Physcomitrium patens: A Single Model to Study Oriented Cell Divisions in 1D to 3D Patterning
Abstract
1. Introduction
Glossary
- Formative cell division: cell division that generates daughters with different identities; also called formative asymmetric cell division (ACD).
- Proliferative cell division: cell division that generates daughters of the same identity; also called symmetric cell division (SCD).
- Cell fate/cell identity: commitment to cell type-specific genetic programs.
- Cell division plane: Actual or forecast plane physically separating two daughter cells.
- Symmetry-breaking/cellular polarization: unequal distribution of molecules and cellular components. Required for important processes like differential cell fate acquisition of two daughter cells.
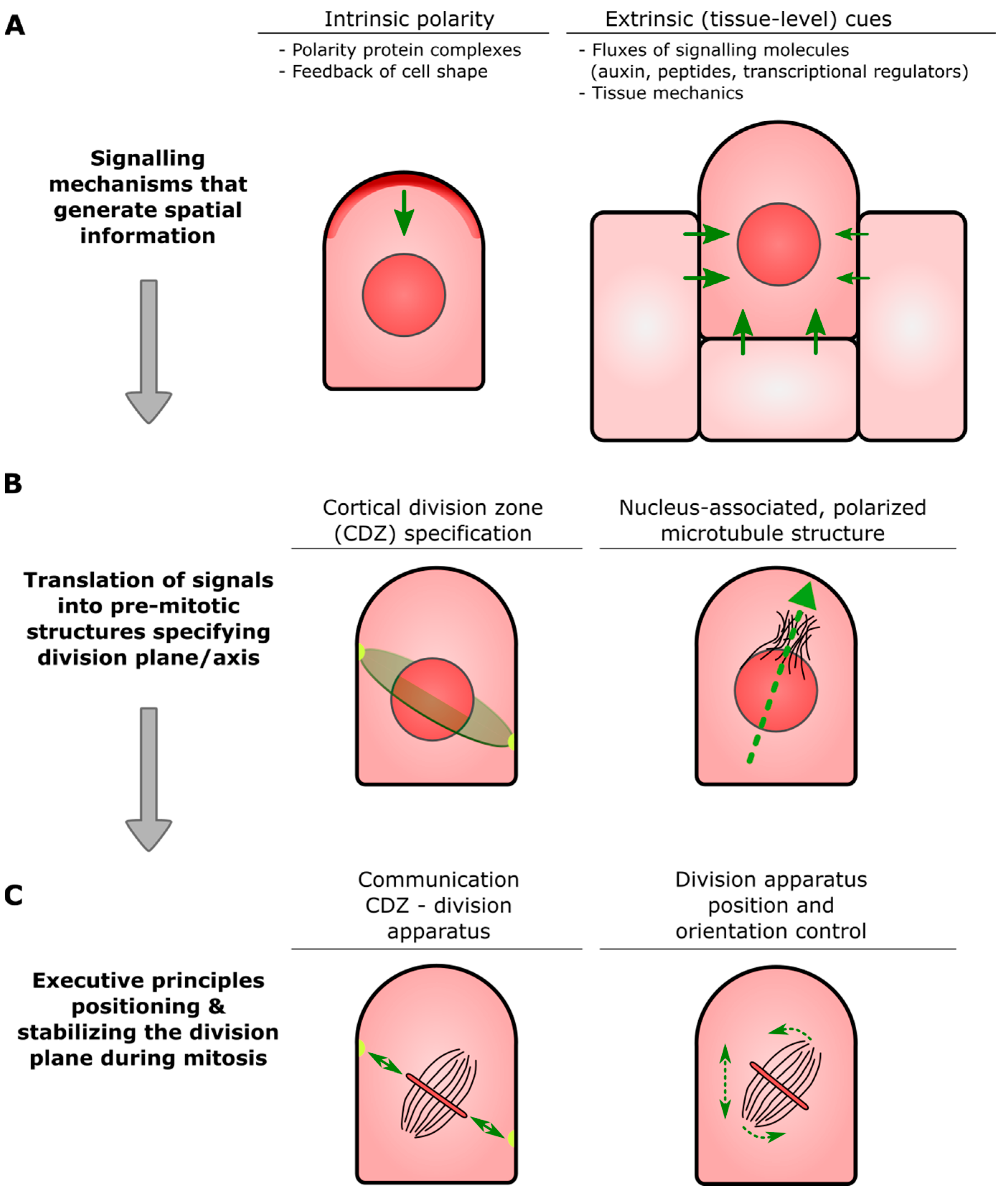
- Cortical Division Zone (CDZ): A membrane and cell wall-associated domain at the cell cortex established at or just before mitotic entry that specifies a plane in the parental cell through which daughter cells will ultimately be partitioned (see also Figure 1B). The CDZ has a dynamic composition that includes cytoskeletal and membrane-bound components, and functions as landmark for the correct insertion of the nascent dividing wall constructed by the phragmoplast.
