Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes
Abstract
1. Introduction
2. Results
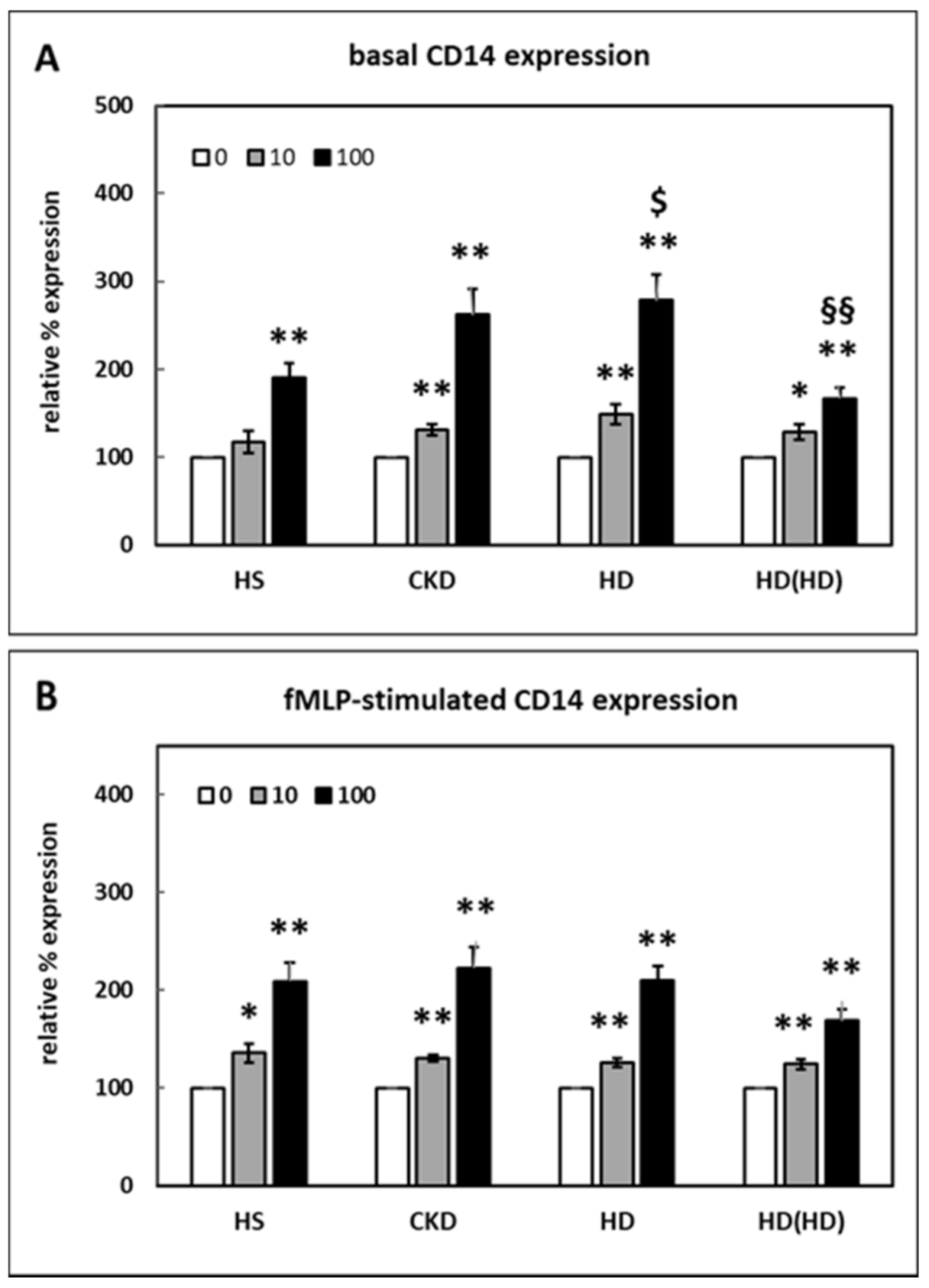
2.1. Effect of HDL on CD14 Surface Expression
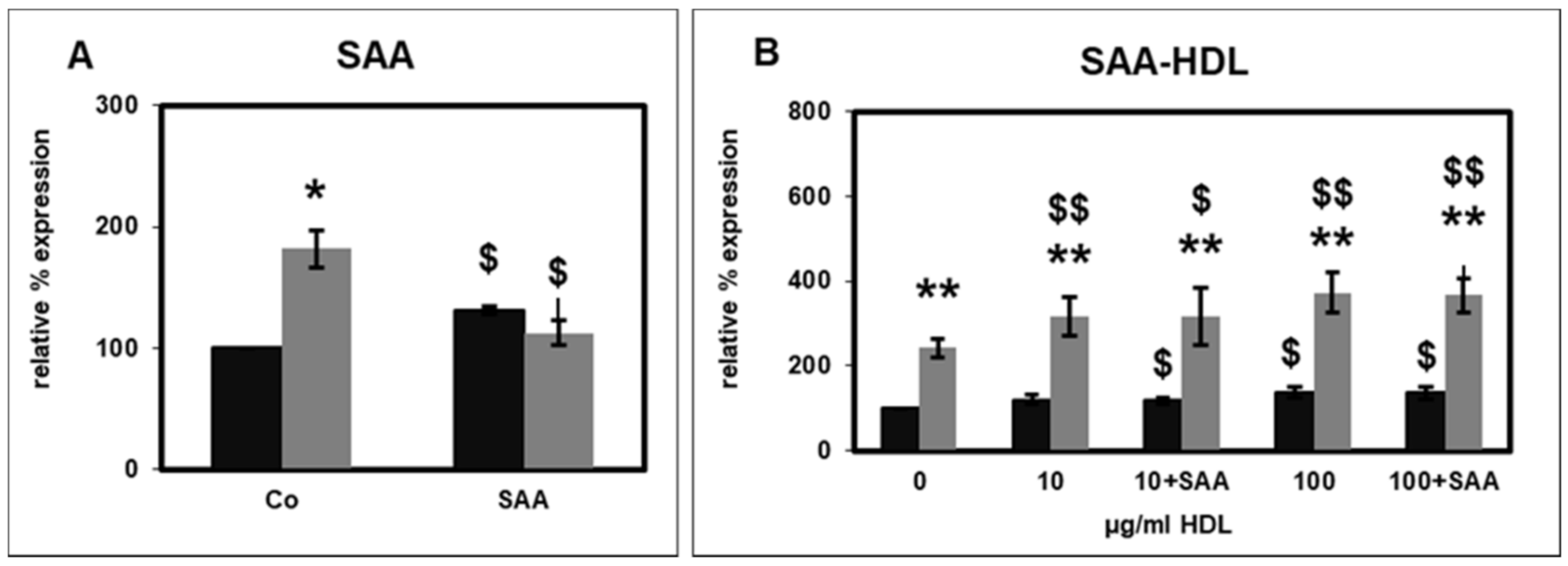
2.2. Effect of SAA on CD14 Surface Expression
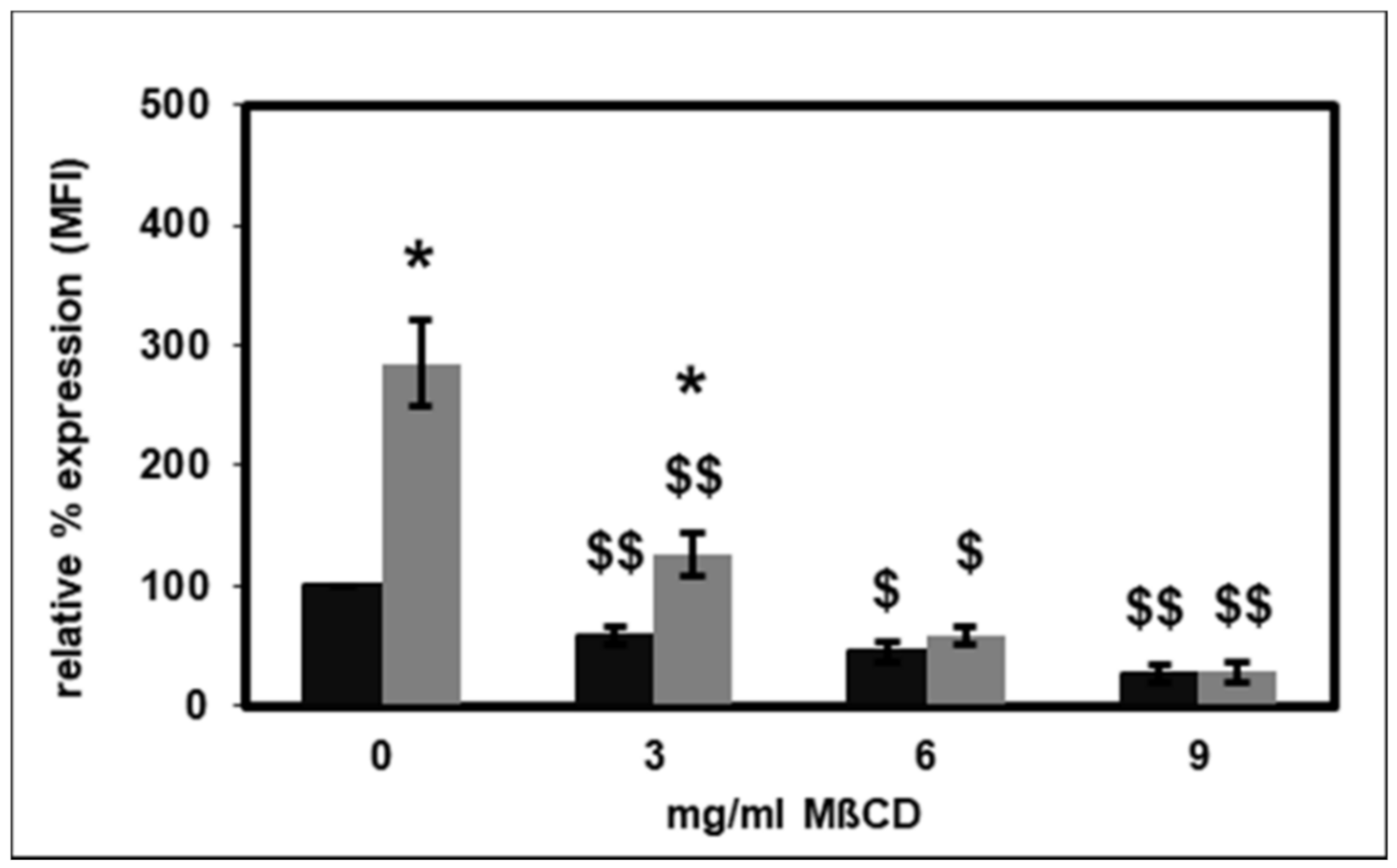
2.3. Effect of Lipid Raft Disruption on CD14 Surface Expression
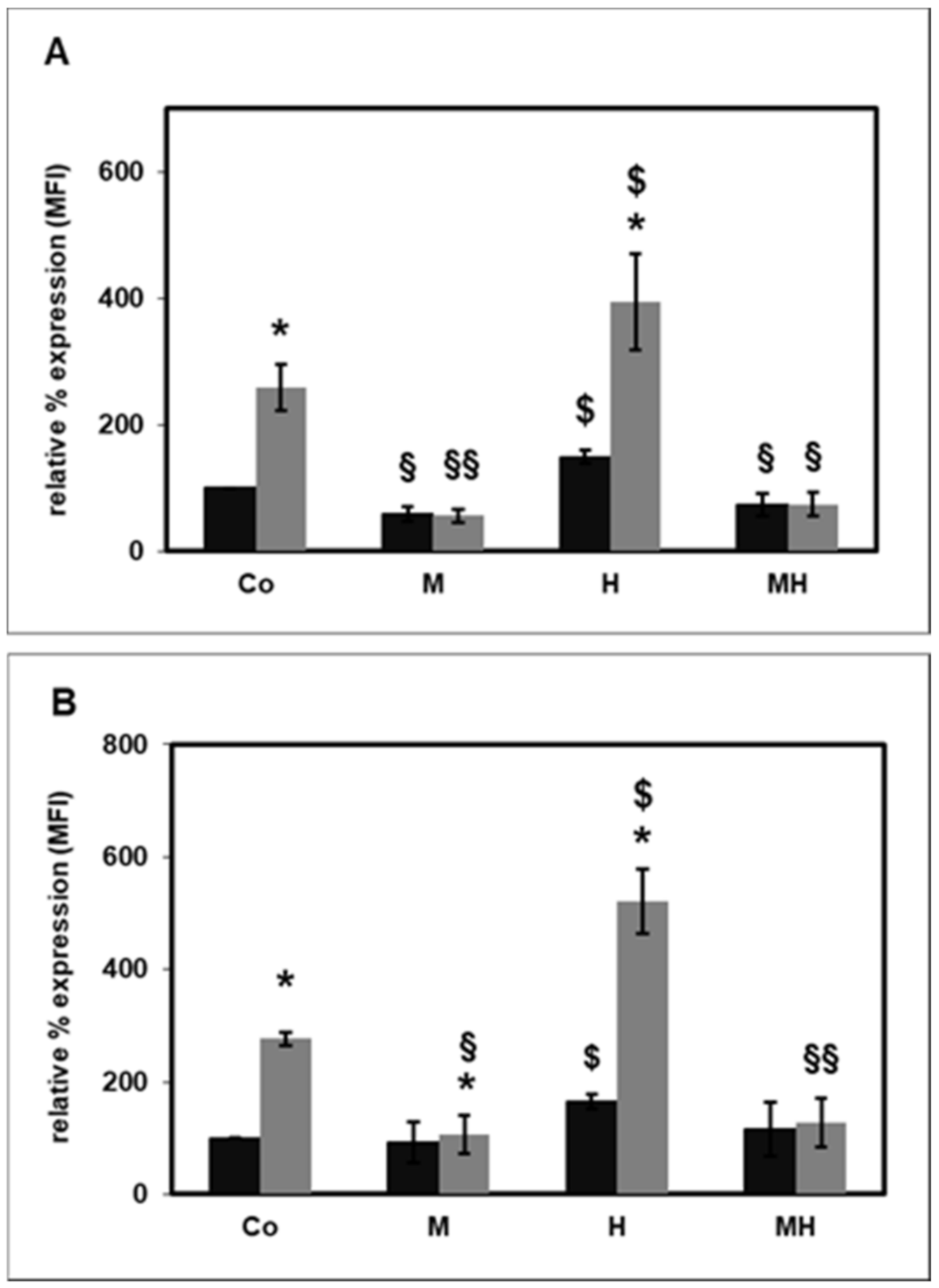
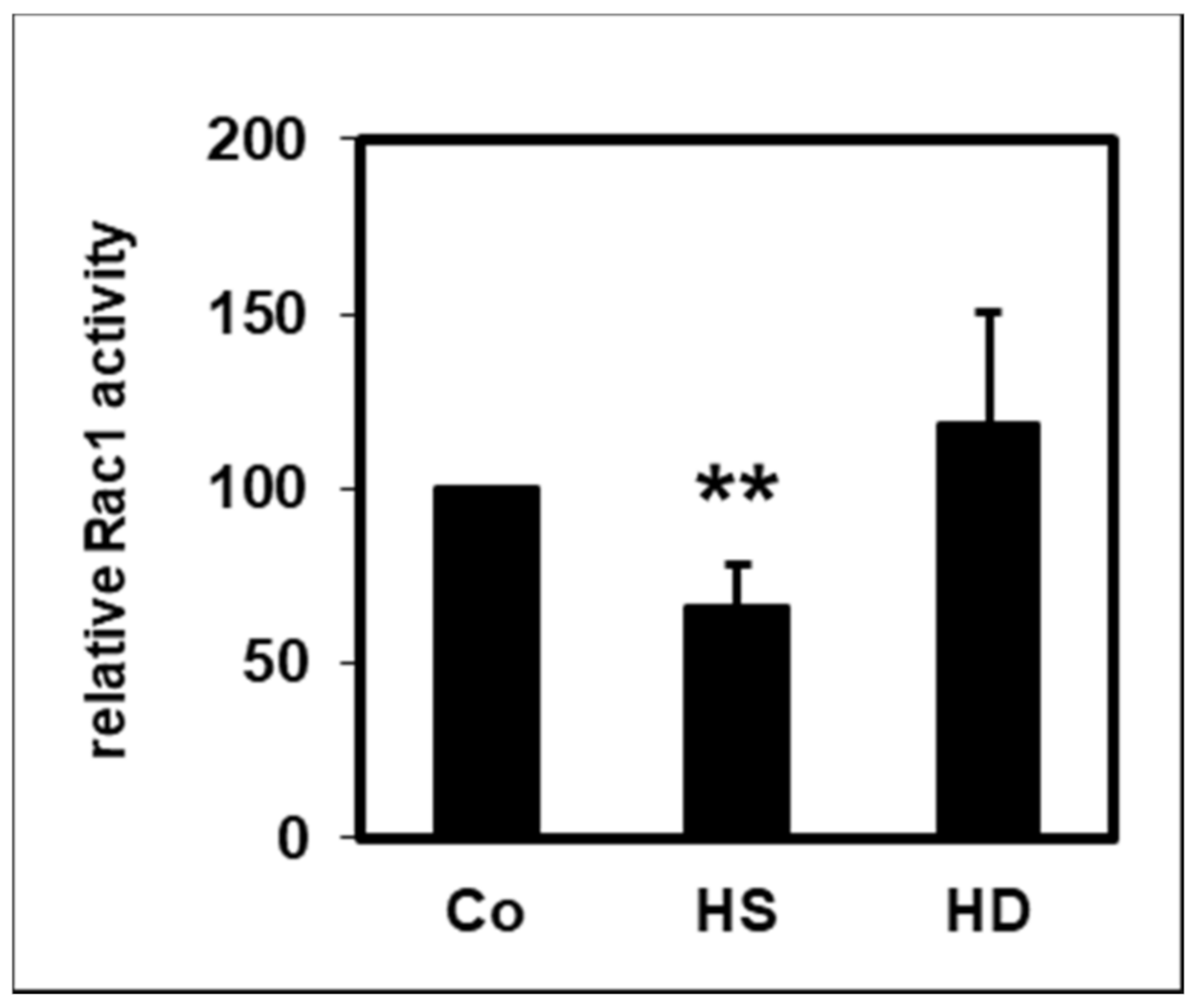
2.4. Effect of HDL on Rac1 Activity
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. High-Density Lipoprotein Isolation
4.3. Isolation of Polymorphonuclear Leukocytes
4.4. Surface CD14 Expression
4.5. Lipid Raft Disintegration
4.6. Rac1 Activity
4.7. Statistical Analysis
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef]
- Creasy, K.T.; Kane, J.P.; Malloy, M.J. Emerging roles of HDL in immune function. Curr. Opin. Lipidol. 2018, 29, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Pirillo, A.; Ammirati, E.; Catapano, A.L. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 2012, 220, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2018, 26, 1644–1664. [Google Scholar] [CrossRef]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Marsche, G.; Saemann, M.D.; Heinemann, A.; Holzer, M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol. Ther. 2013, 137, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Saemann, M.D.; Poglitsch, M.; Kopecky, C.; Haidinger, M.; Horl, W.H.; Weichhart, T. The versatility of HDL: A crucial anti-inflammatory regulator. Eur. J. Clin. Investig. 2010, 40, 1131–1143. [Google Scholar] [CrossRef]
