High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects
Abstract
1. Introduction
2. Results
2.1. HDLs Limit the Infarct Volume in a Mouse Model of Acute Cerebral Ischemia/Reperfusion
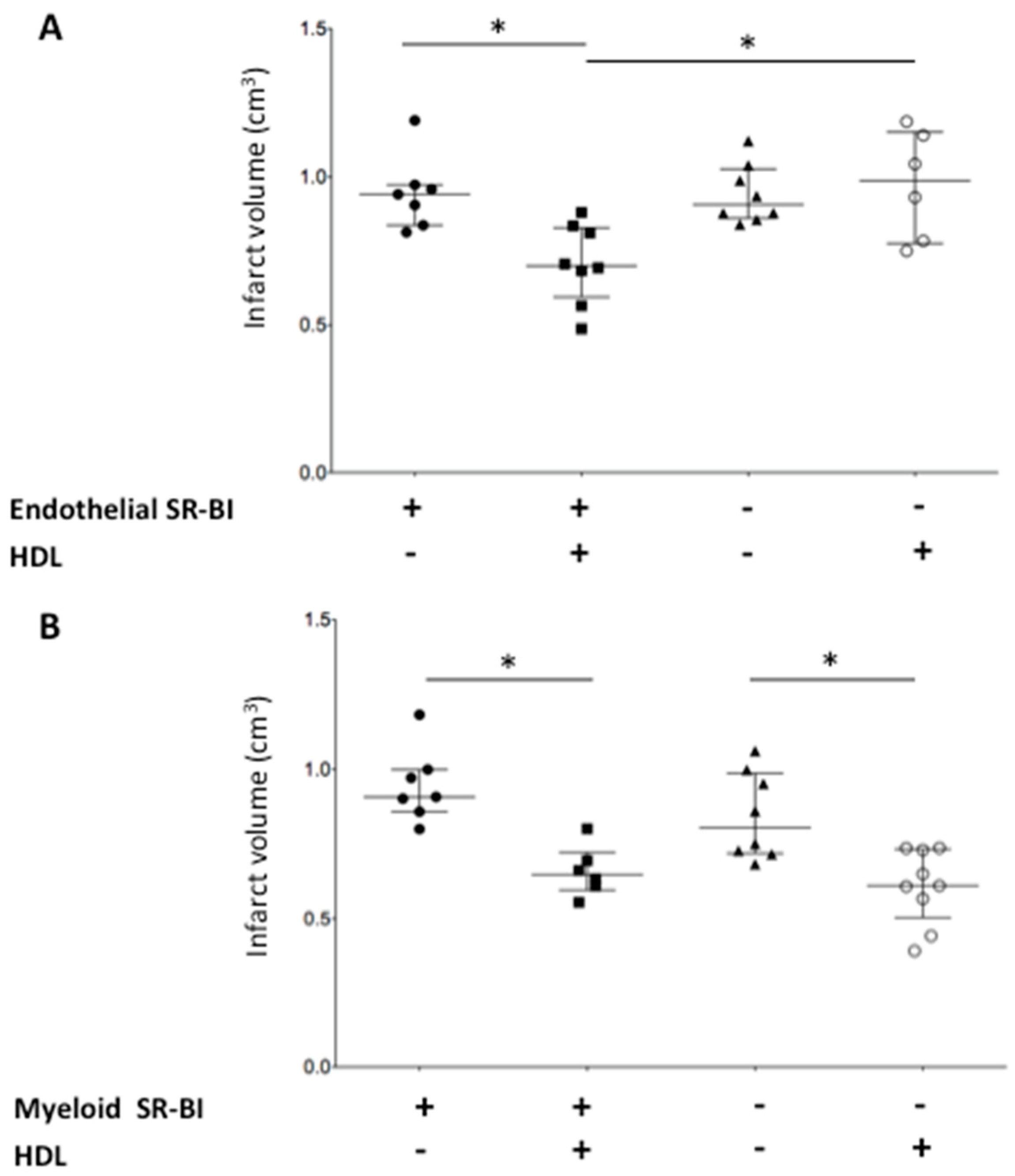
2.2. Endothelial SR-BI Is Involved in the Neuroprotective Effects of HDLs
2.3. Myeloid SR-BI Does Not Mediate HDL Neuroprotective Effects
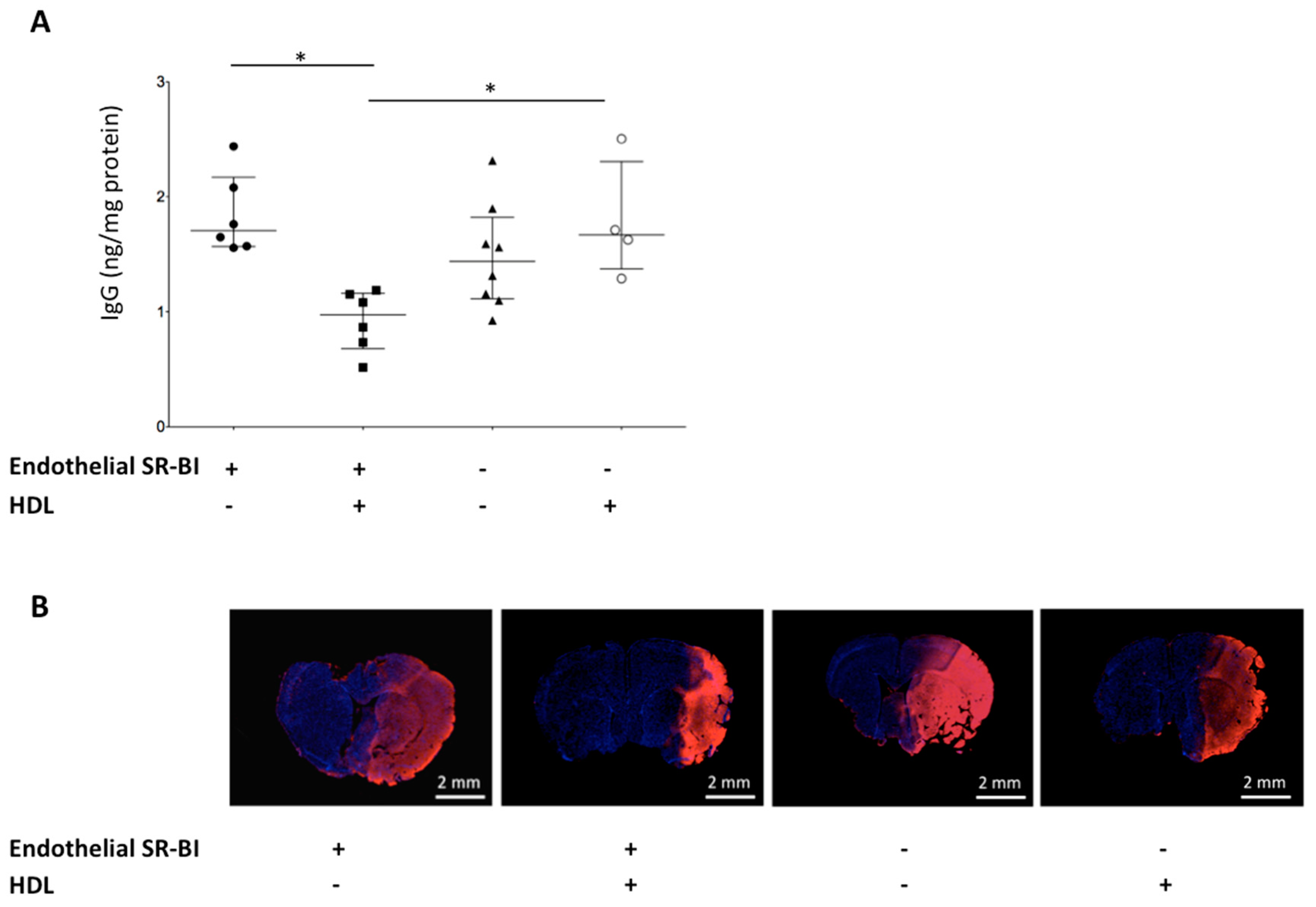
2.4. HDLs Limit the BBB Leakage via Endothelial SR-BI
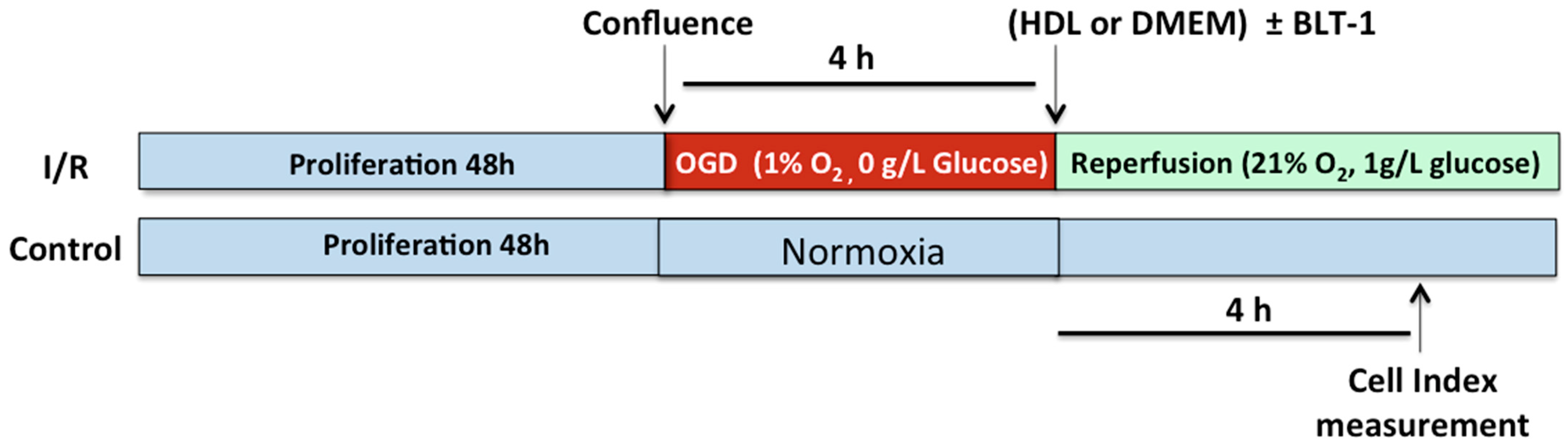
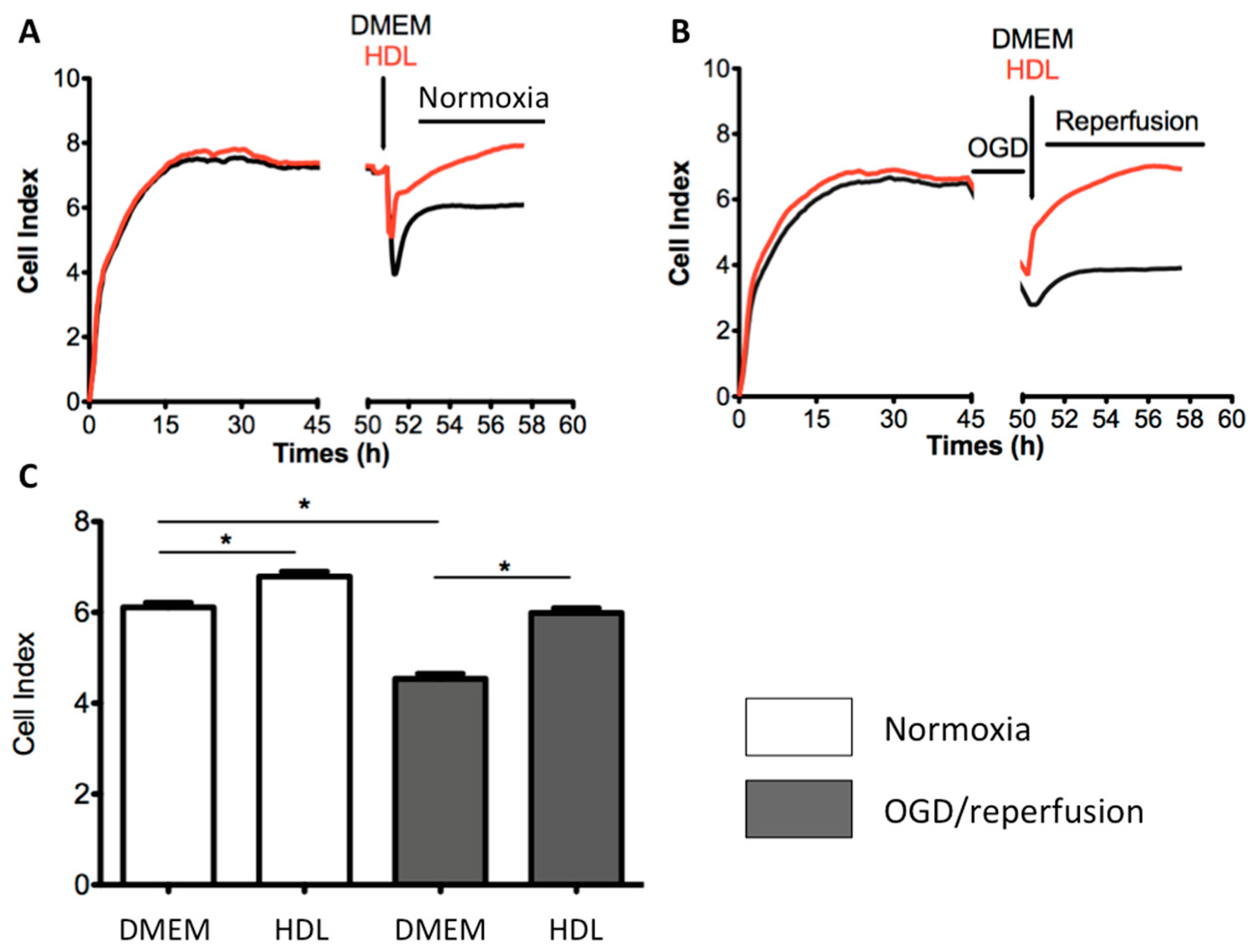
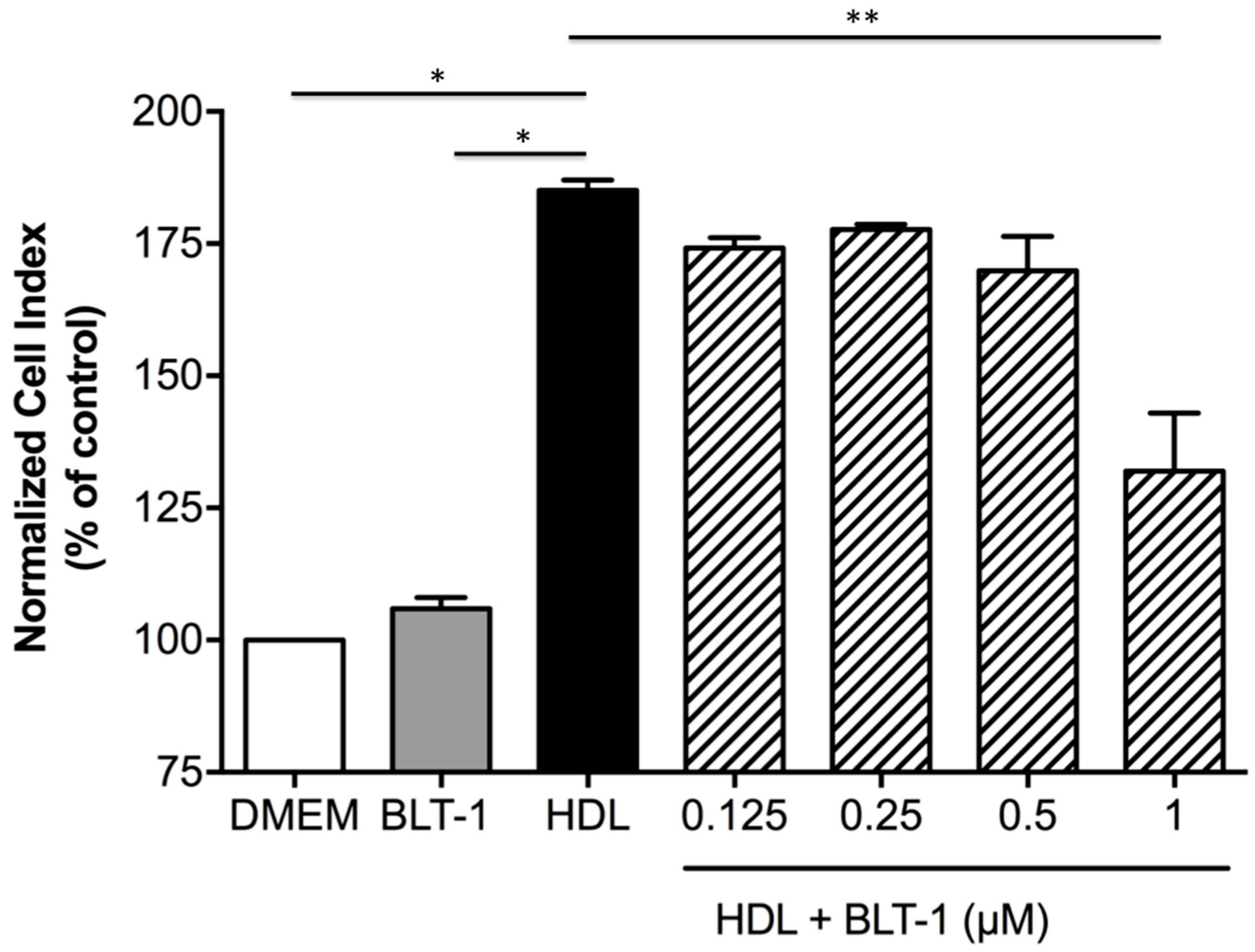
2.5. HDLs Display Protective Effects on the Integrity of a Cellular Model of BBB Under Ischemia/Reperfusion Conditions
2.6. Endothelial SR-BI Is Involved in the Protective Effects of HDLs on the Integrity of a Cellular Model of BBB in Ischemia/Reperfusion Conditions
3. Discussion
4. Methods
4.1. Mouse Models of Endothelial and Myeloid Deletion of SR-BI
4.2. Preparation of HDLs
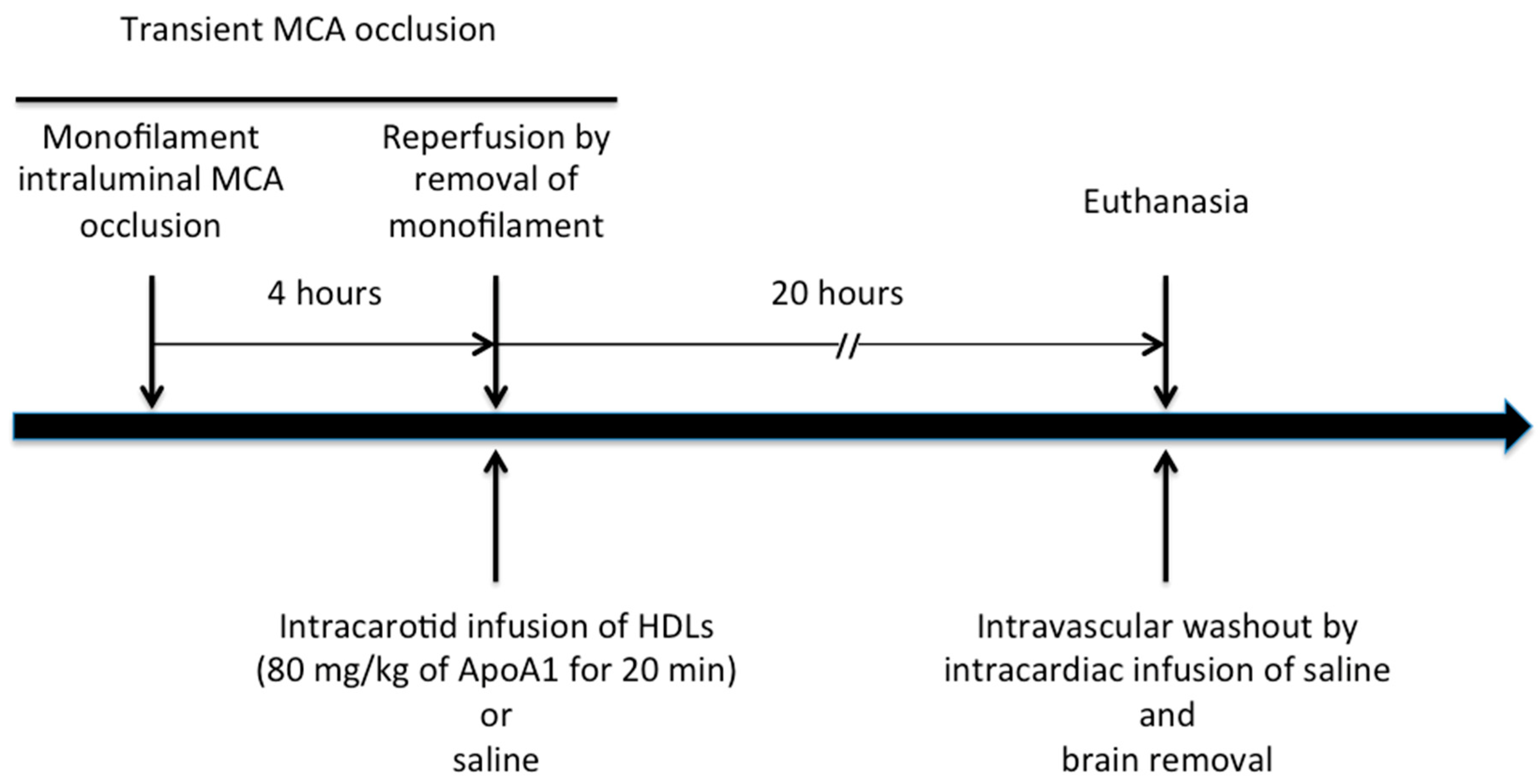
4.3. Mouse Model of Focal Cerebral Ischemia/Reperfusion
4.4. In Vivo Experimental Protocol
4.5. Infarct Volume BBB Leakage
4.6. In Vitro Assessment of the BBB Integrity
4.7. Sample Size Calculation
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Acute ischemic stroke |
| ApoA1 | apolipoprotein A-I |
| BBB | Blood-brain barrier |
| HDL | High-density lipoprotein |
| IgG | Immunoglobulin G |
| MCAO | Middle cerebral artery occlusion |
| OGD | oxygen-glucose deprivation |
| rt-PA | recombinant tissue plaminogen activator |
