Clinical Perspective on Proteomic and Glycomic Biomarkers for Diagnosis, Prognosis, and Prediction of Pancreatic Cancer
Abstract
1. Introduction
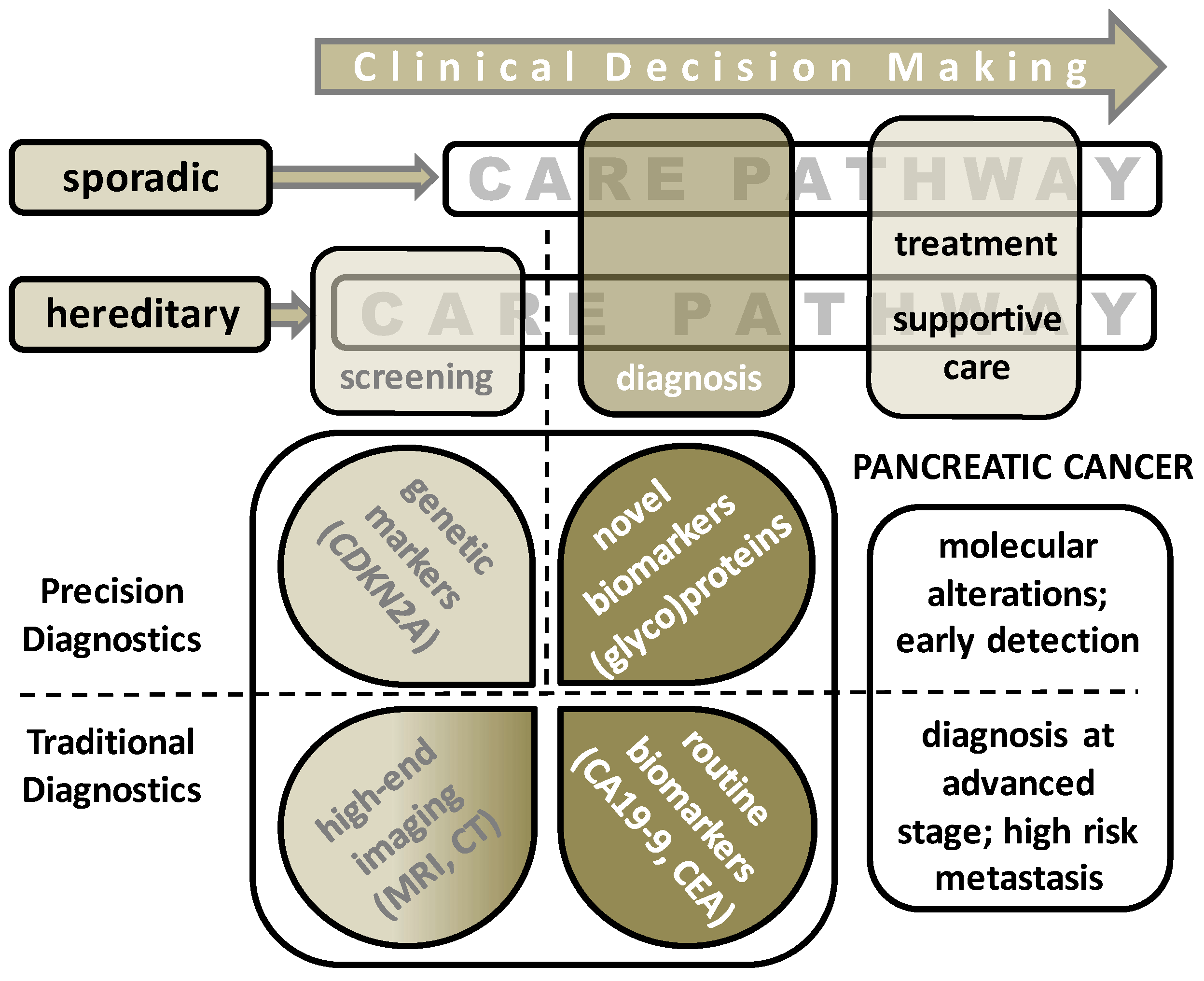
1.1. Sporadic Pancreatic Cancer
1.2. Hereditary Pancreatic Cancer
1.3. Diagnosis of Pancreatic Ductal Adenocarcinoma (PDAC)
1.4. Treatment Strategies for PDAC
2. Current Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma (PDAC)
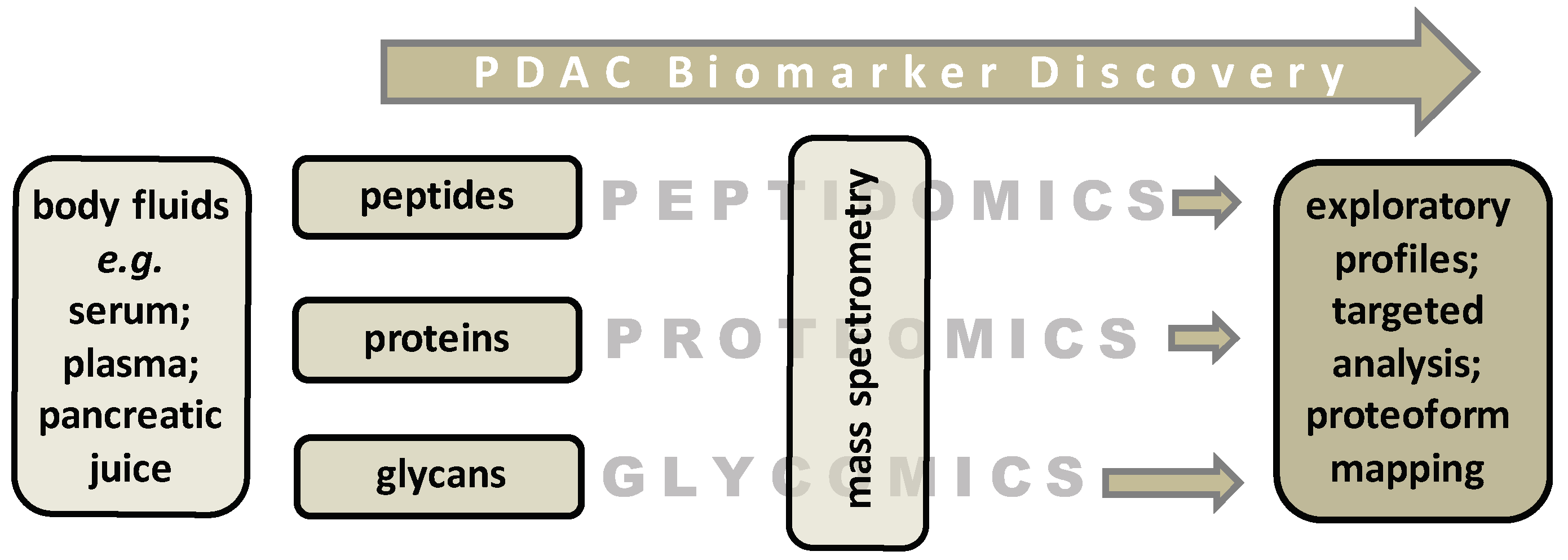
3. The Pursuit of Novel Diagnostic PDAC Biomarkers
3.1. Biomarker Discovery Studies by Mass Spectrometry (MS)-Based Proteomics and Glycomics
3.2. Prognostic Markers
3.3. Predictive Markers
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 28, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Kardosh, A.; Lichtensztajn, D.Y.; Gubens, M.A.; Kunz, P.L.; Fisher, G.A.; Clarke, C.A. Long-Term Survivors of Pancreatic Cancer: A California Population-Based Study. Pancreas 2018, 47, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Maddipati, R.; Lim, K.H.; Tozeren, A. Pancreatic cancer survival analysis defines a signature that predicts outcome. PLoS ONE 2018, 13, e0201751. [Google Scholar] [CrossRef]
- Alyass, A.; Turcotte, M.; Meyre, D. From big data analysis to personalized medicine for all: Challenges and opportunities. BMC Med. Genom. 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lennon, A.M.; Wolfgang, C.L.; Canto, M.I.; Klein, A.P.; Herman, J.M.; Goggins, M.; Fishman, E.K.; Kamel, I.; Weiss, M.J.; Diaz, L.A.; et al. The Early Detection of Pancreatic Cancer: What Will It Take to Diagnose and Treat Curable Pancreatic Neoplasia? Cancer Res. 2014, 74, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Chen, B.C.; Dong, L.; Zhou, M.T.; Andersson, R. Pancreatic cancer: Translational research aspects and clinical implications. World J. Gastroenterol. 2012, 18, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, K.; Levink, I.; Konings, I.; Harinck, F.; Koopmann, B.; Ausems, M.; Wagner, A.; Fockens, P.; Koerkamp, G.; Besselink, M.; et al. 12 Years of Prospective Pancreatic Cancer Surveillance: Results of the Dutch Nationwide Program in High-Risk Individuals. Gastroenterology 2019, 156, S-756–S-757. [Google Scholar] [CrossRef]
- Harsha, H.C.; Kandasamy, K.; Ranganathan, P.; Rani, S.; Ramabadran, S.; Gollapudi, S.; Balakrishnan, L.; Dwivedi, S.B.; Telikicherla, D.; Selvan, L.D.N.; et al. A Compendium of Potential Biomarkers of Pancreatic Cancer. PLoS Med. 2009, 6, e1000046. [Google Scholar] [CrossRef] [PubMed]
- Vreeker, G.C.M.; Hanna-Sawires, R.G.; Mohammed, Y.; Bladergroen, M.R.; Nicolardi, S.; Dotz, V.; Nouta, J.; Bonsing, B.A.; Mesker, W.E.; Van Der Burgt, Y.E.M.; et al. Serum N -Glycome analysis reveals pancreatic cancer disease signatures. Cancer Med. 2020, 9, 8519–8529. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Brentnall, T.A.; Chen, R. Proteomics analysis of bodily fluids in pancreatic cancer. Proteomics 2015, 15, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J.; Wang, J.I.; Kim, Y. Recent advances in proteomic profiling of pancreatic ductal adenocarcinoma and the road ahead. Expert Rev. Proteom. 2017, 14, 963–971. [Google Scholar] [CrossRef]
- Root, A.; Allen, P.; Tempst, P.; Yu, K. Protein Biomarkers for Early Detection of Pancreatic Ductal Adenocarcinoma: Progress and Challenges. Cancers 2018, 10, 67. [Google Scholar] [CrossRef]
- Ansari, D.; Torén, W.; Zhou, Q.; Hu, D.; Andersson, R. Proteomic and genomic profiling of pancreatic cancer. Cell Biol. Toxicol. 2019, 35, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.Y.; Tan, M.L.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Korc, M.; Jeon, C.Y.; Edderkaoui, M.; Pandol, S.J.; Petrov, M.S. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 529–536. [Google Scholar] [CrossRef]