- Tolle, M.; Huang, T.; Schuchardt, M.; Jankowski, V.; Prufer, N.; Jankowski, J.; Tietge, U.J.; Zidek, W.; Van Der Giet, M. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc. Res. 2012, 94, 154–162. [Google Scholar] [CrossRef]
- Weichhart, T.; Kopecky, C.; Kubicek, M.; Haidinger, M.; Doller, D.; Katholnig, K.; Suarna, C.; Eller, P.; Tolle, M.; Gerner, C.; et al. Serum Amyloid A in Uremic HDL Promotes Inflammation. J. Am. Soc. Nephrol. 2012, 23, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Haag-Weber, M.; Hörl, W.H. Dysfunction of polymorphonuclear leukocytes in uremia. Semin. Nephrol. 1996, 16, 192–201. [Google Scholar] [PubMed]
- Raupachova, J.; Kopecky, C.; Cohen, G. High-Density Lipoprotein from Chronic Kidney Disease Patients Modulates Polymorphonuclear Leukocytes. Toxins 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A. Role of inflammation and its treatment in ESRD patients. Blood Purif. 2002, 20, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Crockett-Torabi, E.; Ward, P.A. The role of leukocytes in tissue injury. Eur. J. Anaesthesiol. 1996, 13, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef]
- Leemans, J.C.; Kors, L.; Anders, H.J.; Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 2014, 10, 398–414. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Capuano, G.; Collini, M.; Caccia, M.; Ronchi, A.E.; Rocchetti, M.; Mingozzi, F.; Foti, M.; Chirico, G.; et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 2009, 460, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Antal-Szalmas, P.; Strijp, J.A.; Weersink, A.J.; Verhoef, J.; Van Kessel, K.P. Quantitation of surface CD14 on human monocytes and neutrophils. J. Leukoc. Biol. 1997, 61, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Deppisch, R.; Denefleh, B.; Hug, F.; Andrassy, K.; Hansch, G.M. Expression patterns of the lipopolysaccharide receptor CD14, and the FCgamma receptors CD16 and CD64 on polymorphonuclear neutrophils: Data from patients with severe bacterial infections and lipopolysaccharide-exposed cells. Shock 2003, 19, 5–12. [Google Scholar] [CrossRef]
- Rodeberg, D.A.; Morris, R.E.; Babcock, G.F. Azurophilic granules of human neutrophils contain CD14. Infect. Immun. 1997, 65, 4747–4753. [Google Scholar] [CrossRef]
- Wang, S.H.; Yuan, S.G.; Peng, D.Q.; Zhao, S.P. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis 2012, 225, 105–114. [Google Scholar] [CrossRef]
- Sitrin, R.G.; Sassanella, T.M.; Landers, J.J.; Petty, H.R. Migrating Human Neutrophils Exhibit Dynamic Spatiotemporal Variation in Membrane Lipid Organization. Am. J. Respir. Cell Mol. Biol. 2010, 43, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. NADPH Oxidases, Reactive Oxygen Species, and the Kidney: Friend and Foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Gaus, K.; Rodriguez, M.; Ruberu, K.R.; Gelissen, I.; Sloane, T.M.; Kritharides, L.; Jessup, W. Domain-specific lipid distribution in macrophage plasma membranes. J. Lipid Res. 2005, 46, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Wulf, G.; Bruning, T.; Assmann, G. Flow-cytometric determination of high-density-lipoprotein binding sites on human leukocytes. Clin. Chem. 1987, 33, 2195–2203. [Google Scholar] [CrossRef]