| SR-BI | Scavenger receptor class B type I |
| TTC | 2,3,5-triphenyltetrazolium chloride |
References
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, C.S.; Jahan, R. Endovascular treatment of acute ischemic stroke. Neurol. Clin. 2015, 33, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Amarenco, P.; Goldstein, L.B.; Callahan, A.; Sillesen, H.; Hennerici, M.G.; O’Neill, B.J.; Rudolph, A.E.; Simunovic, L.; Zivin, J.A.; Welch, K.M.A.; et al. Baseline blood pressure, low- and high-density lipoproteins, and triglycerides and the risk of vascular events in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Atherosclerosis 2009, 204, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Munoz, G.; Couret, D.; Lapergue, B.; Bruckert, E.; Meseguer, E.; Amarenco, P.; Meilhac, O. Dysfunctional HDL in acute stroke. Atherosclerosis 2016, 253, 75–80. [Google Scholar] [CrossRef]
- Lapergue, B.; Moreno, J.-A.; Dang, B.Q.; Coutard, M.; Delbosc, S.; Raphaeli, G.; Auge, N.; Klein, I.; Mazighi, M.; Michel, J.-B.; et al. Protective effect of high-density lipoprotein-based therapy in a model of embolic stroke. Stroke J. Cereb. Circ. 2010, 41, 1536–1542. [Google Scholar] [CrossRef]
- Lapergue, B.; Dang, B.Q.; Desilles, J.-P.; Ortiz-Munoz, G.; Delbosc, S.; Loyau, S.; Louedec, L.; Couraud, P.-O.; Mazighi, M.; Michel, J.-B.; et al. High-density lipoprotein-based therapy reduces the hemorrhagic complications associated with tissue plasminogen activator treatment in experimental stroke. Stroke J. Cereb. Circ. 2013, 44, 699–707. [Google Scholar] [CrossRef]