- Vasen, H.; Ibrahim, I.; Ponce, C.G.; Slater, E.P.; Matthäi, E.; Carrato, A.; Earl, J.; Robbers, K.; Van Mil, A.M.; Potjer, T.; et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J. Clin. Oncol. 2016, 34, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Ralph, H.; Hruban, A.M.M.B.B.S.; Scott, E.; Kern, M.D.; Goggins, M.D. Precursors to Pancreatic Cancer. Gastroenterol. Clin. North Am. 2007, 36, 831–836. [Google Scholar]
- Weinrich, M.; Bochow, J.; Kutsch, A.L.; Alsfasser, G.; Weiss, C.; Klar, E.; Rau, B.M. High compliance with guideline recommendations but low completion rates of adjuvant chemotherapy in resected pancreatic cancer: A cohort study. Ann. Med. Surg. (Lond.) 2018, 32, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.H.; Relton, C.L.; Martin, R.M.; Smith, G.D.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2200. [Google Scholar] [CrossRef]
- Carrera, S.; Sancho, A.; Azkona, E.; Azkuna, J.; Lopez-Vivanco, G. Hereditary pancreatic cancer: Related syndromes and clinical perspective. Hered. Cancer Clin. Pr. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.K.; Andren-Sandberg, A.; Duell, E.J.; Goggins, M.; Korc, M.; Petersen, G.; Smith, J.; Whitcomb, D. Pancreatitis-diabetes-pancreatic cancer: Summary of an NIDDK-NCI workshop. Pancreas 2013, 42, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.C.; Wilentz, R.E.; Yeo, C.J.; Sohn, T.A.; Cameron, J.L.; Boitnott, J.K.; Hruban, R.H. Pancreaticoduodenectomy (Whipple Resections) in Patients Without Malignancy: Are They All ‘Chronic Pancreatitis’? Am. J. Surg. Pathol. 2003, 27, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jang, K.M.; Kim, J.H.; Jeong, W.K.; Kim, S.H.; Kang, T.W.; Kim, Y.K.; Cha, D.I.; Kim, K. Differentiation of mass-forming focal pancreatitis from pancreatic ductal adenocarcinoma: Value of characterizing dynamic enhancement patterns on contrast-enhanced MR images by adding signal intensity color mapping. Eur. Radiol. 2016, 27, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Nipp, R.; Tramontano, A.; Ali, A.; Zhan, T.; Pandharipande, P.; Dowling, E.; Ferrone, C.; Hong, T.; Schrag, D.; et al. Neoadjuvant FOLFIRINOX for Patients with Borderline Resectable or Locally Advanced Pancreatic Cancer: Results of a Decision Analysis. Oncologist 2019, 24, 945. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Abdelghani, B.M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, S.H.; Lavelle, L.P.; Hoare, S.M.; O’Neill, A.C.; Awan, F.N.; Malone, D.; Ryan, E.R.; McCann, J.W.; Heffernan, E.J. Pancreaticoduodenectomy: Expected post-operative anatomy and complications. Br. J. Radiol. 2014, 87, 20140050. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Laguna, I.; Hidalgo, M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat. Rev. Clin. Oncol. 2015, 12, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Vasen, H.F.; Wasser, M.; Van Mil, A.; Tollenaar, R.A.; Konstantinovski, M.; Gruis, N.A.; Bergman, W.; Hes, F.J.; Hommes, D.W.; Offerhaus, G.J.A.; et al. Magnetic Resonance Imaging Surveillance Detects Early-Stage Pancreatic Cancer in Carriers of a p16-Leiden Mutation. Gastroenterology 2011, 140, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Giardiello, F.M.; Brensinger, J.D.; Tersmette, A.C.; Goodman, S.N.; Petersen, G.M.; Booker, S.V.; Cruz–Correa, M.; Offerhaus, J.A. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology 2000, 119, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- van Lier, M.G.F.; Wagner, A.; Mathus-Vliegen, E.M.H.; Kuipers, E.J.; Steyerberg, E.W.; van Leerdam, M.E. High Cancer Risk in Peutz–Jeghers Syndrome: A Systematic Review and Surveillance Recommendations. Am. J. Gastroenterol. 