- Blackburn, W.D., Jr.; Dohlman, J.G.; Venkatachalapathi, Y.V.; Pillion, D.J.; Koopman, W.J.; Segrest, J.P.; Anantharamaiah, G.M. Apolipoprotein A-I decreases neutrophil degranulation and superoxide production. J. Lipid Res. 1991, 32, 1911–1918. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Goyert, S.M.; Ferrero, E.; Rettig, W.J.; Yenamandra, A.K.; Obata, F.; Le Beau, M.M. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science 1988, 239, 497–500. [Google Scholar] [CrossRef]
- Keerthivasan, G.; Mei, Y.; Zhao, B.; Zhang, L.; Harris, C.E.; Gao, J.; Basiorka, A.A.; Schipma, M.J.; McElherne, J.; Yang, J.; et al. Aberrant overexpression of CD14 on granulocytes sensitizes the innate immune response in mDia1 heterozygous del(5q) MDS. Blood 2014, 124, 780–790. [Google Scholar] [CrossRef]
- Nakanishi, S.; Vikstedt, R.; Soderlund, S.; Lee-Rueckert, M.; Hiukka, A.; Ehnholm, C.; Muilu, M.; Metso, J.; Naukkarinen, J.; Palotie, L.; et al. Serum, but not monocyte macrophage foam cells derived from low HDL-C subjects, displays reduced cholesterol efflux capacity. J. Lipid Res. 2009, 50, 183–192. [Google Scholar] [CrossRef]
- Persegol, L.; Verges, B.; Foissac, M.; Gambert, P.; Duvillard, L. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2006, 49, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; De Boer, I.; Tang, C.; Mayer, P.S.; Zelnick, L.; Afkarian, M.; Heinecke, J.W.; Himmelfarb, J. A Cluster of Proteins Implicated in Kidney Disease Is Increased in High-Density Lipoprotein Isolated from Hemodialysis Subjects. J. Proteome Res. 2015, 14, 2792–2806. [Google Scholar] [CrossRef]
- Zewinger, S.; Kleber, M.E.; Rohrer, L.; Lehmann, M.; Triem, S.; Jennings, R.T.; Petrakis, I.; Dressel, A.; Lepper, P.M.; Scharnagl, H.; et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 2017, 38, 1597–1607. [Google Scholar] [CrossRef]