- Guo, S.; Lo, E.H. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke J. Cereb. Circ. 2009, 40, S4–S7. [Google Scholar] [CrossRef]
- Tran-Dinh, A.; Diallo, D.; Delbosc, S.; Varela-Perez, L.M.; Dang, Q.B.; Lapergue, B.; Burillo, E.; Michel, J.B.; Levoye, A.; Martin-Ventura, J.L.; et al. HDL and endothelial protection. Br. J. Pharmacol. 2013, 169, 493–511. [Google Scholar] [CrossRef]
- Balazs, Z.; Panzenboeck, U.; Hammer, A.; Sovic, A.; Quehenberger, O.; Malle, E.; Sattler, W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J. Neurochem. 2004, 89, 939–950. [Google Scholar] [CrossRef]
- Saddar, S.; Mineo, C.; Shaul, P.W. Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 144–150. [Google Scholar] [CrossRef]
- Mineo, C.; Yuhanna, I.S.; Quon, M.J.; Shaul, P.W. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J. Biol. Chem. 2003, 278, 9142–9149. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-A.; Guo, L.; Dressman, J.L.; Asmis, R.; Smart, E.J. A novel ligand-independent apoptotic pathway induced by scavenger receptor class B, type I and suppressed by endothelial nitric-oxide synthase and high density lipoprotein. J. Biol. Chem. 2005, 280, 19087–19096. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; van Eck, M.; Van Craeyveld, E.; Jacobs, F.; Carlier, V.; Van Linthout, S.; Erdel, M.; Tjwa, M.; De Geest, B. Critical role of scavenger receptor-BI-expressing bone marrow-derived endothelial progenitor cells in the attenuation