2010, 105, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.J.; Klein, A.P. Genome-wide sequencing to identify the cause of hereditary cancer syndromes: With examples from familial pancreatic cancer. Cancer Lett. 2013, 340, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Shelton, C.A.; Brand, R.E. Genetics and Genetic Testing in Pancreatic Cancer. Gastroenterology 2015, 149, 1252–1264.e4. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, K.A.; Cahen, D.L.; Canto, M.I.; Bruno, M.J. Surveillance for neoplasia in the pancreas. Best Pr. Res. Clin. Gastroenterol. 2016, 30, 971–986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hruban, R.H.; Canto, M.I.; Goggins, M.; Schulick, R.; Klein, A.P. Update on Familial Pancreatic Cancer. Adv. Surg. 2010, 44, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2012, 62, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Healey, P.R. MRI and MRCP for Diagnosis and Staging of Pancreatic Cancer. Adv. Struct. Saf. Stud. 2018, 2018, 711–734. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef]
- Templeton, A.W.; Brentnall, T.A. Screening and Surgical Outcomes of Familial Pancreatic Cancer. Surg. Clin. North Am. 2013, 93, 629–645. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Lang, H. Neoadjuvant Therapy of Pancreatic Cancer: Definitions and Benefits. Int. J. Mol. Sci. 2017, 18, 1622. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Riviere, D.; Gurusamy, K.S.; Kooby, D.A.; Vollmer, C.M.; Besselink, M.G.H.; Davidson, B.R.; Van Laarhoven, C.J.H.M. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst. Rev. 2016, 4, CD011391. [Google Scholar] [CrossRef] [PubMed]
- Gooiker, G.A.; Lemmens, V.E.P.P.; Besselink, M.G.; Busch, O.R.; Bonsing, B.A.; Molenaar, I.Q.; Tollenaar, R.A.E.M.; De Hingh, I.H.J.T.; Wouters, M.W.J.M. Impact of centralization of pancreatic cancer surgery on resection rates and survival. BJS 2014, 101, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, M.; Kourie, H.R.; El Rassy, E.; Haddad, F.G.; Hanna, C.; El Karak, F.; Nasr, D. Where does chemotherapy stands in the treatment of ampullary carcinoma? A review of literature. World J. Gastrointest. Oncol. 2016, 8, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Jäger, C.; Schlitter, A.M.; Konukiewitz, B.; Stecher, L.; Schorn, S.; Tieftrunk, E.; Scheufele, F.; Calavrezos, L.; Schirren, R.; et al. R0 Versus R1 Resection Matters after Pancreaticoduodenectomy, and Less after Distal or Total Pancreatectomy for Pancreatic Cancer. Ann. Surg. 2018, 268, 1058–1068. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, Y.J.; Song, K.B.; Jun, E.; Hwang, D.W.; Lee, J.H.; Park, K.M. Totally laparoscopic or robot-assisted pancreaticoduodenectomy versus open surgery for periampullary neoplasms: Separate systematic reviews and meta-analyses. Surg. Endosc. 2016, 31, 3459–3474. [Google Scholar] [CrossRef] [PubMed]
- Torphy, R.J.; Friedman, C.; Halpern, A.; Chapman, B.C.; Ahrendt, S.S.; McCarter, M.M.; Edil, B.H.; Schulick, R.D.; Gleisner, A. Comparing Short-term and Oncologic Outcomes of Minimally Invasive Versus Open Pancreaticoduodenectomy Across Low and High Volume Centers. Ann. Surg. 2019, 270, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, C.; Senthilnathan, P.; Sabnis, S.C.; Babu, N.S.; Gurumurthy, S.S.; Vijai, N.A.; Nalankilli, V.P.; Raj, P.P.; Parthasarathy, R.; Rajapandian, S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. BJS 2017, 104, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, J.A.; Coppola, A.; Villacreses, D.; Mody, K.; Johnson, E.; Li, Z.; Asbun, H.J. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: Long-term results at a single institution. Surg. Endosc. 2016, 31, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, E.G.; Coveler, A.L. Pancreatic cancer: Optimizing treatment options, new, and emerging targeted therapies. Drug Des. Dev. Ther. 2015, 9, 3529–3545. [Google Scholar] [CrossRef]