- Wang, K.; Zelnick, L.R.; Hoofnagle, A.N.; Vaisar, T.; Henderson, C.M.; Imrey, P.B.; Robinson-Cohen, C.; De Boer, I.H.; Shiu, Y.-T.; Himmelfarb, J.; et al. Alteration of HDL Protein Composition with Hemodialysis Initiation. Clin. J. Am. Soc. Nephrol. 2018, 13, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Sack, G.H., Jr. Serum amyloid A—A review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Drechsler, C.; Kleber, M.E.; Dressel, A.; Riffel, J.; Triem, S.; Lehmann, M.; Kopecky, C.; Saemann, M.D.; Lepper, P.M.; et al. Serum amyloid A: High-density lipoproteins interaction and cardiovascular risk. Eur. Heart J. 2015, 36, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, C.; Genser, B.; Drechsler, C.; Krane, V.; Kaltenecker, C.C.; Hengstschlager, M.; Marz, W.; Wanner, C.; Saemann, M.D.; Weichhart, T. Quantification of HDL Proteins, Cardiac Events, and Mortality in Patients with Type 2 Diabetes on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 224–231. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.D.; Baek, S.-H.; Choi, J.H.; Cho, K.H.; Zabel, B.A.; Bae, Y.S. Serum amyloid A stimulates macrophage foam cell formation via lectin-like oxidized low-density lipoprotein receptor 1 upregulation. Biochem. Biophys. Res. Commun. 2013, 433, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Sun, L. Emerging functions of serum amyloid A in inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef]
- Blackwood, R.A.; Hartiala, K.T.; Kwoh, E.E.; Transue, A.T.; Brower, R.C. Unidirectional heterologous receptor desensitization between both the fMLP and C5a receptor and the IL-8 receptor. J. Leukoc. Biol. 1996, 60, 88–93. [Google Scholar] [CrossRef]