- Pre-prophase band (PPB): Ring-shaped assembly of the microtubule cytoskeleton and associated proteins that transiently appears before the onset of cell division. The overall orientation of the PPB appears to be inherited from that of the interphase cortical microtubules, and its position correlates with that of the CDZ.
- Phragmoplast: Plant-specific cellular apparatus that brings about physical separation of two newly formed daughter cells (cytokinesis) at the end of cell division. It consists of two opposing sets of microtubules, in the center of which, small, membranous building blocks are assembled into a radially expanding precursor of the new dividing wall. Insertion of this precursor at the parental wall occurs at the site specified by the CDZ.
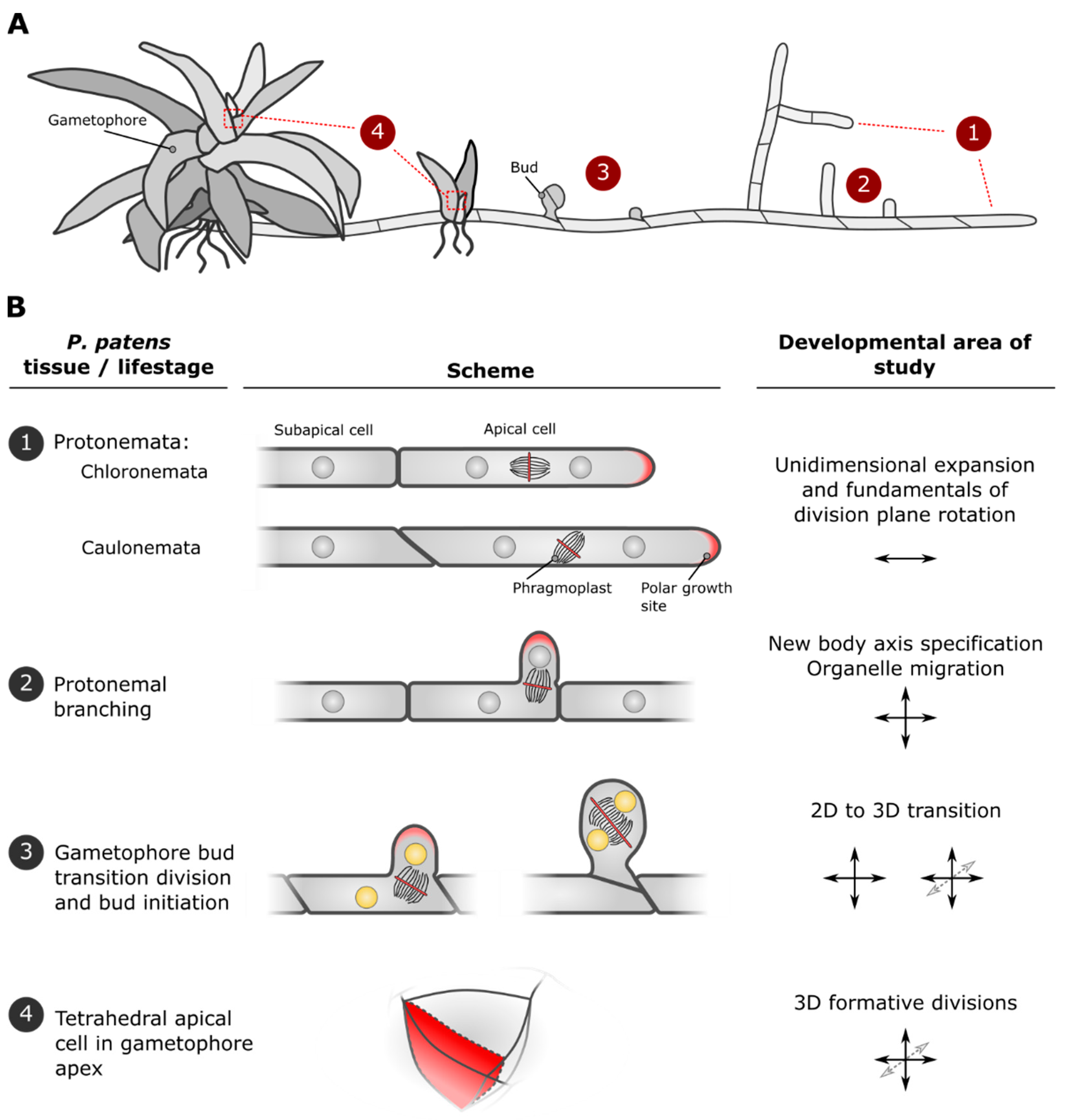
2. Developmental Stages of P. patens Are Marked by Characteristic Cell Divisions and Establishment of New Growth Axes
3. Signaling Molecules Driving Cell Polarization in Moss
3.1. ROPs
3.2. SOSEKIs
4. Peptide-Mediated Intercellular Signaling during Moss Development
5. Role of the Cytoskeleton in Division Plane Control
6. Cellular and Transcriptional Signal Transduction Mechanisms for Asymmetric Cell Divisions
6.1. Defective Kernel1
6.2. Transcriptional Regulation by APBs
7. Hormonal Regulation of Asymmetric Cell Divisions in Moss
7.1. Auxin
7.2. Cytokinin
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Scheres, B. PLETHORA transcription factors orchestrate de novo organ patterning during Arabidopsislateral root outgrowth. Proc. Natl. Acad. Sci. USA 2017, 114, 11709–11714. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 Gene Encodes a Putative Novel Protein Phosphatase 2A Regulatory Subunit Essential for the Control of the Cortical Cytoskeleton. Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Traas, J.; Bellini, C.; Nacry, P.; Kronenberger, J.; Bouchez, D.; Caboche, M. Normal differentiation patterns in plants lacking microtubular preprophase bands. Nat. Cell Biol. 1995, 375, 676–677. [Google Scholar] [CrossRef]
- Torres-Ruiz, R.A.; Jürgens, G. Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development 1994, 120, 2967–2978. [Google Scholar] [PubMed]
- Berleth, T.; Jurgens, G. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 1993, 118, 575–587. [Google Scholar]
- Hove, C.A.T.; Lu, K.-J.; Weijers, D. Building a plant: Cell fate specification in the early Arabidopsis embryo. Development 2015, 142, 420–430. [Google Scholar] [CrossRef]
- Petricka, J.J.; Van Norman, J.M.; Benfey, P.N. Symmetry Breaking in Plants: Molecular Mechanisms Regulating Asymmetric Cell Divisions in Arabidopsis. Cold Spring Harb. Perspect. Biol. 2009, 1, a000497. [Google Scholar] [CrossRef] [PubMed]
- Menand, B.; Calder, G.; Dolan, L. Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J. Exp. Bot. 2007, 58, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Yi, K.; Pires, N.D.; Menand, B.; Dolan, L. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 2011, 138, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Thelander, M.; Landberg, K.; Sundberg, E. Auxin-mediated developmental control in the moss Physcomitrella patens. J. Exp. Bot. 2018, 69, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; de Reuille, P.B.; Lane, B.; Bassel, G.W.; Prusinkiewicz, P.; Smith, R.S.; Weijers, D. Genetic Control of Plant Development by Overriding a Geometric Division Rule. Dev. Cell 2014, 29, 75–87. [Google Scholar] [CrossRef]