- Labori, K.J.; Lassen, K.; Hoem, D.; Grønbech, J.E.; Søreide, J.A.; Mortensen, K.; Smaaland, R.; Sorbye, H.; Verbeke, C.; Dueland, S. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial-1 (NorPACT-1))-study protocol for a national multicentre randomized controlled trial. BMC Surg. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Landau, E.; Kalnicki, S. The Evolving Role of Radiation in Pancreatic Cancer. Surg. Clin. N. Am. 2018, 98, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The Clinical Utility of CA 19-9 in Pancreatic Adenocarcinoma: Diagnostic and Prognostic Updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [PubMed]
- Scarà, S.; Scatena, R. Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar]
- Kim, J.E.; Lee, J.K.; Paik, S.W.; Rhee, J.C.; Choi, K.W. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J. Gastroenterol. Hepatol. 2004, 19, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, J.; Wang, Y.; Tong, M.; Hu, H.; Huang, C.; Li, D. Diagnostic Value of CA 19-9 and Carcinoembryonic Antigen for Pancreatic Cancer: A Meta-Analysis. Gastroenterol. Res. Pr. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Wakabayashi-Nakao, K.; Ohshima, K.; Sakura, N.; Yamaguchi, K.; Mochizuki, T. Novel protein isoforms of carcinoembryonic antigen are secreted from pancreatic, gastric and colorectal cancer cells. BMC Res. Notes 2013, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef]
- Homma, T.R. The study of the mass screening of persons without symptoms and of the screening of outpatients with gastrointestinal complaints or icterus for pancreatic cancer in Japan, using CA19-9 and elastase-1 or ultrasonography. Int. J. Pancreatol. 1991, 9, 119–124. [Google Scholar]
- Detlefsen, S.; de Vos, J.D.; Tanassi, J.T.; Heegaard, N.H.H.; Fristrup, C.; de Muckadell, O.B.S. Value of anti-plasminogen binding peptide, anti-carbonic anhydrase II, immunoglobulin G4, and other serological markers for the differentiation of autoimmune pancreatitis and pancreatic cancer. Medicine 2018, 97, e11641. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, E.S.; Fukuyama, S.; Motoi, F.; Sunamura, M.; Isaji, S.; Imaizumi, T.; Okada, S.; Kato, H.; Suda, K.; Nakao, A.; et al. Pancreatic Cancer Registry in Japan 20 years of experience. Pancreas 2004, 28, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.C.; Wan, X.Y.; Zhu, H.T.; Yu, C.H.; Li, Y.M. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J Gastroenterol. 2015, 21, 8678–8686. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, P.K.J.; Park, H.D.; Kim, J.W.; Woo, H.I.; Lee, K.H.; Lee, K.T.; Lee, J.K.; Park, J.O.; Park, Y.S.; et al. Large-scale clinical validation of biomarkers for pancreatic cancer using a mass spectrometry-based proteomics approach. Oncotarget 2017, 28, 42761–42771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bamlet, W.R.; Oberg, A.L.; Chaffee, K.G.; Donahue, G.; Cao, X.J.; Chari, S.; Garcia, B.A.; Petersen, G.M.; Zaret, K.S. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci. Transl. Med. 2017, 9, eaah5583. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, W.; Wang, W.; Shen, H.; Liu, L.; Lou, W.; Wang, X.; Yang, P. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry-based pipeline. Br. J. Cancer 2017, 117, 1846–1854. [Google Scholar] [CrossRef]