of allograft vasculopathy after human apo A-I transfer. Blood 2009, 113, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.P.; Cheng, J.; Gospodarowicz, D. Effect of high and low density lipoproteins on proliferation of cultured bovine vascular endothelial cells. J. Clin. Investig. 1980, 66, 696–708. [Google Scholar] [CrossRef]
- Brodde, M.F.; Korporaal, S.J.A.; Herminghaus, G.; Fobker, M.; Van Berkel, T.J.C.; Tietge, U.J.F.; Robenek, H.; Van Eck, M.; Kehrel, B.E.; Nofer, J.-R. Native high-density lipoproteins inhibit platelet activation via scavenger receptor BI: Role of negatively charged phospholipids. Atherosclerosis 2011, 215, 374–382. [Google Scholar] [CrossRef]
- Rohrer, L.; Ohnsorg, P.M.; Lehner, M.; Landolt, F.; Rinninger, F.; von Eckardstein, A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circ. Res. 2009, 104, 1142–1150. [Google Scholar] [CrossRef]
- Sag, C.M.; Schnelle, M.; Zhang, J.; Murdoch, C.E.; Kossmann, S.; Protti, A.; Santos, C.X.C.; Sawyer, G.; Zhang, X.; Mongue-Din, H.; et al. Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood PressureClinical Perspective. Circulation 2017, 135, 2163–2177. [Google Scholar] [CrossRef]
- Hirano, K.; Yamashita, S.; Nakagawa, Y.; Ohya, T.; Matsuura, F.; Tsukamoto, K.; Okamoto, Y.; Matsuyama, A.; Matsumoto, K.; Miyagawa, J.; et al. Expression of Human Scavenger Receptor Class B Type I in Cultured Human Monocyte-Derived Macrophages and Atherosclerotic Lesions. Circ. Res. 1999, 85, 108–116. [Google Scholar] [CrossRef]