- Serra, L.; Robinson, S. Plant cell divisions: Variations from the shortest symmetric path. Biochem. Soc. Trans. 2020, 48, 2743–2752. [Google Scholar] [CrossRef]
- Coudert, Y.; Harris, S.; Charrier, B. Design Principles of Branching Morphogenesis in Filamentous Organisms. Curr. Biol. 2019, 29, R1149–R1162. [Google Scholar] [CrossRef] [PubMed]
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Nogué, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Thelander, M.; Olsson, T.; Ronne, H. Effect of the energy supply on filamentous growth and development in Physcomitrella patens. J. Exp. Bot. 2004, 56, 653–662. [Google Scholar] [CrossRef]
- Schmiedel, G.; Schnepf, E. Side branch formation and orientation in the caulonema of the moss, Funaria hygrometrica: Experiments with inhibitors and with centrifugation. Protoplasma 1979, 101, 47–59. [Google Scholar] [CrossRef]
- Uenaka, H.; Wada, M.; Kadota, A. Four distinct photoreceptors contribute to light-induced side branch formation in the moss Physcomitrella patens. Planta 2005, 222, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Mwaura, B.W.; Stauffer, S.R.C.; Bezanilla, M. A Fully Functional ROP Fluorescent Fusion Protein Reveals Roles for This GTPase in Subcellular and Tissue-Level Patterning. Plant Cell 2020, 32, 3436–3451. [Google Scholar] [CrossRef]
- Yi, P.; Goshima, G. Rho of Plants GTPases and Cytoskeletal Elements Control Nuclear Positioning and Asymmetric Cell Division during Physcomitrella patens Branching. Curr. Biol. 2020, 30, 2860–2868.e3. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Roeder, A.H.; Meyerowitz, E.M.; Langdale, J.A. Local Cues and Asymmetric Cell Divisions Underpin Body Plan Transitions in the Moss Physcomitrella patens. Curr. Biol. 2009, 19, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kosetsu, K.; Murata, T.; Yamada, M.; Nishina, M.; Boruc, J.; Hasebe, M.; Van Damme, D.; Goshima, G. Cytoplasmic MTOCs control spindle orientation for asymmetric cell division in plants. Proc. Natl. Acad. Sci. USA 2017, 114, E8847–E8854. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Duijts, K.; Bezanilla, M.; Scheres, B.; Vermeer, J.E.M.; Willemsen, V. Geometric cues forecast the switch from two- to three-dimensional growth in Physcomitrella patens. New Phytol. 2019, 225, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.A. The 2D to 3D growth transition in the moss Physcomitrella patens. Curr. Opin. Plant Biol. 2019, 47, 88–95. [Google Scholar] [CrossRef]
- Zagórska-Marek, B.; Sokołowska, K.; Turzańska, M. Chiral events in developing gametophores of Physcomitrella patens and other moss species are driven by an unknown, universal direction-sensing mechanism. Am. J. Bot. 2018, 105, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Véron, E.; Vernoux, T.; Coudert, Y. Phyllotaxis from a Single Apical Cell. Trends Plant Sci. 2021, 26, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Feiguelman, G.; Fu, Y.; Yalovsky, S. ROP GTPases Structure-Function and Signaling Pathways. Plant Physiol. 2018, 176, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, H.; Chen, B.; Zhang, R.; Huang, S.; Fu, Y. Arabidopsis RIC1 Severs Actin Filaments at the Apex to Regulate Pollen Tube Growth. Plant Cell 2015, 27, 1140–1161. [Google Scholar] [CrossRef]
- Li, C.; Lu, H.; Li, W.; Yuan, M.; Fu, Y. A ROP2-RIC1 pathway fine-tunes microtubule reorganization for salt tolerance in Arabidopsis. Plant Cell Environ. 2017, 40, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Eklund, D.M.; Svensson, E.M.; Kost, B. Physcomitrella patens: A model to investigate the role of RAC/ROP GTPase signalling in tip growth. J. Exp. Bot. 2010, 61, 1917–1937. [Google Scholar] [CrossRef]
