Flowering and Seed Production across the Lemnaceae
Abstract
1. Introduction
2. Results
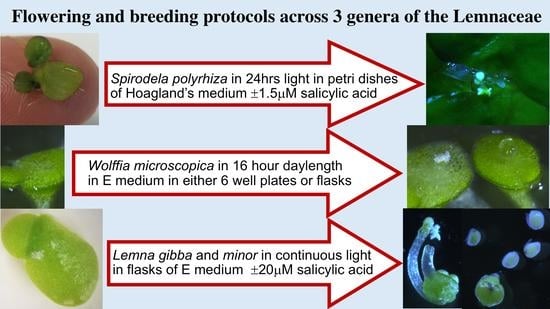
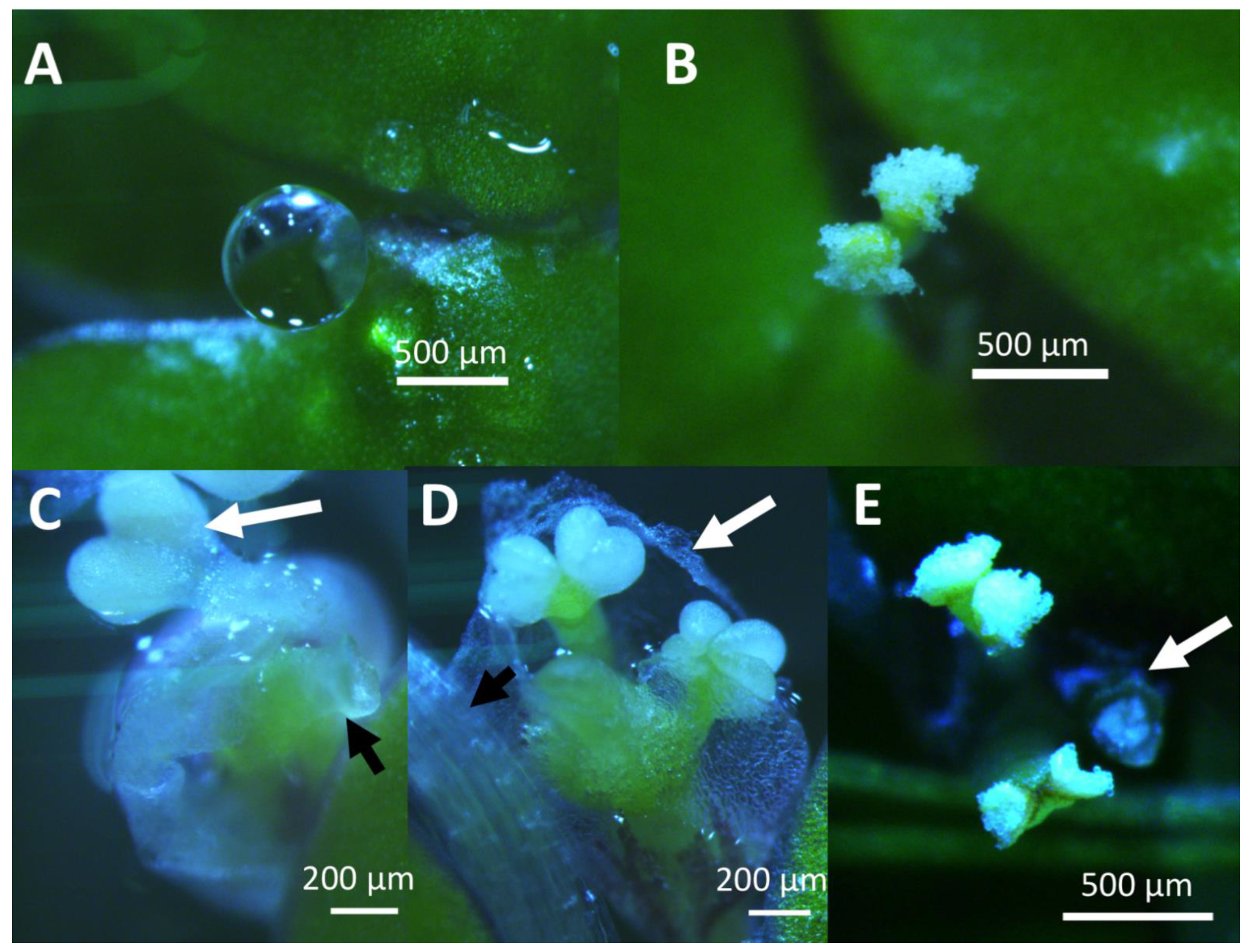
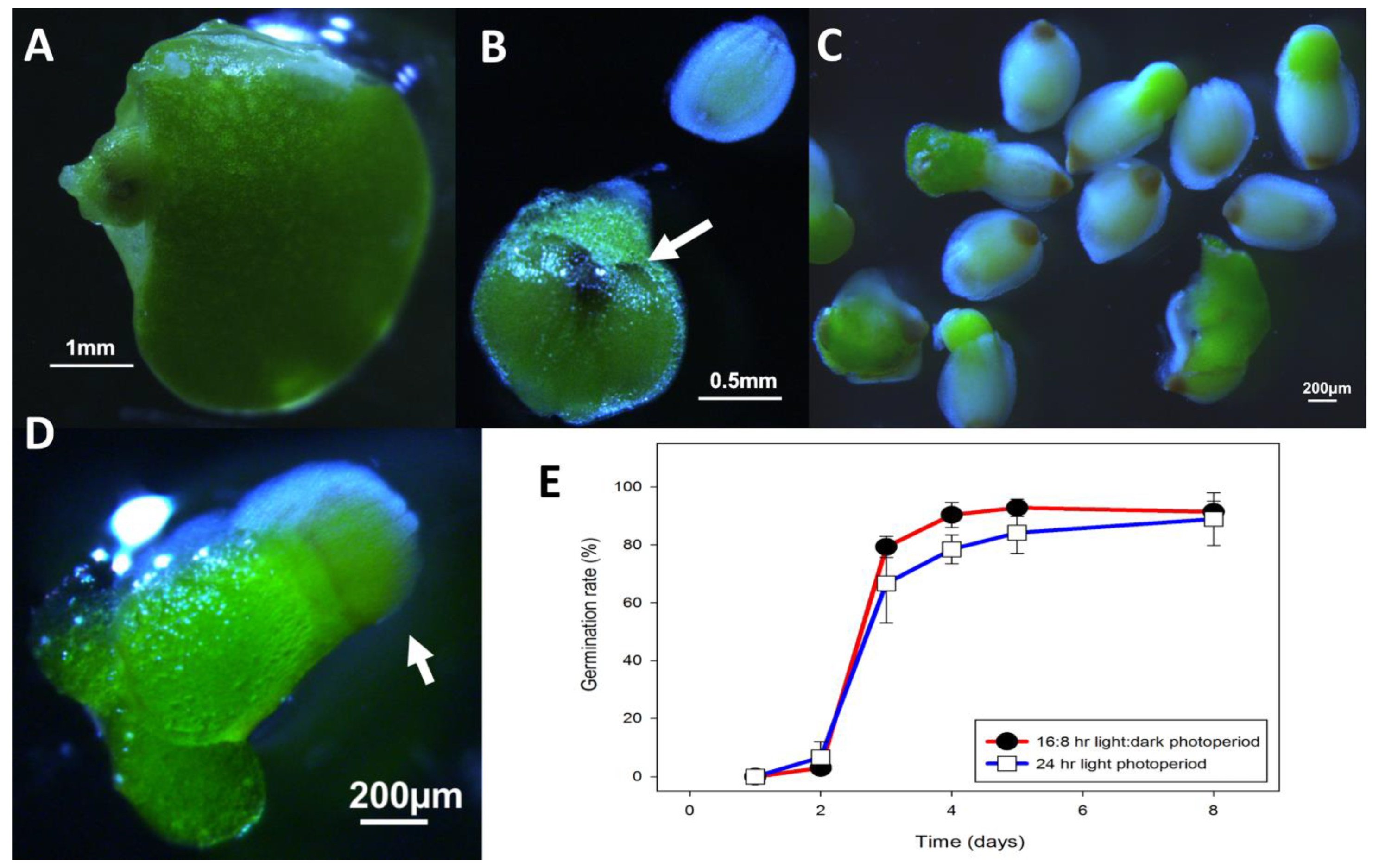
2.1. Spirodela Polyrhiza
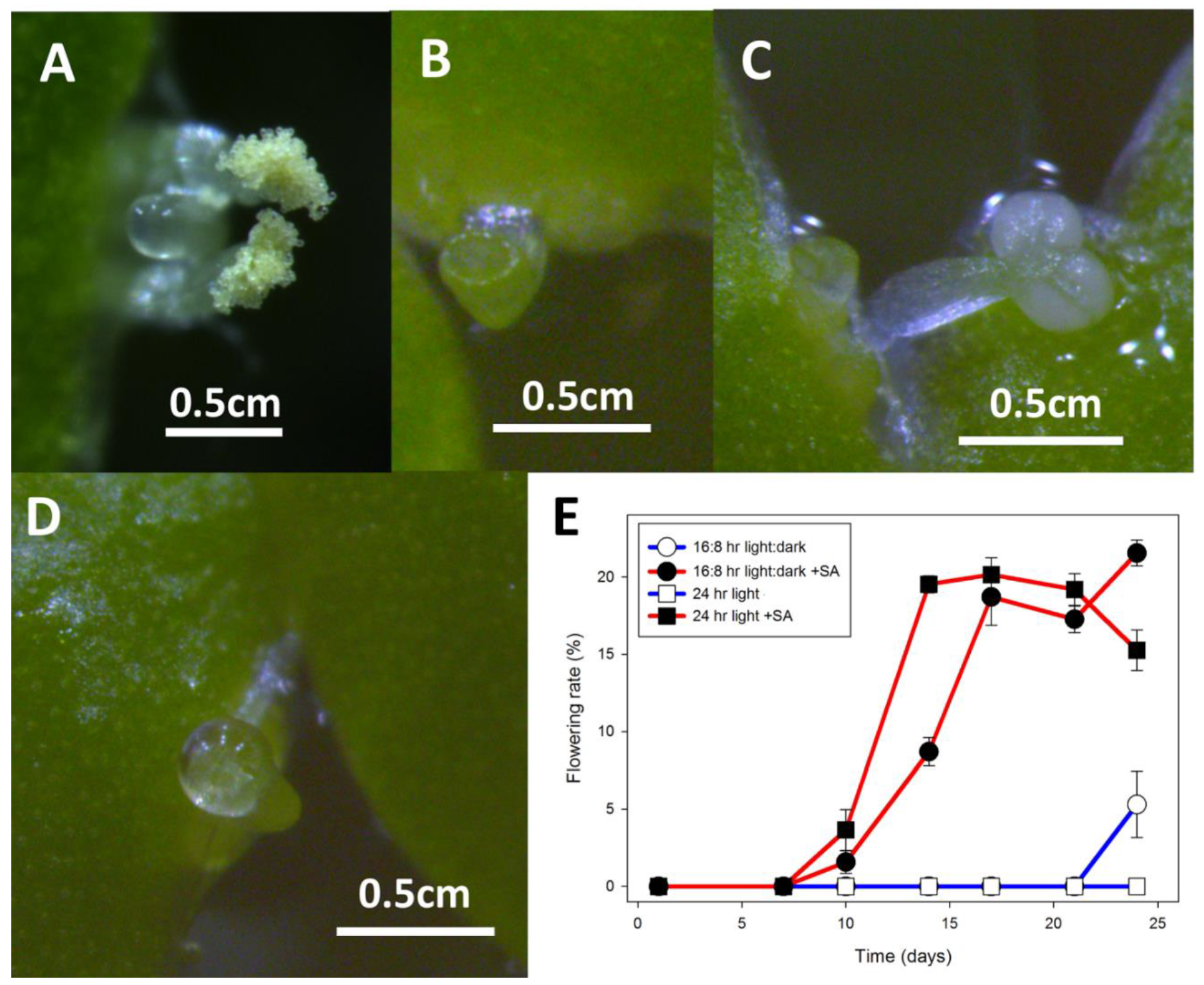
2.2. Wolffia Microscopica
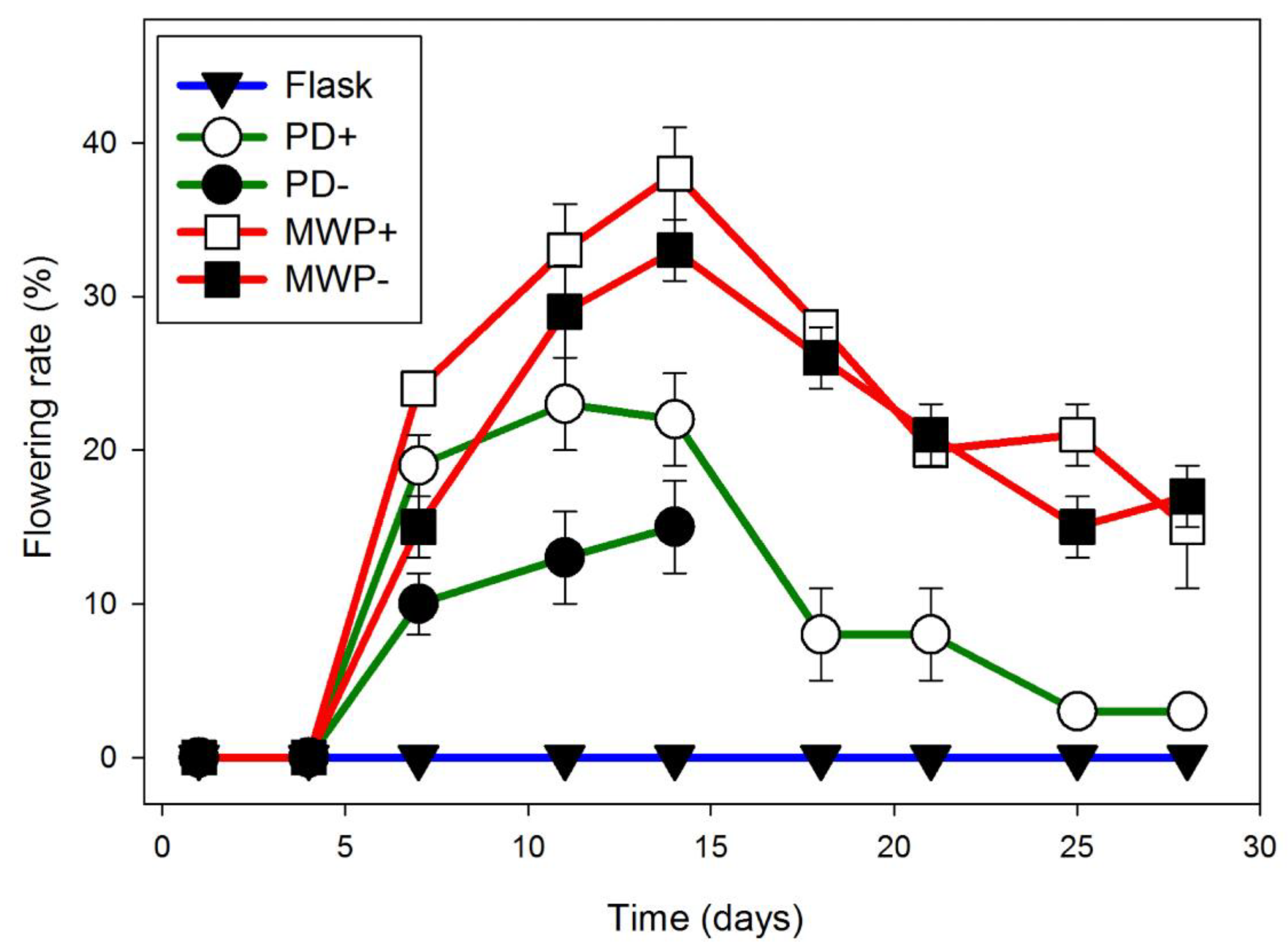
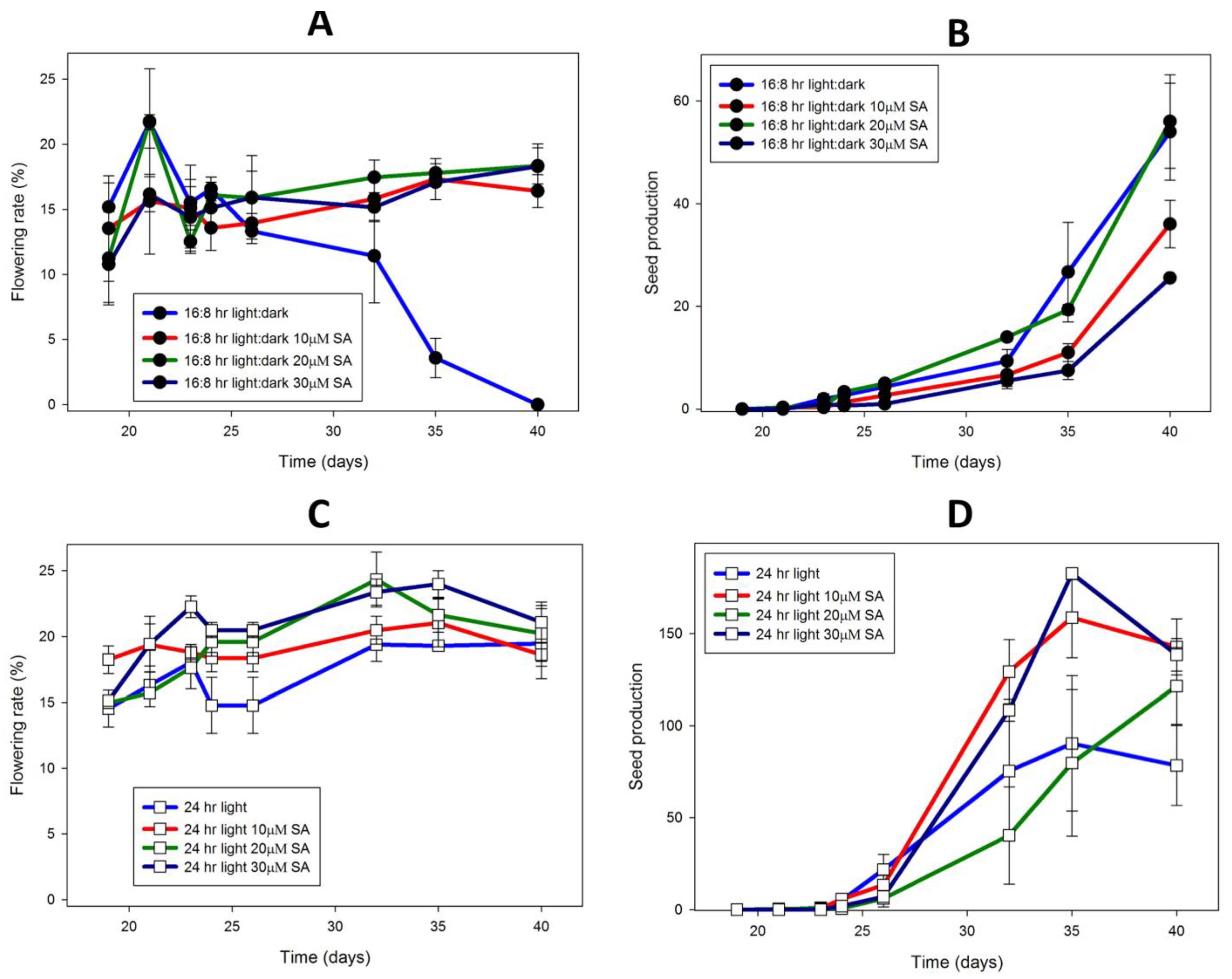
2.3. Lemna
3. Discussion
3.1. Spirodela Polyrhiza
3.2. Wolffia Microscopica
3.3. Lemna
4. Summary
5. Materials and Methods
5.1. Plant Material and Culture Media
5.2. Flowering, Seed Production and Seed Storage
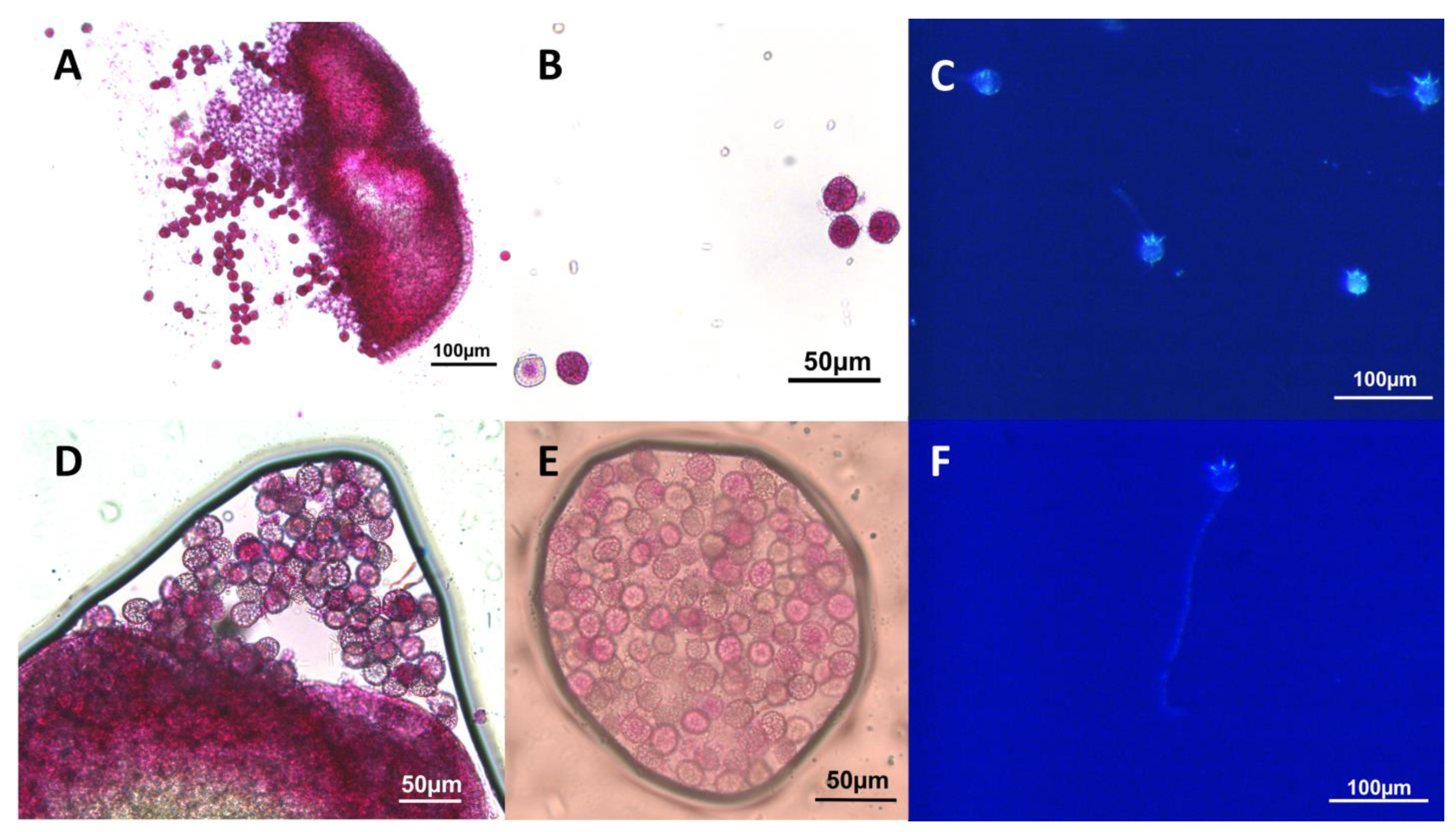
5.3. Pollen Viability and Fertility
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landolt, E.; Kandeler, R. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae); The Family of Lemnaceae—Monographic Study Volume 2; ETH Zurich: Zurich, Switzerland, 1987. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Landolt, E. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae); The Family of Lemnaceae—Monographic Study Volume 1; ETH Zurich: Zurich, Switzerland, 1986. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Cao, H.X.; Fourounjian, P.; Wang, W. The Importance and Potential of Duckweeds as a Model and Crop Plant for Biomass-Based Applications and Beyond. In Handbook of Environmental Materials Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar]
- Fourounjian, P.; Fakhoorian, T.; Cao, X.H. Importance of Duckweeds in Basic Research and Their Industrial Applications. In Compendium of Plant Genomes; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–17. [Google Scholar]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries; World Bank: Washington, DC, USA, 1993. [Google Scholar]
- Food and Agriculture Organization. FAOP Statistics; Food and Agriculture Organization: Quebec City, QC, Canada, 2021. [Google Scholar]
- Sree, K.S.; Maheshwari, S.C.; Bóka, K.; Khurana, J.P.; Keresztes, Á.; Appenroth, K.-J. The duckweed Wolffia microscopica: A unique aquatic monocot. Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 210, 31–39. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Messing, J. RNA-Seq transcriptome analysis of Spirodela dormancy without reproduction. BMC Genom. 2014, 15, 60. [Google Scholar] [CrossRef]
- Tanaka, O.; Cleland, C.F. Influence of Ammonium on the Ability of Salicylic Acid to Induce Flowering in the Short-Day Plant Lemna paucicostata 6746. Plant Cell Physiol. 1981, 22, 597–602. [Google Scholar] [CrossRef]
- Micheli, P.A. Nova Plantarum Genera Iuxta Tournefortii Methodum Disposita Quibus Plantae Recensentur, Scilicet Fere Nondum Observatae, Reliquae Suis Sedibus, Florentiae; Typis Bernardi Paperini: Florentiae, Italy, 1729. [Google Scholar]
- Hicks, L. Flower Production in the Lemnaceae; Ohio State University: Columbus, OH, USA, 1932. [Google Scholar]