- Gowdy, K.M.; Madenspacher, J.H.; Azzam, K.M.; Gabor, K.A.; Janardhan, K.S.; Aloor, J.J.; Fessler, M.B. Key role for scavenger receptor B-I in the integrative physiology of host defense during bacterial pneumonia. Mucosal Immunol. 2015, 8, 559–571. [Google Scholar] [CrossRef]
- Benakis, C.; Garcia-Bonilla, L.; Iadecola, C.; Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 2015, 8. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Nieland, T.J.F.; Penman, M.; Dori, L.; Krieger, M.; Kirchhausen, T. Nonlinear partial differential equations and applications: Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc. Natl. Acad. Sci. USA 2002, 99, 15422–15427. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Button, E.B.; Stukas, S.; Boyce, G.K.; Gibbs, E.; Cowan, C.M.; Gilmour, M.; Cheng, W.H.; Soo, S.K.; Yuen, B.; et al. High-density lipoproteins suppress Aβ-induced PBMC adhesion to human endothelial cells in bioengineered vessels and in monoculture. Mol. Neurodegener. 2017, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.F.; Wilson, G.K.; Murray, J.; Hu, K.; Lewis, A.; Reynolds, G.M.; Stamataki, Z.; Meredith, L.W.; Rowe, I.A.; Luo, G.; et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology 2012, 142, 634–643.e6. [Google Scholar] [CrossRef] [PubMed]
- Lindner, C.; Sigrüner, A.; Walther, F.; Bogdahn, U.; Couraud, P.O.; Schmitz, G.; Schlachetzki, F. ATP-binding cassette transporters in immortalised human brain microvascular endothelial cells in normal and hypoxic conditions. Exp. Transl. Stroke Med. 