- Humphries, J.A.; Vejlupkova, Z.; Luo, A.; Meeley, R.B.; Sylvester, A.W.; Fowler, J.E.; Smith, L.G. ROP GTPases Act with the Receptor-Like Protein PAN1 to Polarize Asymmetric Cell Division in Maize. Plant Cell 2011, 23, 2273–2284. [Google Scholar] [CrossRef]
- Goldstein, B.; Macara, I.G. The PAR Proteins: Fundamental Players in Animal Cell Polarization. Dev. Cell 2007, 13, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Hiwatashi, T.; Goh, H.; Yasui, Y.; Koh, L.Q.; Takami, H.; Kajikawa, M.; Kirita, H.; Kanazawa, T.; Minamino, N.; Togawa, T.; et al. The RopGEF KARAPPO Is Essential for the Initiation of Vegetative Reproduction in Marchantia polymorpha. Curr. Biol. 2019, 29, 3525–3531.e7. [Google Scholar] [CrossRef] [PubMed]
- Van Dop, M.; Fiedler, M.; Mutte, S.; de Keijzer, J.; Olijslager, L.; Albrecht, C.; Liao, C.-Y.; Janson, M.E.; Bienz, M.; Weijers, D. DIX Domain Polymerization Drives Assembly of Plant Cell Polarity Complexes. Cell 2020, 180, 427–439.e12. [Google Scholar] [CrossRef]
- Yoshida, S.; Van Der Schuren, A.; Van Dop, M.; Van Galen, L.; Saiga, S.; Adibi, M.; Möller, B.; Hove, C.A.T.; Marhavy, P.; Smith, R.; et al. A SOSEKI-based coordinate system interprets global polarity cues in Arabidopsis. Nat. Plants 2019, 5, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T.; Tsukaya, H.; Uchimiya, H. Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 1996, 122, 1589–1600. [Google Scholar] [PubMed]
- Bai, Y.; Vaddepalli, P.; Fulton, L.; Bhasin, H.; Hülskamp, M.; Schneitz, K. ANGUSTIFOLIA is a central component of tissue morphogenesis mediated by the atypical receptor-like kinase STRUBBELIG. BMC Plant Biol. 2013, 13, 16. [Google Scholar] [CrossRef]
- Shao, W.; Dong, J. Polarity in plant asymmetric cell division: Division orientation and cell fate differentiation. Dev. Biol. 2016, 419, 121–131. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Tang, G.; Huang, T. Small but powerful: Function of microRNAs in plant development. Plant Cell Rep. 2018, 37, 515–528. [Google Scholar] [CrossRef]
- Fletcher, J.C. Recent Advances in Arabidopsis CLE Peptide Signaling. Trends Plant Sci. 2020, 25, 1005–1016. [Google Scholar] [CrossRef]
- Yamaguchi, Y.L.; Ishida, T.; Sawa, S. CLE peptides and their signaling pathways in plant development. J. Exp. Bot. 2016, 67, 4813–4826. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.; Jürgens, G.; Laux, T. The Stem Cell Population of Arabidopsis Shoot Meristems Is Maintained by a Regulatory Loop between the CLAVATA and WUSCHEL Genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef]
- Stahl, Y.; Simon, R. Peptides and receptors controlling root development. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1453–1460. [Google Scholar] [CrossRef][Green Version]
- Etchells, J.P.; Turner, S.R.; Perez-Alcala, S.; Nieto, M.A.; Barbas, J.A. The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 2010, 137, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Uchida, N.; Yamaguchi, Y.L.; Tabata, R.; Ishida, S.; Ishizaki, K.; Nishihama, R.; Kohchi, T.; Sawa, S.; Bowman, J.L. Control of proliferation in the haploid meristem by CLE peptide signaling in Marchantia polymorpha. PLoS Genet. 2019, 15, e1007997. [Google Scholar] [CrossRef]