- Baldi, B.G.; Maher, B.R.; Slovin, J.P.; Cohen, J.D. Stable Isotope Labeling, in vivo, of d-and l-Tryptophan Pools in Lemna gibba and the Low Incorporation of Label into Indole-3-Acetic Acid. Plant Physiol. 1991, 95, 1203–1208. [Google Scholar] [CrossRef]
- Slovin, J.P.; Cohen, J.D. Levels of Indole-3-Acetic Acid in Lemna gibba G-3 and in a Large Lemna Mutant Regenerated from Tissue Culture. Plant Physiol. 1988, 86, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, J.; Veluthambi, K.; Thompson, G.A.; Mudd, S.H.; Datko, A.H.; Jameel, S.; Reddy, V.M.; Rhodes, W.G.; McFadden, B.A. Threonine Synthase of Lemna paucicostata Hegelm. 6746. Plant Physiol. 1984, 76, 285–292. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Hoffman-Falk, H.; Marder, J.B.; Edelman, M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 1380–1384. [Google Scholar] [CrossRef]
- Slovin, J.P.; Tobin, E.M. Synthesis and turnover of the light-harvesting chlorophylla/b-protein in Lemna gibba grown with intermittent red light: Possible translational control. Planta 1982, 154, 465–472. [Google Scholar] [CrossRef]
- Zhao, H.; Appenroth, K.; Landesman, L.; Salmeán, A.A.; Lam, E. Duckweed rising at Chengdu: Summary of the 1st International Conference on Duckweed Application and Research. Plant Mol. Biol. 2012, 78, 627–632. [Google Scholar] [CrossRef]
- Hillman, W.S. The Lemnaceae, or duckweeds. Bot. Rev. 1961, 27, 221–287. [Google Scholar] [CrossRef]
- Oláh, V.; Hepp, A.; Vaca, N.Y.G.; Tamás, M.; Mészáros, I. Retrospective analyses of archive phytotoxicity test data can help in assessing internal dynamics and stability of growth in laboratory duckweed cultures. Aquat. Toxicol. 2018, 201, 40–46. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for the Testing of Chemicals, Revised Proposal for a New Guideline 221, Lemna sp. Growth Inhibition Test; OECD: Paris, France, 2006.