2012, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Harrington, A.; Yang, X.; Friesel, R.E.; Liaw, L. The contribution of the Tie2+ lineage to primitive and definitive hematopoietic cells. Genesis 2010, 48, 563–567. [Google Scholar] [CrossRef]
- Murphy, A.J.; Chin-Dusting, J.P.F.; Sviridov, D.; Woollard, K.J. The anti inflammatory effects of high density lipoproteins. Curr. Med. Chem. 2009, 16, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Dusting, G.J.; Cutri, B.; Bao, S.; Drummond, G.R.; Rye, K.-A.; Barter, P.J. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 2005, 111, 1543–1550. [Google Scholar] [CrossRef]
- Puranik, R.; Bao, S.; Nobecourt, E.; Nicholls, S.J.; Dusting, G.J.; Barter, P.J.; Celermajer, D.S.; Rye, K.-A. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis 2008, 196, 240–247. [Google Scholar] [CrossRef]
- Murphy, A.J.; Woollard, K.J.; Suhartoyo, A.; Stirzaker, R.A.; Shaw, J.; Sviridov, D.; Chin-Dusting, J.P.F. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1333–1341. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.A.; Bobik, A.; Murphy, A.; Kanellakis, P.; Blombery, P.; Mukhamedova, N.; Woollard, K.; Lyon, S.; Sviridov, D.; Dart, A.M. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 2008, 103, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.G.; Fidge, N.H.; Gallon-Beaumier, G.; Kemp, B.E.; Kingwell, B.A. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc. Natl. Acad. Sci. USA 2004, 101, 6999–7004. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wilson, M.; Kelly, T.; Su, W.; Dressman, J.; Kincer, J.; Matveev, S.V.; Guo, L.; Guerin, T.; Li, X.-A.; et al. HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner. J. Clin. Investig. 2003, 111, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- McGrath, K.C.Y.; Li, X.H.; Puranik, R.; Liong, E.C.; Tan, J.T.M.; Dy, V.M.; DiBartolo, B.A.; Barter, P.J.; Rye, K.A.; Heather, A.K. Role of 3beta-hydroxysteroid-delta 24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 877–882. [Google Scholar] [CrossRef]
- Muñoz-Vega, M.; Massó, F.; Páez, A.; Vargas-Alarcón, G.; Coral-Vázquez, R.; Mas-Oliva, J.; Carreón-Torres, E.; Pérez-Méndez, Ó. HDL-Mediated Lipid Influx to Endothelial Cells Contributes to Regulating Intercellular Adhesion Molecule (ICAM)-1 Expression and eNOS Phosphorylation. Int. J. Mol. Sci. 2018, 19, 3394. [Google Scholar] [CrossRef]
- Saddar, S.; Carriere, V.; Lee, W.-R.; Tanigaki, K.; Yuhanna, I.S.; Parathath, S.; Morel, E.; Warrier, M.; Sawyer, J.K.; Gerard, R.D.; et al. Scavenger receptor class B type I is a plasma membrane cholesterol sensor. Circ. Res. 2013, 112, 140–151. [Google Scholar] [CrossRef]
- Huby, T.; Doucet, C.; Dachet, C.; Ouzilleau, B.; Ueda, Y.; Afzal, V.; Rubin, E.; Chapman, M.J.; Lesnik, P. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J. Clin. Investig. 2006, 116, 2767–2776. [Google Scholar] [CrossRef]
- Kisanuki, Y.Y.; Hammer, R.E.; Miyazaki, J.; Williams, S.C.; Richardson, J.A.; Yanagisawa, M. Tie2-Cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001, 230, 230–242. [Google Scholar] [CrossRef]