- Whitewoods, C.D.; Cammarata, J.; Venza, Z.N.; Sang, S.; Crook, A.D.; Aoyama, T.; Wang, X.Y.; Waller, M.; Kamisugi, Y.; Cuming, A.C.; et al. CLAVATA Was a Genetic Novelty for the Morphological Innovation of 3D Growth in Land Plants. Curr. Biol. 2018, 28, 2365–2376.e5. [Google Scholar] [CrossRef]
- Smertenko, A. Phragmoplast expansion: The four-stroke engine that powers plant cytokinesis. Curr. Opin. Plant Biol. 2018, 46, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Müller, S. Plant cell division—Defining and finding the sweet spot for cell plate insertion. Curr. Opin. Cell Biol. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Livanos, P.; Müller, S. Division Plane Establishment and Cytokinesis. Annu. Rev. Plant Biol. 2019, 70, 239–267. [Google Scholar] [CrossRef]
- Buschmann, H.; Müller, S. Update on plant cytokinesis: Rule and divide. Curr. Opin. Plant Biol. 2019, 52, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.G.; Bellinger, M. An overview of plant division-plane orientation. New Phytol. 2018, 219, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Komis, G.; Luptovčiak, I.; Ovečka, M.; Samakovli, D.; Šamajová, O.; Šamaj, J. Katanin Effects on Dynamics of Cortical Microtubules and Mitotic Arrays in Arabidopsis thaliana Revealed by Advanced Live-Cell Imaging. Front. Plant Sci. 2017, 8, 866. [Google Scholar] [CrossRef]
- Vos, J.W.; Dogterom, M.; Emons, A.M.C. Microtubules become more dynamic but not shorter during preprophase band formation: A possible “search-and-capture” mechanism for microtubule translocation. Cell Motil. Cytoskelet. 2004, 57, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Mineyuki, Y. The Preprophase Band of Microtubules: Its Function as a Cytokinetic Apparatus in Higher Plants. Adv. Appl. Microbiol. 1999, 187, 1–49. [Google Scholar] [CrossRef]
- Chakrabortty, B.; Willemsen, V.; de Zeeuw, T.; Liao, C.-Y.; Weijers, D.; Mulder, B.; Scheres, B. A Plausible Microtubule-Based Mechanism for Cell Division Orientation in Plant Embryogenesis. Curr. Biol. 2018, 28, 3031–3043.e2. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, M.; Julien, J.-D.; Mirabet, V.; Boudaoud, A.; Hamant, O. Cell division plane orientation based on tensile stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, E4294–E4303. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.; Belcram, K.; Uyttewaal, M.; Duroc, Y.; Goussot, M.; Legland, D.; Laruelle, E.; de Tauzia-Moreau, M.L.; Pastuglia, M.; Bouchez, D. The preprophase band of microtubules controls the robustness of division orientation in plants. Science 2017, 356, 186–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Iakovidis, M.; Costa, S. Control of patterns of symmetric cell division in the epidermal and cortical tissues of the Arabidopsis root. Development 2016, 143, 978–982. [Google Scholar] [CrossRef]
- Doonan, J.H.; Cove, D.J.; Corke, F.M.K.; Lloyd, C.W. Pre-prophase band of microtubules, absent from tip-growing moss filaments, arises in leafy shoots during transition to intercalary growth. Cell Motil. Cytoskelet. 1987, 7, 138–153. [Google Scholar] [CrossRef]
- Doonan, J.H.; Cove, D.J.; Lloyd, C.W. Immunofluorescence microscopy of microtubules in intact cell lineages of the moss, Physcomitrella patens. I. Normal and CIPC-treated tip cells. J. Cell Sci. 1985, 75, 131–147. [Google Scholar]
- Spinner, L.; Pastuglia, M.; Belcram, K.; Pegoraro, M.; Goussot, M.; Bouchez, D.; Schaefer, D.G. The function of TONNEAU1 in moss reveals ancient mechanisms of division plane specification and cell elongation in land plants. Development 2010, 137, 2733–2742. [Google Scholar] [CrossRef]