- ISO. Water quality determination of the toxic effect of water constituents and waste water on duckweed (Lemna minor)—Duckweed growth inhibition test. ISO 2005, 3, 23. [Google Scholar]
- Mazur, R.; Szoszkiewicz, K.; Lewicki, P.; Bedla, D. The use of computer image analysis in a Lemna minor L. bioassay. Hydrobiologia 2018, 812, 193–201. [Google Scholar] [CrossRef]
- Haffner, O.; Kučera, E.; Drahoš, P.; Cigánek, J.; Kozáková, A.; Urminská, B. Lemna minor Bioassay Evaluation Using Computer Image Analysis. Water 2020, 12, 2207. [Google Scholar] [CrossRef]
- Sree, K.S.; Appenroth, K.-J. Worldwide Genetic Resources of Duckweed: Stock Collections. In Compendium of Plant Genomes; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 39–46. [Google Scholar]
- Wang, W.; Wu, Y.; Yan, Y.; Ermakova, M.; Kerstetter, R.; Messing, J. DNA barcoding of the Lemnaceae, a family of aquatic monocots. BMC Plant Biol. 2010, 10, 205. [Google Scholar] [CrossRef]
- Wang, W.; Haberer, G.; Gundlach, H.; Gläßer, C.; Nussbaumer, T.; Luo, M.; Lomsadze, A.; Borodovsky, M.; Kerstetter, R.; Shanklin, J.D.; et al. The Spirodela polyrhiza genome reveals insights into its neotenous reduction fast growth and aquatic lifestyle. Nat. Commun. 2014, 5, 3311. [Google Scholar] [CrossRef]
- Michael, T.P.; Bryant, D.; Gutierrez, R.; Borisjuk, N.; Chu, P.; Zhang, H.; Xia, J.; Zhou, J.; Peng, H.; El Baidouri, M.; et al. Comprehensive definition of genome features in Spirodela polyrhiza by high-depth physical mapping and short-read DNA sequencing strategies. Plant J. 2017, 89, 617–635. [Google Scholar] [CrossRef]
- Van Hoeck, A.; Horemans, N.; Monsieurs, P.; Cao, H.X.; Vandenhove, H.; Blust, R. The first draft genome of the aquatic model plant Lemna minor opens the route for future stress physiology research and biotechnological applications. Biotechnol. Biofuels 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, W.; Messing, J. Status of duckweed genomics and transcriptomics. Plant Biol. 2014, 17, 10–15. [Google Scholar] [CrossRef]
- Fang, Y.; Du, A.; Tan, L.; He, K.; Jin, Y.; Ding, Y.; Guo, L.; Zhao, H. The Transcriptome in Landoltia punctata. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 125–131. [Google Scholar]
- Fourounjian, P.; Tang, J.; Tanyolac, B.; Feng, Y.; Gelfand, B.; Kakrana, A.; Tu, M.; Wakim, C.; Meyers, B.C.; Ma, J.; et al. Post-transcriptional adaptation of the aquatic plant Spirodela polyrhiza under stress and hormonal stimuli. Plant J. 2019, 98. [Google Scholar] [CrossRef]
- Harkess, A.; McLoughlin, F.; Bilkey, N.; Elliott, K.; Emenecker, R.; Mattoon, E.; Miller, K.; Czymmek, K.; Vierstra, R.; Meyers, B.C.; et al. A new Spirodela polyrhiza genome and proteome reveal a conserved chromosomal structure with high abundances of proteins favoring energy production. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yang, R.; Niu, L.; Wang, W. Comparison of protein extraction methods for 2DE-based proteomic analysis of duckweed Spirodela polyrhiza, a small aquatic model plant. Aquat. Bot. 2020, 163, 103216. [Google Scholar] [CrossRef]
- Hoang, P.N.; Michael, T.P.; Gilbert, S.; Chu, P.; Motley, S.T.; Appenroth, K.J.; Schubert, I.; Lam, E. Generating a high-confidence reference genome map of the Greater Duckweed by integration of cytogenomic, optical mapping, and Oxford Nanopore technologies. Plant J. 2018, 96, 670–684. [Google Scholar] [CrossRef]
- An, D.; Zhou, Y.; Li, C.; Xiao, Q.; Wang, T.; Zhang, Y.; Wu, Y.; Li, Y.; Chao, D.-Y.; Messing, J.; et al. Plant evolution and environmental adaptation unveiled by long-read whole-genome sequencing of Spirodela. Proc. Natl. Acad. Sci. USA 2019, 116, 18893–18899. [Google Scholar] [CrossRef] [PubMed]
- Fourounjian, P. Repetitive Sequences: Impacts and Uses in the Spirodela Genome. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 87–90. [Google Scholar]
- Yang, J.; Hu, S.; Li, G.; Khan, S.; Kumar, S.; Yao, L.; Duan, P.; Hou, H. Transformation Development in Duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 143–155. [Google Scholar]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Evaluation of environmental bacterial communities as a factor affecting the growth of duckweed Lemna minor. Biotechnol. Biofuels 2017, 10, 62. [Google Scholar] [CrossRef]
- Ishizawa, H.; Kuroda, M.; Inoue, D.; Morikawa, M.; Ike, M. Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2020, 96, 101. [Google Scholar] [CrossRef]
- Spanudakis, E.; Jackson, S. The role of microRNAs in the control of flowering time. J. Exp. Bot. 2014, 65, 365–380. [Google Scholar] [CrossRef]
- Van Dijk, A.D.J.; Molenaar, J. Floral pathway integrator gene expression mediates gradual transmission of environmental and endogenous cues to flowering time. PeerJ 2017, 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chaïlakhyan, M.K. Hormonal Theory of Plant Development; Akademii Nauk SSSR (Academy of Sciences of the USSR): Moscow, Russia, 1937. [Google Scholar]
- Pieterse, A.H. Is flowering in Lemnaceae stress-induced? A review. Aquat. Bot. 2013, 104, 1–4. [Google Scholar] [CrossRef]
- Maheshwari, S.C. Endosperm and Seed of Wolffia. Nat. Cell Biol. 1956, 178, 925–926. [Google Scholar] [CrossRef]