- Bradley, S.D.; Talukder, A.H.; Lai, I.; Davis, R.; Alvarez, H.; Tiriac, H.; Zhang, M.; Chiu, Y.; Melendez, B.; Jackson, K.R.; et al. Vestigial-like 1 is a shared targetable cancer-placenta antigen expressed by pancreatic and basal-like breast cancers. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Chung, K.H.; Lee, J.C.; Cho, I.K.; Kim, J.; Jang, W.; Yoo, B.C.; Hwang, J.H. Serum fibrinogen as a diagnostic and prognostic biomarker for pancreatic ductal adenocarcinoma. Pancreatology 2020, 20, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Krisp, C.; Molloy, M.P.; Nahm, C.; Maloney, S.; Gillson, J.; Gill, A.J.; Samra, J.; Mittal, A. PSMD11, PTPRM and PTPRB as novel biomarkers of pancreatic cancer progression. Biochim. Biophys. Acta (BBA) Gen. Subj. 2020, 1864, 129682. [Google Scholar] [CrossRef] [PubMed]
- Mandili, G.; Follia, L.; Ferrero, G.; Katayama, H.; Hong, W.; Momin, A.A.; Capello, M.; Giordano, D.; Spadi, R.; Satolli, M.A.; et al. Immune-Complexome Analysis Identifies Immunoglobulin-Bound Biomarkers That Predict the Response to Chemotherapy of Pancreatic Cancer Patients. Cancers 2020, 12, 746. [Google Scholar] [CrossRef]
- Zhou, Q.; Andersson, R.; Hu, D.; Bauden, M.; Sasor, A.; Bygott, T.; Pawlowski, K.; Pla, I.; Marko-Varga, G.; Ansari, D. Alpha-1-acid glycoprotein 1 is upregulated in pancreatic ductal adenocarcinoma and confers a poor prognosis. Transl. Res. 2019, 212, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Z.; Chen, X.; Xu, Y.; Yin, N.; Yang, J.; Zhu, D.; Li, D.; Zhou, J. A Panel of Three Biomarkers Identified by iTRAQ for the Early Diagnosis of Pancreatic Cancer. Proteom. Clin. Appl. 2019, 13, e1800195. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.C.; Chang, M.C.; Chen, C.H.; Tsai, I.L.; Wang, S.Y.; Kuo, Y.P.; Chang, Y.T.; Chen, C.H. High accuracy differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma by immunoglobulin G glycosylation. Clin. Proteom. 2019, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.V.; Piersma, S.R.; Oudgenoeg, G.; Jimenez, C.R. Label-free mass spectrometry-based proteomics for biomarker discovery and validation. Expert Rev. Mol. Diagn. 2012, 12, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Velstra, B.; Bonsing, B.A.; Mertens, B.J.; Burgt, Y.E.M.; Huijbers, A.; Vasen, H.; Mesker, W.E.; Deelder, A.M.; Tollenaar, R.A.E.M. Detection of pancreatic cancer using serum protein profiling. HPB 2013, 15, 602–610. [Google Scholar] [CrossRef]
- Nicolardi, S.; Velstra, B.; Mertens, B.J.; Bonsing, B.; Mesker, W.E.; Tollenaar, R.A.; Deelder, A.M.; Van Der Burgt, Y.E. Ultrahigh resolution profiles lead to more detailed serum peptidome signatures of pancreatic cancer. Transl. Proteom. 2014, 2, 39–51. [Google Scholar] [CrossRef]
- Potjer, T.P.; Mertens, B.J.; Nicolardi, S.; Van Der Burgt, Y.E.M.; Bonsing, B.A.; Mesker, W.E.; Tollenaar, R.A.E.M.A.; Vasen, H.F. Application of a Serum Protein Signature for Pancreatic Cancer to Separate Cases from Controls in a Pancreatic Surveillance Cohort. Transl. Oncol. 2016, 9, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kinsinger, C.R.; Apffel, J.; Baker, M.; Bian, X.; Borchers, C.H.; Bradshaw, R.; Brusniak, M.Y.; Chan, D.W.; Deutsch, E.W.; Domon, B.; et al. Recommendations for mass spectrometry data quality metrics for open access data (corollary to the Amsterdam principles). Proteom. Clin. Appl. 2011, 5, 580–589. [Google Scholar] [CrossRef]
- Anne, K.C.; Werner, V.; Per, E.J.; Søren, C.; Ole, M.; Torben, A.; Kruse, O.N.J.; Jonna, S.M. Reproducibility of Mass Spectrometry Based Protein Profiles for Diagnosis of Breast Cancer across Clinical Studies: A Systematic Review. J. Proteome Res. 2008, 7, 1395–1402. [Google Scholar]
