamache, D.E.; Manivel, J.C.; Furcht, L.T. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am. J. Pathol. 2001, 158, 639–646. [Google Scholar] [CrossRef]
- Bendre, M.S.; Gaddy-Kurten, D.; Mon-Foote, T.; Akel, N.S.; Skinner, R.A.; Nicholas, R.W.; Suva, L.J. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002, 62, 5571–5579. [Google Scholar] [PubMed]
- Remeniuk, B.; King, T.; Sukhtankar, D.; Nippert, A.; Li, N.; Li, F.; Cheng, K.; Rice, K.C.; Porreca, F. Disease modifying actions of interleukin-6 blockade in a rat model of bone cancer pain. Pain 2018, 159, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef]
- Al-Ejeh, F.; Smart, C.E.; Morrison, B.J.; Chenevix-Trench, G.; López, J.A.; Lakhani, S.R.; Brown, M.P.; Khanna, K.K. Breast cancer stem cells: Treatment resistance and therapeutic opportunities. Carcinogenesis 2011, 32, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Eyre, R.; Alférez, D.G.; Santiago-Gómez, A.; Spence, K.; McConnell, J.C.; Hart, C.; Simões, B.M.; Lefley, D.; Tulotta, C.; Storer, J.; et al. Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat. Commun. 2019, 10, 5016. [Google Scholar] [CrossRef]
- Sarmiento-Castro, A.; Caamaño-Gutiérrez, E.; Sims, A.H.; Hull, N.J.; James, M.I.; Santiago-Gómez, A.; Eyre, R.; Clark, C.; Brown, M.E.; Brooks, M.D.; et al. Increased Expression of Interleukin-1 Receptor Characterizes Anti-estrogen-Resistant ALDH+ Breast Cancer Stem Cells. Stem Cell Rep. 2020, 15, 307–316. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol. Cancer 2017, 16, 57. [Google Scholar] [CrossRef]
- Sansone, P.; Ceccarelli, C.; Berishaj, M.; Chang, Q.; Rajasekhar, V.K.; Perna, F.; Bowman, R.L.; Vidone, M.; Daly, L.; Nnoli, J.; et al. Self-renewal of CD133hi cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat. Commun. 2016, 7, 10442. [Google Scholar] [CrossRef]
- Xie, G.; Yao, Q.; Liu, Y.; Du, S.; Liu, A.; Guo, Z.; Sun, A.; Ruan, J.; Chen, L.; Ye, C.; et al. IL-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int. J. Oncol. 2012, 40, 1171–1179. [Google Scholar] [CrossRef]
- Bernfield, M.; Götte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Greve, B.; Pavao, M.S.G.; Kiesel, L.; Ibrahim, S.A.; Götte, M. Syndecan-1 modulates β-integrin-dependent and interleukin-6-dependent functions in breast cancer cell adhesion, migration, and resistance to irradiation. FEBS J. 2013, 280, 2216–2227. [Google Scholar] [CrossRef]
- Singh, J.K.; Farnie, G.; Bundred, N.J.; Simões, B.M.; Shergill, A.; Landberg, G.; Howell, S.J.; Clarke, R.B. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin. Cancer Res. 2013, 19, 643–656. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Wicinski, J.; Cervera, N.; Finetti, P.; Hur, M.H.; Diebel, M.E.; Monville, F.; Dutcher, J.; et al. Breast cancer cell lines contain functional cancer stem sells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009, 69, 1302–1313. [Google Scholar] [CrossRef]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D.; et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Patanè, S.; Grange, C.; Bussolati, B.; Isella, C.; Fontani, L.; Godio, L.; Cilli, M.; D’Amelio, P.; Isaia, G.; et al. Primary breast cancer stem-like cells metastasise to bone, switch phenotype and acquire a bone tropism signature. Br. J. Cancer 2013, 108, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Toloudi, M.; Apostolou, P.; Chatziioannou, M.; Papasotiriou, I. Correlation between cancer stem cells and circulating tumor cells and their value. Case Rep. Oncol. 2011, 4, 44–54. [Google Scholar] [CrossRef]