- Hamidi, H.; Lilja, J.; Ivaska, J. Using xCELLigence RTCA Instrument to Measure Cell Adhesion. Bio-Protocol 2017, 7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran-Dinh, A.; Levoye, A.; Couret, D.; Galle-Treger, L.; Moreau, M.; Delbosc, S.; Hoteit, C.; Montravers, P.; Amarenco, P.; Huby, T.; et al. High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects. Int. J. Mol. Sci. 2021, 22, 106. https://doi.org/10.3390/ijms22010106
Tran-Dinh A, Levoye A, Couret D, Galle-Treger L, Moreau M, Delbosc S, Hoteit C, Montravers P, Amarenco P, Huby T, et al. High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects. International Journal of Molecular Sciences. 2021; 22(1):106. https://doi.org/10.3390/ijms22010106
Chicago/Turabian StyleTran-Dinh, Alexy, Angélique Levoye, David Couret, Lauriane Galle-Treger, Martine Moreau, Sandrine Delbosc, Camille Hoteit, Philippe Montravers, Pierre Amarenco, Thierry Huby, and et al. 2021. "High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects" International Journal of Molecular Sciences 22, no. 1: 106. https://doi.org/10.3390/ijms22010106
APA StyleTran-Dinh, A., Levoye, A., Couret, D., Galle-Treger, L., Moreau, M., Delbosc, S., Hoteit, C., Montravers, P., Amarenco, P., Huby, T., & Meilhac, O. (2021). High-Density Lipoprotein Therapy in Stroke: Evaluation of Endothelial SR-BI-Dependent Neuroprotective Effects. International Journal of Molecular Sciences, 22(1), 106. https://doi.org/10.3390/ijms22010106