- Park, J.C.Y.; Namkung, J.; Yi, S.G.; Kim, H.; Yu, J.; Kim, Y.; Kwon, M.S.; Kwon, W.; Oh, D.Y.; Kim, S.W.; et al. Diagnostic performance enhancement of pancreatic cancer using proteomic multimarker panel. Oncotarget 2017, 8, 93117–93130. [Google Scholar] [CrossRef]
- van der Burgt, Y.E.M.; Cobbaert, C.M. Proteoform Analysis to Fulfill Unmet Clinical Needs and Reach Global Standardization of Protein Measurands in Clinical Chemistry Proteomics. Clin. Lab. Med. 2018, 38, 487–497. [Google Scholar] [CrossRef]
- Jimenez-Luna, C.; Torres, C.; Ortiz, R.; Dieguez, C.; Martinez-Galan, J.; Melguizo, C.; Prados, J.C.; Caba, O. Proteomic biomarkers in body fluids associated with pancreatic cancer. Oncotarget 2018, 9, 16573–16587. [Google Scholar] [CrossRef]
- Pan, S.A.; Brentnall, T.; Chen, R. Glycoproteins and glycoproteomics in pancreatic cancer. World J. Gastroenterol. 2016, 22, 9288–9299. [Google Scholar] [CrossRef] [PubMed]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanov, U.; Kosanam, H.; Diamandis, E.P. The sweet and sour of serological glycoprotein tumor biomarker quantification. BMC Med. 2013, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Llop, E.; Guerrero, P.; Duran, A.; Barrabés, S.; Massaguer, A.; Ferri, M.J.; Albiol-Quer, M.; De Llorens, R.; Peracaula, R. Glycoprotein biomarkers for the detection of pancreatic ductal adenocarcinoma. World J. Gastroenterol. 2018, 24, 2537–2554. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Hwang, M.P.; Kim, Y.W.; Kim, K.J.; Jin, J.M.; Kim, Y.H.; Yang, Y.H.; Lee, K.H.; Kim, Y.G. Mass spectrometry-based N-linked glycomic profiling as a means for tracking pancreatic cancer metastasis. Carbohydr. Res. 2015, 413, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef]
- Nagata, K.; Horinouchi, M.; Saitou, M.; Higashi, M.; Nomoto, M.; Goto, M.; Yonezawa, S. Mucin expression profile in pancreatic cancer and the precursor lesions. J. Hepato-Biliary-Pancreatic Surg. 2007, 14, 243–254. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernández, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nat. Cell Biol. 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Lucien, F.; Lac, V.; Billadeau, D.D.; Borgida, A.; Gallinger, S.; Leong, H.S. Glypican-1 and glycoprotein 2 bearing extracellular vesicles do not discern pancreatic cancer from benign pancreatic diseases. Oncotarget 2019, 10, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, S.; Hayakawa, T.; Kando, T.; Shibata, T.; Kitagawa, M.; Ono, H.; Sakai, Y. Usefulness of a new tumor marker, span-1, for the diagnosis of pancreatic cancer. Cancer 1990, 65, 1557–1561. [Google Scholar] [CrossRef]
- Nigjeh, E.N.; Chen, R.; Allen-Tamura, Y.; Brand, R.E.; Brentnall, T.A.; Pan, S. Spectral library-based glycopeptide analysis-detection of circulating galectin-3 binding protein in pancreatic cancer. Proteom. Clin. Appl. 2017, 11, 1700064. [Google Scholar] [CrossRef]
- Krishnan, S.; Whitwell, H.J.; Cuenco, J.; Gentry-Maharaj, A.; Menon, U.; Pereira, S.P.; Gaspari, M.; Timms, J.F. Evidence of Altered Glycosylation of Serum Proteins Prior to Pancreatic Cancer Diagnosis. Int. J. Mol. Sci. 2017, 18, 2670. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Lo, A.; Wu, J.; Zhu, J.; Tan, Z.; Simeone, D.M.; Anderson, M.A.; Shedden, K.A.; Ruffin, M.T.; Lubman, D.M. Glycoprotein Biomarker Panel for Pancreatic Cancer Discovered by Quantitative Proteomics Analysis. J. Proteome Res. 2014, 13, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Kontro, H.; Joenväärä, S.; Haglund, C.; Renkonen, R. Comparison of sialylated N -glycopeptide levels in serum of pancreatic cancer patients, acute pancreatitis patients, and healthy controls. Proteomics 2014, 14, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yin, H.; Nie, S.; Lin, Z.; Zhu, J.; Ruffin, M.T.; Anderson, M.A.; Simeone, D.M.; Lubman, D.M. Large-Scale Identification of Core-Fucosylated Glycopeptide Sites in Pancreatic Cancer Serum Using Mass Spectrometry. J. Proteome Res. 2015, 14, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, L.; Andersson, R.; Bauden, M.; Andersson, B.; Bygott, T.; Ansari, D. High-density and targeted glycoproteomic profiling of serum proteins in pancreatic cancer and intraductal papillary mucinous neoplasm. Scand. J. Gastroenterol. 2018, 53, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Sund, M.; Vinci, A.; Beyer, G.; Javed, M.A.; Krug, S.; Neessee, A.; Schober, M. Prognostic and predictive markers in pancreatic adenocarcinoma. Dig. Liver Dis. 2016, 48, 223–230. [Google Scholar] [CrossRef] [PubMed]
- de Vroome, S.W.; Girondo, M.R.; van der Burgt, Y.E.M.; Mesker, W.E.; Tollenaar, R.A.E.M.; Wuhrer, M. Serum N-glycome alterations in colorectal cancer associate with survival. Oncotarget 2018, 9, 30610–30623. [Google Scholar] [CrossRef] [PubMed]
- Anugraham, M.; Jacob, F.; Everest-Dass, A.V.; Schoetzau, A.; Nixdorf, S.; Hacker, N.F.; Fink, D.; Heinzelmann-Schwarz, V.; Packer, N.H. Tissue glycomics distinguish tumour sites in women with advanced serous adenocarcinoma. Mol. Oncol. 2017, 11, 1595–1615. [Google Scholar] [CrossRef]
- (Saldova), R.F.; Haakensen, V.D.; Rødland, E.; Walsh, I.; Stockmann, H.; Engebraaten, O.; Børresen-Dale, A.L.; Rudd, P.M. Serum N-glycome alterations in breast cancer during multimodal treatment and follow-up. Mol. Oncol. 2017, 11, 1361–1379. [Google Scholar] [CrossRef]
- Jenkinson, C.; Elliott, V.L.; Evans, A.; Oldfield, L.; Jenkins, R.E.; O’Brien, D.P.; Apostolidou, S.; Gentry-Maharaj, A.; Fourkala, E.O.; Jacobs, I.J.; et al. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin. Cancer Res. 2016, 22, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Boeck, S.; Wittwer, C.; Heinemann, V.; Haas, M.; Kern, C.; Stieber, P.; Nagel, D.; Holdenrieder, S. Cytokeratin 19-fragments (CYFRA 21-1) as a novel serum biomarker for response and survival in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: A systematic review and meta-analysis. OncoTargets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, C.; Jiang, J.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Zhang, Z.; Xu, J.; Liu, L.; et al. Analysis of ctDNA to predict prognosis and monitor treatment responses in metastatic pancreatic cancer patients. Int. J. Cancer 2017, 140, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- van der Sijde, F.; Vietsch, E.E.; Mustafa, D.A.M.; Besselink, M.G.; Groot, K.B.; van Eijck, C.H.J. Circulating Biomarkers for Prediction of Objective Response to Chemotherapy in Pancreatic Cancer Patients. Cancers. 2019, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Kawamoto, H.; Hirao, K.; Sakakihara, I.; Yamamoto, N.; Noma, Y.; Fujii, M.; Kato, H.; Ogawa, T.; Ishida, E.; et al. Monitoring of CA19-9 and SPan-1 can facilitate the earlier confirmation of progressing pancreatic cancer during chemotherapy. Pancreatol. 2012, 12, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Santucci, N.; Facy, O.; Ortega-Deballon, P.; Lequeu, J.B.; Rat, P.; Rat, P. CA 19–9 predicts resectability of pancreatic cancer even in jaundiced patients. Pancreatol. 2018, 18, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, S.; Aihara, T.; Yamanaka, N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia-Pacific J. Clin. Oncol. 2019, 15, e109–e114. [Google Scholar] [CrossRef] [PubMed]