- Rasmussen, C.G.; Wright, A.J.; Mueller, S. The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J. 2013, 75, 258–269. [Google Scholar] [CrossRef]
- Kozgunova, E.; Yoshida, M.W.; Goshima, G. Spindle position dictates division site during asymmetric cell division in moss. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wu, S.-Z.; Bezanilla, M. Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division. eLife 2014, 3, e03498. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Tamaoki, D.; Mineyuki, Y.; Yasuhara, H.; Nakai, T.; Shimmen, T.; Yoshihisa, T.; Sonobe, S. Displacement of the mitotic apparatuses by centrifugation reveals cortical actin organization during cytokinesis in cultured tobacco BY-2 cells. J. Plant Res. 2018, 131, 803–815. [Google Scholar] [CrossRef]
- Chugh, M.; Reißner, M.; Bugiel, M.; Lipka, E.; Herrmann, A.; Roy, B.; Müller, S.; Schäffer, E. Phragmoplast Orienting Kinesin 2 Is a Weak Motor Switching between Processive and Diffusive Modes. Biophys. J. 2018, 115, 375–385. [Google Scholar] [CrossRef]
- Neuffer, M.G.; Sheridan, W.F. Defective Kernel Mutants of Maize. I. Genetic and Lethality Studies. Genetics 1980, 95, 929–944. [Google Scholar] [PubMed]
- Lid, S.E.; Gruis, D.; Jung, R.; Lorentzen, J.A.; Ananiev, E.; Chamberlin, M.; Niu, X.; Meeley, R.; Nichols, S.; Olsen, O.-A. The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proc. Natl. Acad. Sci. USA 2002, 99, 5460–5465. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Galletti, R.; Neumann, E.D.; Dubois-Maheo, A.; Sharif-Naeini, R.; Geitmann, A.; Frachisse, J.-M.; Hamant, O.; Ingram, G.C. A mechanosensitive Ca2+ channel activity is dependent on the developmental regulator DEK1. Nat. Commun. 2017, 8, 1009. [Google Scholar] [CrossRef]
- Liang, Z.; Demko, V.; Wilson, R.C.; Johnson, K.A.; Ahmad, R.; Perroud, P.-F.; Quatrano, R.; Zhao, S.; Shalchian-Tabrizi, K.; Otegui, M.S.; et al. The catalytic domain CysPc of the DEK1 calpain is functionally conserved in land plants. Plant J. 2013, 75, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Faulkner, C.; Jeffree, C.E.; Ingram, G.C. The Phytocalpain Defective Kernel 1 Is a Novel Arabidopsis Growth Regulator Whose Activity Is Regulated by Proteolytic Processing. Plant Cell 2008, 20, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Galletti, R.; Johnson, K.L.; Scofield, S.; San-Bento, R.; Watt, A.M.; Murray, J.A.H.; Ingram, G.C. DEFECTIVE KERNEL 1 promotes and maintains plant epidermal differentiation. Development 2015, 142, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Perroud, P.-F.; Demko, V.; Johansen, W.; Wilson, R.C.; Olsen, O.-A.; Quatrano, R.S. Defective Kernel 1 (DEK1) is required for three-dimensional growth in Physcomitrella patens. New Phytol. 2014, 203, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Lid, S.E.; Olsen, L.; Nestestog, R.; Aukerman, M.; Brown, R.C.; Lemmon, B.; Mucha, M.; Opsahl-Sorteberg, H.-G.; Olsen, O.-A. Mutation in the Arabidopisis thaliana DEK1 calpain gene perturbs endosperm and embryo development while over-expression affects organ development globally. Planta 2005, 221, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Becraft, P.W.; Li, K.; Dey, N.; Asuncion-Crabb, Y. The maize dek1 gene functions in embryonic pattern formation and cell fate specification. Development 2002, 129, 5217–5225. [Google Scholar] [PubMed]
- Johansen, W.; Ako, A.E.; Demko, V.; Perroud, P.-F.; Rensing, S.A.; Mekhlif, A.K.; Olsen, O.-A. The DEK1 calpain Linker functions in three-dimensional body patterning in Physcomitrella patens. Plant Physiol. 2016, 172, 1089–1104. [Google Scholar] [CrossRef][Green Version]