- Fu, L.; Huang, M.; Han, B.; Sun, X.; Sree, K.S.; Appenroth, K.-J.; Zhang, J. Flower induction, microscope-aided cross-pollination, and seed production in the duckweed Lemna gibba with discovery of a male-sterile clone. Sci. Rep. 2017, 7, 3047. [Google Scholar] [CrossRef] [PubMed]
- Khurana, J.P.; Maheshwari, S.C. Some effects of salicylic acid on growth and flowering in Spirodela polyrrhiza SP20. Plant Cell Physiol. 1980, 21, 923–927. [Google Scholar] [CrossRef]
- Lacor, M.A.M. Flowering of Spirodela Polyrhiza (L.) Schleiden. Acta Bot. Neerl. 1968, 17, 357–359. [Google Scholar] [CrossRef]
- Halliday, K.J.; Koornneef, M.; Whitelam, G.C. Phytochrome B and at Least One Other Phytochrome Mediate the Accelerated Flowering Response of Arabidopsis thaliana L. to Low Red/Far-Red Ratio. Plant Physiol. 1994, 104, 1311–1315. [Google Scholar] [CrossRef]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 2010, 1, 13. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kaul, S.; Koo, H.L.; Jenkins, J.; Rizzo, M.; Rooney, T.; Tallon, L.J.; Feldblyum, T.; Nierman, W.; Benito, M.I.; Lin, X.; et al. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Consortium, I.H.G.S. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in plants. Genes Dev. 2002, 16, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Barks, P.M.; Laird, R.A. Senescence in duckweed: Age-related declines in survival, reproduction and offspring quality. Funct. Ecol. 2015, 29, 540–548. [Google Scholar] [CrossRef]
- Barks, P.M.; Laird, R.A. A multigenerational effect of parental age on offspring size but not fitness in common duckweed (Lemna minor). J. Evol. Biol. 2016, 29, 748–756. [Google Scholar] [CrossRef]
- Mejbel, H.S.; Simons, A.M. Aberrant clones: Birth order generates life history diversity in Greater Duckweed, Spirodela polyrhiza. Ecol. Evol. 2018, 8, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Cleland, C.F.; Briggs, W.R. Flowering responses of the long-day plant Lemna gibba G3. Plant Physiol. 1967, 42, 1553–1561. [Google Scholar] [CrossRef]
- Lemon, G.D.; Posluszny, U. Comparative Shoot Development and Evolution in the Lemnaceae. Int. J. Plant Sci. 2000, 161, 733–748. [Google Scholar] [CrossRef]
- Basu, S. An Insight into the Factors Regulating Flowering in Rice: From Genetics to Epigenetics. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 233–247. [Google Scholar]
- Yaish, M.W.; Colasanti, J.; Rothstein, S.J. The role of epigenetic processes in controlling flowering time in plants exposed to stress. J. Exp. Bot. 2011, 62, 3727–3735. [Google Scholar] [CrossRef]
- Hébrard, C.; Peterson, D.G.; Willems, G.; Delaunay, A.; Jesson, B.; Lefèbvre, M.; Barnes, S.; Maury, S. Epigenomics and bolting tolerance in sugar beet genotypes. J. Exp. Bot. 2016, 67, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.-J. Useful Methods 1. Determination of Growth Rates in Duckweed. Int. Conf. Duckweed Res. Appl. 2015, 3, 34–80. [Google Scholar]
- Fourounjian, P. Small RNAs in Duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 157–164. [Google Scholar]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential actions of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of Flowering Time and Floral Organ Identity by a MicroRNA and Its APETALA2-Like Target Genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Krajnčič, B.; Devidé, Z. Flower development in Spirodela polyrrhiza (Lemnaceae). Plant Syst. Evol. 1979, 132, 305–312. [Google Scholar] [CrossRef]
- Takeno, K. Stress-induced flowering: The third category of flowering response. J. Exp. Bot. 2016, 67, 4925–4934. [Google Scholar] [CrossRef]
- Cho, L.-H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2016, 90, 708–719. [Google Scholar] [CrossRef]
- Hegelmaier, F. The Lemnaceen: A Monographic Study; Utersuchung: Liepzig, Germany, 1868. [Google Scholar]
- Khurana, J.; Maheshwari, S. Floral Induction in Wolffia microscopica by Salicylic Acid and Related Compounds under Non-inductive Long Days. Plant Cell Physiol. 1983, 24, 907–912. [Google Scholar] [CrossRef]
- Maheshwari, P.S. Induction of flowering in Wolffia microscopica by iron salt of ethylenediamine-di-o-hydroxyphenylacetic acid (Fe-EDDHA). Z. Pflanzenphysiol. 1966, 55, 89–95. [Google Scholar]
- Khurana, J.; Maheshwari, S. A Comparison of the Effects of Chelates, Salicylic Acid and Benzoic Acid on Growth and Flowering of Spirodela polyrrhiza. Plant Cell Physiol. 1986, 27, 919–924. [Google Scholar] [CrossRef]
- Pieterse, A.H.; Müller, L.J. Induction of flowering in Lemna gibba G3 under short-day conditions. Plant Cell Physiol. 1977, 18, 45–53. [Google Scholar] [CrossRef]
- Hernández-Apaolaza, L.; Lucena, J.J. Influence of irradiation time and solution concentration on the photochemical degradation of EDDHA/Fe3+: Effect of its photodecomposition products on soybean growth. J. Sci. Food Agric. 2011, 91, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Serikawa, M.; Suzuki, S.; Kondo, T.; Oyama, T. Conserved Expression Profiles of Circadian Clock-related Genes in Two Lemna Species Showing Long-day and Short-day Photoperiodic Flowering Responses. Plant Cell Physiol. 2006, 47, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.Y.; Slovin, J.P.; Cohen, J.D. Continuous light alters indole-3-acetic acid metabolism in Lemna gibba. Phytochemistry 1998, 49, 17–21. [Google Scholar] [CrossRef]