- Koch, C.; Kuske, A.; Joosse, S.A.; Yigit, G.; Sflomos, G.; Thaler, S.; Smit, D.J.; Werner, S.; Borgmann, K.; Gärtner, S.; et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol. Med. 2020, 12, e11908. [Google Scholar] [CrossRef]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.M.; Bertucci, F.; Jacquemier, J.; et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.R.; Jin, W.L.; Lou, G. Circulating tumor cells in precision oncology: Clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019, 19, 341. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef]
- Smit, D.J.; Cayrefourcq, L.; Haider, M.T.; Hinz, N.; Pantel, K.; Alix-Panabières, C.; Jücker, M. High Sensitivity of Circulating Tumor Cells Derived from a Colorectal Cancer Patient for Dual Inhibition with AKT and mTOR Inhibitors. Cells 2020, 9, 2129. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Dowling, R.J.O.; Ennis, M.; Amir, E.; Elser, C.; Brezden-Masley, C.; Vandenberg, T.; Lee, E.; Fazaee, K.; Stambolic, V.; et al. Association of metabolic, inflammatory, and tumor markers with circulating tumor cells in metastatic breast cancer. JNCI Cancer Spectr. 2018, 2, pky028. [Google Scholar] [CrossRef] [PubMed]
- Vilsmaier, T.; Rack, B.; König, A.; Friese, K.; Janni, W.; Jeschke, U.; Weissenbacher, T. Influence of circulating tumour cells on production of il-1, il-1 and il-12 in sera of patients with primary diagnosis of breast cancer before treatment. Anticancer Res. 2016, 36, 5227–5236. [Google Scholar] [CrossRef][Green Version]
- König, A.; Vilsmaier, T.; Rack, B.; Friese, K.; Janni, W.; Jeschke, U.; Andergassen, U.; Trapp, E.; Jückstock, J.; Jäger, B.; et al. Determination of interleukin-4,-5,-6,-8 and-13 in serum of patients with breast cancer before treatment and its correlation to circulating tumor cells. Anticancer Res. 2016, 36, 3123–3130. [Google Scholar]
- Zhang, Q.; Gerratana, L.; Flaum, L.; Shah, A.N.; Helfand, B.; Zhang, Y.; Gradishar, W.J.; Platanias, L.C.; Cristofanilli, M. Dynamic changes of interleukin 2 (IL-2) and circulating tumor cells (CTCs) in patients with advanced breast cancer (BCa) after systemic therapies. J. Clin. Oncol. 2018, 36, 1090. [Google Scholar] [CrossRef]
- Kim, M.Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.F.; Norton, L.; Massagué, J. Tumor Self-Seeding by Circulating Cancer Cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Mon, N.N.; Senga, T.; Ito, S. Interleukin-1β activates focal adhesion kinase and Src to induce matrix metalloproteinase-9 production and invasion of MCF-7 breast cancer cells. Oncol. Lett. 2017, 13, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Clément-Demange, L.; Mulcrone, P.L.; Tabarestani, T.Q.; Sterling, J.A.; Elefteriou, F. β2ARs stimulation in osteoblasts promotes breast cancer cell adhesion to bone marrow endothelial cells in an IL-1β and selectin-dependent manner. J. Bone Oncol. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Storr, S.J.; Safuan, S.; Ahmad, N.; El-Refaee, M.; Jackson, A.M.; Martin, S.G. Macrophage-derived interleukin-1beta promotes human breast cancer cell migration and lymphatic adhesion in vitro. Cancer Immunol. Immunother. 2017, 66, 1287–1294. [Google Scholar] [CrossRef]
- Vazquez Rodriguez, G.; Abrahamsson, A.; Jensen, L.D.E.; Dabrosin, C. Adipocytes Promote Early Steps of Breast Cancer Cell Dissemination via Interleukin-8. Front. Immunol. 2018, 9, 1767. [Google Scholar] [CrossRef]
- Chen, X.; Lu, J.; Ji, Y.; Hong, A.; Xie, Q. Cytokines in osteoblast-conditioned medium promote the migration of breast cancer cells. Tumor Biol. 2014, 35, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Studebaker, A.W.; Storci, G.; Werbeck, J.L.; Sansone, P.; Sasser, A.K.; Tavolari, S.; Huang, T.; Chan, M.W.Y.; Marini, F.C.; Roso, T.J.; et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res. 2008, 68, 9087–9095. [Google Scholar] [CrossRef]
- Queen, M.M.; Ryan, R.E.; Holzer, R.G.; Keller-Peck, C.R.; Jorcyk, C.L. Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Res. 2005, 65, 8896–8904. [Google Scholar] [CrossRef] [PubMed]