- Demko, V.; Perroud, P.-F.; Johansen, W.; Delwiche, C.F.; Cooper, E.D.; Remme, P.; Ako, A.E.; Kugler, K.G.; Mayer, K.F.; Quatrano, R.; et al. Genetic Analysis of DEFECTIVE KERNEL1 Loop Function in Three-Dimensional Body Patterning in Physcomitrella patens. Plant Physiol. 2014, 166, 903–919. [Google Scholar] [CrossRef]
- Perroud, P.; Meyberg, R.; Demko, V.; Quatrano, R.S.; Olsen, O.; Rensing, S.A. DEK1 displays a strong subcellular polarity during Physcomitrella patens 3D growth. New Phytol. 2020, 226, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Hiwatashi, Y.; Shigyo, M.; Kofuji, R.; Kubo, M.; Ito, M.; Hasebe, M. AP2-type transcription factors determine stem cell identity in the moss Physcomitrella patens. Development 2012, 139, 3120–3129. [Google Scholar] [CrossRef]
- Moody, L.A.; Kelly, S.; Rabbinowitsch, E.; Langdale, J.A. Genetic Regulation of the 2D to 3D Growth Transition in the Moss Physcomitrella patens. Curr. Biol. 2018, 28, 473–478.e5. [Google Scholar] [CrossRef] [PubMed]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nat. Cell Biol. 2007, 449, 1053–1057. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.-S.; Amasino, R.; Scheres, B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef]
- Mähönen, A.P.; Tusscher, K.T.; Siligato, R.; Smetana, O.; Díaz-Triviño, S.; Salojärvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. PLETHORA gradient formation mechanism separates auxin responses. Nat. Cell Biol. 2014, 515, 125–129. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans-Hereijgers, J.L.; et al. The PLETHORA Gene Regulatory Network Guides Growth and Cell Differentiation in Arabidopsis Roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.W.; Grimsley, N.H.; Cove, D.J. Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 1979, 144, 427–435. [Google Scholar] [CrossRef]
- Causier, B.; Ashworth, M.; Guo, W.; Davies, B. The TOPLESS Interactome: A Framework for Gene Repression in Arabidopsis. Plant Physiol. 2011, 158, 423–438. [Google Scholar] [CrossRef]
- Causier, B.; Lloyd, J.; Stevens, L.; Davies, B. TOPLESS co-repressor interactions and their evolutionary conservation in plants. Plant Signal. Behav. 2012, 7, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Eklund, D.M.; Ishizaki, K.; Flores-Sandoval, E.; Kikuchi, S.; Takebayashi, Y.; Tsukamoto, S.; Hirakawa, Y.; Nonomura, M.; Kato, H.; Kouno, M.; et al. Auxin Produced by the Indole-3-Pyruvic Acid Pathway Regulates Development and Gemmae Dormancy in the Liverwort Marchantia polymorpha. Plant Cell 2015, 27, 1650–1669. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sandoval, E.; Eklund, D.M.; Bowman, J.L. A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort Marchantia polymorpha. PLoS Genet. 2015, 11, e1005207. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ishizaki, K.; Kouno, M.; Shirakawa, M.; Bowman, J.L.; Nishihama, R.; Kohchi, T. Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet. 2015, 11, e1005084. [Google Scholar]
- Kato, H.; Kouno, M.; Takeda, M.; Suzuki, H.; Ishizaki, K.; Nishihama, R.; Kohchi, T. The Roles of the Sole Activator-Type Auxin Response Factor in Pattern Formation of Marchantia polymorpha. Plant Cell Physiol. 2017, 58, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Mutte, S.K.; Suzuki, H.; Crespo, I.; Das, S.; Radoeva, T.; Fontana, M.; Yoshitake, Y.; Hainiwa, E.; Berg, W.V.D.; et al. Design principles of a minimal auxin response system. Nat. Plants 2020, 6, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishihama, R.; Weijers, D.; Kohchi, T. Evolution of nuclear auxin signaling: Lessons from genetic studies with basal land plants. J. Exp. Bot. 2017, 69, 291–301. [Google Scholar] [CrossRef]