- Westfall, C.S.; Sherp, A.M.; Zubieta, C.; Alvarez, S.; Schraft, E.; Marcellin, R.; Ramirez, L.; Jez, J.M. Arabidopsis thaliana GH3.5 acyl acid amido synthetase mediates metabolic crosstalk in auxin and salicylic acid homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 13917–13922. [Google Scholar] [CrossRef]
- He, Y.; Michaels, S.D.; Amasino, R.M. Regulation of Flowering Time by Histone Acetylation in Arabidopsis. Science 2003, 302, 1751–1754. [Google Scholar] [CrossRef]
- Liu, F.; Quesada, V.; Crevillén, P.; Bäurle, I.; Swiezewski, S.; Dean, C. The Arabidopsis RNA-Binding Protein FCA requires a Lysine-specific Demethylase 1 Homolog to Downregulate FLC. Mol. Cell 2007, 28, 398–407. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Amasino, R.M. Role of chromatin modification in flowering-time control. Trends Plant Sci. 2005, 10, 30–35. [Google Scholar] [CrossRef]
- Singh, V.; Roy, S.; Giri, M.K.; Chaturvedi, R.; Chowdhury, Z.; Shah, J.; Nandi, A.K. Arabidopsis thaliana FLOWERING LOCUS D Is Required for Systemic Acquired Resistance. Mol. Plant Microbe Interact. 2013, 26, 1079–1088. [Google Scholar] [CrossRef]
- Singh, V.; Roy, S.; Singh, D.; Nandi, A.K. Arabidopsis Flowering Locus D influences systemic-acquired-resistance-induced expression and histone modifications of WRKY genes. J. Biosci. 2014, 39, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Tan, D.; Sun, X.; Ding, Z.; Zhang, J. Transcriptional analysis reveals potential genes and regulatory networks involved in salicylic acid-induced flowering in duckweed (Lemna gibba). Plant Physiol. Biochem. 2020, 155, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Rejmánková, E. Germination of seeds of Lemna gibba. Folia Geobot. Phytotaxon. 1976, 11, 261–267. [Google Scholar] [CrossRef]
- Bertin, R.I. Incidence of Monoecy and Dichogamy in Relation to Self-Fertilization in Angiosperms. Am. J. Bot. 1993, 80, 557–560. [Google Scholar] [CrossRef]
- Ruiz, L.A. La floración de Lemna gibba L. y Lemna parodiana Giard. (Lemnaceae) en Mendoza. Rev. Fac. Cienc. Agrar. 1951, 3, 1. [Google Scholar]
- Maheshwari, S.C. The embryology of Wolffia. Phytomorphology 1954, 4, 355–365. [Google Scholar]
- Brooks, J.S. The Cytology and Morphology of the Lemnaceae; Cornell University: Ithaca, NY, USA, 1940. [Google Scholar]
- Fujii, S.; Kubo, K.-I.; Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 16130. [Google Scholar] [CrossRef]
- Niu, S.-C.; Huang, J.; Zhang, Y.-Q.; Li, P.-X.; Zhang, G.-Q.; Xu, Q.; Chen, L.-J.; Wang, J.-Y.; Luo, Y.-B.; Liu, Z.-J. Lack of S-RNase-Based Gametophytic Self-Incompatibility in Orchids Suggests That This System Evolved after the Monocot-Eudicot Split. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.E. Zur Kultur von Wolffiella welwitschii Monod. Aqua Planta 1985, 10, 7. [Google Scholar]
- Caldwell, O.W. On the Life-History of Lemna minor. Int. J. Plant Sci. 1899, 27, 37–66. [Google Scholar] [CrossRef]
- Hillman, W.S. Experimental control of flowering in Lemna III. A relationship between medium composition and the opposite photoperiodic responses of L. perpusilla 6746 and L. gibba G3. Am. J. Bot. 1961, 48, 413–419. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 32. [Google Scholar]
- Shenk, R.U.; Hildebrandt, A.C. Production of Protoplasts from Plant Cells in Liquid Culture Using Purified Commercial Cellulases 1. Crop. Sci. 1969, 9, 629–631. [Google Scholar] [CrossRef]
- Steinberg, R.A. Mineral Requirements of Lemna minor. Plant Physiol. 1946, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]




| Strain | 1st Match | Accession | 2nd Match | Accession |
|---|---|---|---|---|
| DWC114 | Lemna gibba (5504) | KX212889.1 | Lemna gibba (7741) | KX212887.1 |
| DWC130 | Lemna minor (7210) | KX212888.1 | Lemna minor | DQ400350.1 |
| DWC131 | Lemna minor (7210) | KX212888.1 | Lemna minor | DQ400350.1 |
| DWC132 | Lemna japonica (0216) | KJ921747.1 | Lemna minor (7210) | KX212888.1 |
| Media | Abbreviation | Reference | pH | Supplier |
|---|---|---|---|---|
| E a | E | [60,99] | 4.6 | Chemicals from Sigma-Aldrich, St. Louis, MO, USA |
| Hoagland’s a | Hg | [100] | 5.8 | Cassion Labs, Smithfield, UT, USA |
| Shenk Hildebrandt a | SH | [101] | 5.8 | Sigma-Aldrich |
| Container | Container Size | Volume of Media | Supplier |
|---|---|---|---|
| Flask | 250 mL | 100 mL | VWR, Bridgeport, PA, USA |
| Small flask | 125 mL | 50 mL | VWR |
| Petri dish | 100 × 15 mm | 50 mL | Kord-Valmark, Bridgeport PA, USA |
| Multi-well Plate | 6 well | 10 mL | Corning, NY, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fourounjian, P.; Slovin, J.; Messing, J. Flowering and Seed Production across the Lemnaceae. Int. J. Mol. Sci. 2021, 22, 2733. https://doi.org/10.3390/ijms22052733
Fourounjian P, Slovin J, Messing J. Flowering and Seed Production across the Lemnaceae. International Journal of Molecular Sciences. 2021; 22(5):2733. https://doi.org/10.3390/ijms22052733
Chicago/Turabian StyleFourounjian, Paul, Janet Slovin, and Joachim Messing. 2021. "Flowering and Seed Production across the Lemnaceae" International Journal of Molecular Sciences 22, no. 5: 2733. https://doi.org/10.3390/ijms22052733
APA StyleFourounjian, P., Slovin, J., & Messing, J. (2021). Flowering and Seed Production across the Lemnaceae. International Journal of Molecular Sciences, 22(5), 2733. https://doi.org/10.3390/ijms22052733