- Bolin, C.; Tawara, K.; Sutherland, C.; Redshaw, J.; Aranda, P.; Moselhy, J.; Anderson, R.; Jorcyk, C.L. Oncostatin M Promotes Mammary Tumor Metastasis to Bone and Osteolytic Bone Degradation. Genes Cancer 2012, 3, 117–130. [Google Scholar] [CrossRef]
- Oh, K.; Lee, O.Y.; Shon, S.Y.; Nam, O.; Ryu, P.M.; Seo, M.W.; Lee, D.S. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013, 15, R79. [Google Scholar] [CrossRef] [PubMed]
- Felipe Lima, J.; Nofech-Mozes, S.; Bayani, J.; Bartlett, J. EMT in Breast Carcinoma—A Review. J. Clin. Med. 2016, 5, 65. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011, 71, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Sosnoski, D.M.; Norgard, R.J.; Grove, C.D.; Foster, S.J.; Mastro, A.M. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin. Exp. Metastasis 2015, 32, 335–344. [Google Scholar] [CrossRef]
- Tivari, S.; Lu, H.; Dasgupta, T.; De Lorenzo, M.S.; Wieder, R. Reawakening of dormant estrogen-dependent human breast cancer cells by bone marrow stroma secretory senescence. Cell Commun. Signal. 2018, 16, 48. [Google Scholar] [CrossRef]
- Park, S.Y.; Nam, J.S. The force awakens: Metastatic dormant cancer cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef]
- Kinder, M.; Chislock, E.; Bussard, K.M.; Shuman, L.; Mastro, A.M. Metastatic breast cancer induces an osteoblast inflammatory response. Exp. Cell Res. 2008, 314, 173–183. [Google Scholar] [CrossRef]
- Wang, H.; Yu, C.; Gao, X.; Welte, T.; Muscarella, A.M.; Tian, L.; Zhao, H.; Zhao, Z.; Du, S.; Tao, J.; et al. The Osteogenic Niche Promotes Early-Stage Bone Colonization of Disseminated Breast Cancer Cells. Cancer Cell 2015, 27, 193–210. [Google Scholar] [CrossRef]
- Bussard, K.M.; Venzon, D.J.; Mastro, A.M. Osteoblasts are a major source of inflammatory cytokines in the tumor microenvironment of bone metastatic breast cancer. J. Cell. Biochem. 2010, 111, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.T.; Saito, H.; Zarrer, J.; Uzhunnumpuram, K.; Nagarajan, S.; Kari, V.; Horn-Glander, M.; Werner, S.; Hesse, E.; Taipaleenmäki, H. Breast cancer bone metastases are attenuated in a Tgif1-deficient bone microenvironment. Breast Cancer Res. 2020, 22. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.D.; Shupp, A.B.; Mukhopadhyay, D.; Marini, F.C.; Bussard, K.M. Osteoblasts are “educated” by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Okita, N.; Sharkey, N.; Neuberger, T.; Webb, A.; Mastro, A.M. Localization of osteoblast inflammatory cytokines MCP-1 and VEGF to the matrix of the trabecula of the femur, a target area for metastatic breast cancer cell colonization. Clin. Exp. Metastasis 2010, 27, 331–340. [Google Scholar] [CrossRef]
- Romero-Moreno, R.; Curtis, K.J.; Coughlin, T.R.; Cristina Miranda-Vergara, M.; Dutta, S.; Natarajan, A.; Facchine, B.A.; Jackson, K.M.; Nystrom, L.; Li, J.; et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat. Commun. 2019, 10, 4404. [Google Scholar] [CrossRef]
- Johnson, R.W.; Finger, E.C.; Olcina, M.M.; Vilalta, M.; Aguilera, T.; Miao, Y.; Merkel, A.R.; Johnson, J.R.; Sterling, J.A.; Wu, J.Y.; et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol. 2016, 18, 1078–1089. [Google Scholar] [CrossRef]
- Sims, N.A.; Johnson, R.W. Leukemia inhibitory factor: A paracrine mediator of bone metabolism. Growth Factors 2012, 30, 76–87. [Google Scholar] [CrossRef]
- Khazali, A.S.; Clark, A.M.; Wells, A. Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. Br. J. Cancer 2018, 118, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Szmulewitz, R.; Taylor, J.; Rinker-Schaffer, C. Metastatic Colonization. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 2271–2273. ISBN 978-3-642-16483-5. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef]