- Shridas, P.; De Beer, M.C.; Webb, N.R. High-density lipoprotein inhibits serum amyloid A-mediated reactive oxygen species generation and NLRP3 inflammasome activation. J. Biol. Chem. 2018, 293, 13257–13269. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; De Beer, M.C.; Wroblewski, J.M.; Webb, N.R.; De Beer, F.C. SAA does not induce cytokine production in physiological conditions. Cytokine 2013, 61, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Badolato, R.; Wang, J.M.; Murphy, W.J.; Lloyd, A.R.; Michiel, D.F.; Bausserman, L.L.; Kelvin, D.J.; Oppenheim, J.J.; Fava, R.A.; Olsen, N.J.; et al. Serum amyloid A is a chemoattractant: Induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J. Exp. Med. 1994, 180, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Mohler, E.R., 3rd; Tian, A.; Baumgart, T.; Diamond, S.L. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1290–1297. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- Harder, T.; Engelhardt, K.R. Membrane Domains in Lymphocytes—From Lipid Rafts to Protein Scaffolds. Traffic 2004, 5, 265–275. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Gomez-Mouton, C.; Abad, J.L.; Mira, E.; LaCalle, R.A.; Gallardo, E.; Jimenez-Baranda, S.; Illa, I.; Bernad, A.; Manes, S.; Martinez, A.C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA 2001, 98, 9642–9647. [Google Scholar] [CrossRef]
- Schmitz, G.; Orso, E. CD14 signalling in lipid rafts: New ligands and co-receptors. Curr. Opin. Lipidol. 2002, 13, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Patino, R.; Ibarra, J.; Rodriguez, A.; Yague, M.R.; Pintor, E.; Fernandez-Cruz, A.; Figueredo, A. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am. J. Cardiol. 2000, 85, 1288–1291. [Google Scholar] [CrossRef]