| Marker | Type | Study Size N | Sensitivity (%) | Specificity (%) | Methodology | Reference |
|---|---|---|---|---|---|---|
| CA 19-9 (meta-analysis of 13 studies) | Diagnostic | 25–641 PDAC | 72–86 | 68–80 | Routine Diagnostics | H. Xing et al. |
| CEA (meta-analysis of 23 studies) | Diagnostic | 17–123 PDAC 15–58 BD | 38–50 | 82–91 | Routine Diagnostics | K. Poruk et al. |
CA 19-9 combined with:
| Diagnostic | 42 early stage (I/II) PDAC 72 advanced stage (III/IV) PDAC 31 pancreatitis 35 controls | 86 | 90 | Routine Diagnostics & MS-based Proteomics | J. Park et al. |
CA 19-9 combined with:
| Diagnostic | 81 PDAC 80 controls | 87 | 98 | Routine Diagnostics & ELISA | J. Kim et al. |
CA 19-9 combined with:
| Diagnostic | 80 PDAC 30 BD 40 controls | 95 | 94 | Routine Diagnostics & MS-based Proteomics | X. Liu et al. |
Peptide signature:
| Diagnostic | Calibration set: 50 PDAC 110 controls Validation set: 39 PDAC 75 controls | 78 74 | 89 91 | MS-based Peptidomics | B. Velstra et al. S. Nicolardi et al. |
Multimarker panel:
| Diagnostic | 50 PDAC 34 PL 50 controls | 82 | 92 | MS-based Proteomics | J. Park et al. |
| SPAN-1 | Diagnostic | 64 PDAC 90 other cancers 254 BD 55 controls | 81 | 76 | Radioimmuno assay | S. Kiriyama et al. |
Multimarker panel:
| Diagnostic | 154 PDAC 154 controls | Not reported | Not reported | MS-based Proteomics | S. Krishnan et al. |
Multimarker panel:
| Diagnostic | 37 PDAC 30 CP 30 DMII 30 PC 22 OJ 30 controls | 91 | 78 | MS-based Proteomics | S. Nie et al. |
| Thrombospondin-1 | Prognostic | 152 PDAC 57 CP 13 DMII 20 BD 56 controls | Not reported | Not reported | MS-based Proteomics | Jenkinson et al. |
| CEA | Prognostic | Not reported | Not reported | Routine Diagnostics | S. Boeck et al. | |
| Cytokeratin-19 | Prognostic | Not reported | Not reported | Routine Diagnostics | S. Boeck et al. | |
| SPAN-1 | Predictive | 90 | 77 | immunoassay | K. Tsutsumi et al. | |
| CA 19-9 | Predictive | 85 | 81 | Routine Diagnostics | N. Santucci et al. | |
| CAR | Predictive | Not reported | Not reported | Routine Diagnostics | S. Ikuta et al. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna-Sawires, R.G.; Schiphuis, J.H.; Wuhrer, M.; Vasen, H.F.A.; van Leerdam, M.E.; Bonsing, B.A.; Mesker, W.E.; van der Burgt, Y.E.M.; Tollenaar, R.A.E.M. Clinical Perspective on Proteomic and Glycomic Biomarkers for Diagnosis, Prognosis, and Prediction of Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2655. https://doi.org/10.3390/ijms22052655
Hanna-Sawires RG, Schiphuis JH, Wuhrer M, Vasen HFA, van Leerdam ME, Bonsing BA, Mesker WE, van der Burgt YEM, Tollenaar RAEM. Clinical Perspective on Proteomic and Glycomic Biomarkers for Diagnosis, Prognosis, and Prediction of Pancreatic Cancer. International Journal of Molecular Sciences. 2021; 22(5):2655. https://doi.org/10.3390/ijms22052655
Chicago/Turabian StyleHanna-Sawires, Randa G., Jorinde H. Schiphuis, Manfred Wuhrer, Hans F. A. Vasen, Monique E. van Leerdam, Bert A. Bonsing, Wilma E. Mesker, Yuri E. M. van der Burgt, and Rob A. E. M. Tollenaar. 2021. "Clinical Perspective on Proteomic and Glycomic Biomarkers for Diagnosis, Prognosis, and Prediction of Pancreatic Cancer" International Journal of Molecular Sciences 22, no. 5: 2655. https://doi.org/10.3390/ijms22052655
APA StyleHanna-Sawires, R. G., Schiphuis, J. H., Wuhrer, M., Vasen, H. F. A., van Leerdam, M. E., Bonsing, B. A., Mesker, W. E., van der Burgt, Y. E. M., & Tollenaar, R. A. E. M. (2021). Clinical Perspective on Proteomic and Glycomic Biomarkers for Diagnosis, Prognosis, and Prediction of Pancreatic Cancer. International Journal of Molecular Sciences, 22(5), 2655. https://doi.org/10.3390/ijms22052655