- Lefley, D.; Howard, F.; Arshad, F.; Bradbury, S.; Brown, H.; Tulotta, C.; Eyre, R.; Alférez, D.; Wilkinson, J.M.; Holen, I.; et al. Development of clinically relevant in vivo metastasis models using human bone discs and breast cancer patient-derived xenografts. Breast Cancer Res. 2019, 21, 130. [Google Scholar] [CrossRef]
- Zhao, C.; Cai, X.; Wang, Y.; Wang, D.; Wang, T.; Gong, H.; Sun, H.; Jia, Q.; Zhou, W.; Wu, Z.; et al. Original Article NAT1 promotes osteolytic metastasis in luminal breast cancer by regulating the bone metastatic niche via NF-κB/IL-1B signaling pathway. Am. J. Cancer Res. 2020, 10, 2464–2479. [Google Scholar]
- Sasser, A.K.; Sullivan, N.J.; Studebaker, A.W.; Hendey, L.F.; Axel, A.E.; Hall, B.M. Interleukin-6 is a potent growth factor for ER-α-positive human breast cancer. FASEB J. 2007, 21, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Rajski, M.; Vogel, B.; Baty, F.; Rochlitz, C.; Buess, M. Global gene expression analysis of the interaction between cancer cells and osteoblasts to predict bone metastasis in breast cancer. PLoS ONE 2012, 7, e29743. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Shuman, L.A.; Sosnoski, D.M.; Dhurjati, R.; Vogler, E.A.; Mastro, A.M. Dynamic interaction between breast cancer cells and osteoblastic tissue: Comparison of Two- and Three-dimensional cultures. J. Cell. Physiol. 2011, 226, 2150–2158. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Hamaguchi, T.; Nagao, N.; Kato, S.; Iino, T.; Nakamura, T.; Sudo, A. Interleukin-6 receptor inhibitor suppresses bone metastases in a breast cancer cell line. Breast Cancer 2018, 25, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fu, Y.; Loza, A.J.; Murali, B.; Leahy, K.M.; Ruhland, M.K.; Gang, M.; Su, X.; Zamani, A.; Shi, Y.; et al. Stromal-Initiated Changes in the Bone Promote Metastatic Niche Development. Cell Rep. 2016, 14, 82–92. [Google Scholar] [CrossRef]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-Derived Jagged1 Promotes Osteolytic Bone Metastasis of Breast Cancer by Engaging Notch Signaling in Bone Cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Pederson, L.; Winding, B.; Foged, N.T.; Spelsberg, T.C.; Oursler, M.J. Identification of breast cancer cell line-derived paracrine factors that stimulate osteoclast activity. Cancer Res. 1999, 59, 5849–5855. [Google Scholar] [PubMed]
- Shin, E.; Koo, J.S. The role of adipokines and bone marrow adipocytes in breast cancer bone metastasis. Int. J. Mol. Sci. 2020, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Margulies, A.G.; Walser, B.; Akel, N.S.; Bhattacharrya, S.; Skinner, R.A.; Swain, F.; Ramani, V.; Mohammad, K.S.; Wessner, L.L.; et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-κB ligand pathway. Cancer Res. 2005, 65, 11001–11009. [Google Scholar] [CrossRef]
- Fong, Y.C.; Maa, M.C.; Tsai, F.J.; Chen, W.C.; Lin, J.G.; Jeng, L.B.; Yang, R.S.; Fu, W.M.; Tang, C.H. Osteoblast-derived TGF-β1 stimulates IL-8 release through AP-1 and NF-κB in human cancer cells. J. Bone Miner. Res. 2008, 23, 961–970. [Google Scholar] [CrossRef]
- Yang, Y.H.; Buhamrah, A.; Schneider, A.; Lin, Y.L.; Zhou, H.; Bugshan, A.; Basile, J.R. Semaphorin 4D promotes skeletal metastasis in breast cancer. PLoS ONE 2016, 11, e0150151. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Miller, L.J.; Kurtzman, S.H.; Wang, Y.; Anderson, K.H.; Lindquist, R.R.; Kreutzer, D.L. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998, 18, 77–81. [Google Scholar]
- Strieter, R.M.; Kunkel, S.L.; Elner, V.M.; Martonyi, C.L.; Koch, A.E.; Polverini, P.J.; Elner, S.G. Interleukin-8: A corneal factor that induces neovascularization. Am. J. Pathol. 1992, 141, 1279–1284. [Google Scholar] [PubMed]