- Smythies, L.E.; White, C.R.; Maheshwari, A.; Palgunachari, M.N.; Anantharamaiah, G.M.; Chaddha, M.; Kurundkar, A.R.; Datta, G. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am. J. Physiol. Cell Physiol. 2010, 298, C1538–C1548. [Google Scholar] [CrossRef]
- Olsson, S.; Sundler, R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 2006, 43, 607–612. [Google Scholar] [CrossRef]
- Carrizzo, A.; Forte, M.; Lembo, M.; Formisano, L.; Puca, A.A.; Vecchione, C. Rac-1 as a New Therapeutic Target in Cerebro- and Cardio-Vascular Diseases. Curr. Drug Targets 2014, 15, 1231–1246. [Google Scholar] [CrossRef]
- Pantarelli, C.; Welch, H.C.E. Rac-GTPases and Rac-GEFs in neutrophil adhesion, migration and recruitment. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12939. [Google Scholar] [CrossRef]
- Moissoglu, K.; Kiessling, V.; Wan, C.; Hoffman, B.D.; Norambuena, A.; Tamm, L.K.; Schwartz, M.A. Regulation of Rac1 translocation and activation by membrane domains and their boundaries. J. Cell Sci. 2014, 127, 2565–2576. [Google Scholar] [CrossRef]
- Van Helden, S.F.; Anthony, E.C.; Dee, R.; Hordijk, P.L. Rho GTPase Expression in Human Myeloid Cells. PLoS ONE 2012, 7, e42563. [Google Scholar] [CrossRef] [PubMed]
- Tolle, M.; Pawlak, A.; Schuchardt, M.; Kawamura, A.; Tietge, U.J.; Lorkowski, S.; Keul, P.; Assmann, G.; Chun, J.; Levkau, B.; et al. HDL-Associated Lysosphingolipids Inhibit NAD(P)H Oxidase-Dependent Monocyte Chemoattractant Protein-1 Production. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1542–1548. [Google Scholar] [CrossRef]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, G. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.Y.; Sun, J.T.; Yang, K.; Shen, W.F.; Lu, L.; Zhang, R.Y.; Tong, X.; Liu, Y. Serum amyloid A enrichment impairs the anti-inflammatory ability of HDL from diabetic nephropathy patients. J. Diabetes Complicat. 2017, 31, 1538–1543. [Google Scholar] [CrossRef]
- Cohen, G.; Rudnicki, M.; Walter, F.; Niwa, T.; Horl, W.H. Glucose-modified proteins modulate essential functions and apoptosis of polymorphonuclear leukocytes. J. Am. Soc. Nephrol. 2001, 12, 1264–1271. [Google Scholar] [PubMed]
- Cohen, G.; Raupachova, J.; Horl, W.H. The uraemic toxin phenylacetic acid contributes to inflammation by priming polymorphonuclear leucocytes. Nephrol. Dial. Transplant. 2013, 28, 421–429. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, G. Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes. Int. J. Mol. Sci. 2021, 22, 2830. https://doi.org/10.3390/ijms22062830
Cohen G. Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes. International Journal of Molecular Sciences. 2021; 22(6):2830. https://doi.org/10.3390/ijms22062830
Chicago/Turabian StyleCohen, Gerald. 2021. "Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes" International Journal of Molecular Sciences 22, no. 6: 2830. https://doi.org/10.3390/ijms22062830
APA StyleCohen, G. (2021). Effect of High-Density Lipoprotein from Healthy Subjects and Chronic Kidney Disease Patients on the CD14 Expression on Polymorphonuclear Leukocytes. International Journal of Molecular Sciences, 22(6), 2830. https://doi.org/10.3390/ijms22062830