- Morinaga, Y.; Fujtta, N.; Ohishi, K.; Tsuruo, T. Stimulation of interleukin-11 production from osteoblast-like cells by transforming growth factor-β and tumor cell factors. Int. J. Cancer 1997, 71, 422–428. [Google Scholar] [CrossRef]
- Morinaga, Y.; Fujita, N.; Ohishi, K.; Zhang, Y.; Tsuruo, T. Suppression of interleukin-11-mediated bone resorption by cyclooxygenases inhibitors. J. Cell. Physiol. 1998, 175, 247–254. [Google Scholar] [CrossRef]
- McCoy, E.M.; Hong, H.; Pruitt, H.C.; Feng, X. IL-11 produced by breast cancer cells augments osteoclastogenesis by sustaining the pool of osteoclast progenitor cells. BMC Cancer 2013, 13, 16. [Google Scholar] [CrossRef]
- Cai, W.L.; Huang, W.D.; Li, B.; Chen, T.R.; Li, Z.X.; Zhao, C.L.; Li, H.Y.; Wu, Y.M.; Yan, W.J.; Xiao, J.R. microRNA-124 inhibits bone metastasis of breast cancer by repressing Interleukin-11. Mol. Cancer 2018, 17, 9. [Google Scholar] [CrossRef]
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.M.; Curé, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456. [Google Scholar] [CrossRef]
- Mombelli, S.; Cochaud, S.; Merrouche, Y.; Garbar, C.; Antonicelli, F.; Laprevotte, E.; Alberici, G.; Bonnefoy, N.; Eliaou, J.F.; Bastid, J.; et al. IL-17A and its homologs IL-25/IL-17E recruit the c-RAF/S6 kinase pathway and the generation of pro-oncogenic LMW-E in breast cancer cells. Sci. Rep. 2015, 5, 11874. [Google Scholar] [CrossRef]
- Das Roy, L.; Pathangey, L.B.; Tinder, T.L.; Schettini, J.L.; Gruber, H.E.; Mukherjee, P. Breast cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Res. 2009, 11, R56. [Google Scholar] [CrossRef]
- Roy, L.D.; Sahraei, M.; Schettini, J.L.; Gruber, H.E.; Besmer, D.M.; Mukherjee, P. Systemic neutralization of IL-17A significantly reduces breast cancer associated metastasis in arthritic mice by reducing CXCL12/SDF-1 expression in the metastatic niches. BMC Cancer 2014, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Hsing, C.-H.; Li, C.-F.; Chan, C.-H.; Chang, M.-C.; Yan, J.-J.; Chang, M.-S. Anti–IL-20 Monoclonal Antibody Suppresses Breast Cancer Progression and Bone Osteolysis in Murine Models. J. Immunol. 2012, 188, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
| Receptor Family | Interleukins |
|---|---|
| Type I cytokine receptor family | IL-12 family: IL-12, IL-23, IL-27, IL-30, IL-35 Glycoprotein gp130/IL-6 family: IL-6, IL-11, IL-31 β chain cytokine family: IL-3, IL-5 γ chain cytokine family: IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, IL-21 others: IL-16, IL-32, IL-34 |
| Type II cytokine receptor family | IL-10 family: IL-10 IL-20 subfamily: IL-19, IL-20, IL-22, IL-24, IL-26 Type III interferons (IFNs): IL-28A, IL-28B, IL-29 |
| IL-1/Toll like receptor | IL-1 family: IL-1α, IL-1β, IL-1Ra, IL-18, IL-33, IL-36α, IL-36β, IL-36γ, IL-36Ra, IL-37, IL-38 |
| Others | IL-14 IL-17 family: IL-17A-F, IL-25 (IL-17E) CXC chemokine family: IL-8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, M.-T.; Ridlmaier, N.; Smit, D.J.; Taipaleenmäki, H. Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2021, 22, 2898. https://doi.org/10.3390/ijms22062898
Haider M-T, Ridlmaier N, Smit DJ, Taipaleenmäki H. Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. International Journal of Molecular Sciences. 2021; 22(6):2898. https://doi.org/10.3390/ijms22062898
Chicago/Turabian StyleHaider, Marie-Therese, Nicole Ridlmaier, Daniel J. Smit, and Hanna Taipaleenmäki. 2021. "Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis" International Journal of Molecular Sciences 22, no. 6: 2898. https://doi.org/10.3390/ijms22062898
APA StyleHaider, M.-T., Ridlmaier, N., Smit, D. J., & Taipaleenmäki, H. (2021). Interleukins as Mediators of the Tumor Cell—Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. International Journal of Molecular Sciences, 22(6), 2898. https://doi.org/10.3390/ijms22062898