- Paponov, I.A.; Teale, W.; Lang, D.; Paponov, M.; Reski, R.; Rensing, S.A.; Palme, K. The evolution of nuclear auxin signalling. BMC Evol. Biol. 2009, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Prigge, M.J.; Lavy, M.; Ashton, N.W.; Estelle, M. Physcomitrella patens Auxin-Resistant Mutants Affect Conserved Elements of an Auxin-Signaling Pathway. Curr. Biol. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.-F.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella Genome Reveals Evolutionary Insights into the Conquest of Land by Plants. Science 2007, 319, 64–69. [Google Scholar] [CrossRef]
- Sugano, S.S.; Shirakawa, M.; Takagi, J.; Matsuda, Y.; Shimada, T.; Hara-Nishimura, I.; Kohchi, T. CRISPR/Cas9-Mediated Targeted Mutagenesis in the Liverwort Marchantia polymorpha L. Plant Cell Physiol. 2014, 55, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Prigge, M.J.; Tigyi, K.; Estelle, M. The cyclophilin DIAGEOTROPICA has a conserved role in auxin signaling. Development 2012, 139, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.A.; Liu, M.M.; Aoyama, T.; Bierfreund, N.M.; Braun, M.; Coudert, Y.; Dennis, R.J.; O’Connor, D.; Wang, X.Y.; White, C.D.; et al. Plasma Membrane-Targeted PIN Proteins Drive Shoot Development in a Moss. Curr. Biol. 2014, 24, 2776–2785. [Google Scholar] [CrossRef]
- Viaene, T.; Landberg, K.; Thelander, M.; Medvecka, E.; Pederson, E.; Feraru, E.; Cooper, E.D.; Karimi, M.; Delwiche, C.F.; Ljung, K.; et al. Directional auxin transport mechanisms in early diverging land plants. Curr. Biol. 2014, 24, 2786–2791. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nat. Cell Biol. 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Lavy, M.; Prigge, M.J.; Tao, S.; Shain, S.; Kuo, A.; Kirchsteiger, K.; Estelle, M. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 2016, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Nagawa, S.; Chen, J.; Cao, L.; Chen, X.; Xu, T.; Li, H.; Dhonukshe, P.; Yamamuro, C.; Friml, J.; et al. A ROP GTPase-Dependent Auxin Signaling Pathway Regulates the Subcellular Distribution of PIN2 in ArabidopsisRoots. Curr. Biol. 2012, 22, 1319–1325. [Google Scholar] [CrossRef]
- Pan, X.; Fang, L.; Liu, J.; Senay-Aras, B.; Lin, W.; Zheng, S.; Zhang, T.; Guo, J.; Manor, U.; Van Norman, J.; et al. Auxin-induced signaling protein nanoclustering contributes to cell polarity formation. Nat. Commun. 2020, 11, 3914. [Google Scholar] [CrossRef]
- Platre, M.P.; Bayle, V.; Armengot, L.; Bareille, J.; Marquès-Bueno, M.D.M.; Creff, A.; Maneta-Peyret, L.; Fiche, J.-B.; Nollmann, M.; Miège, C.; et al. Developmental control of plant Rho GTPase nano-organization by the lipid phosphatidylserine. Science 2019, 364, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.G.; Naseer, S.; Ursache, R.; Wybouw, B.; Smet, W.; De Rybel, B.; Vermeer, J.E.M.; Geldner, N. Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells. Nat. Cell Biol. 2018, 555, 529–533. [Google Scholar] [CrossRef]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariutta, Y. A Mutually Inhibitory Interaction between Auxin and Cytokinin Specifies Vascular Pattern in Roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef]
- Salvi, E.; Rutten, J.P.; Di Mambro, R.; Polverari, L.; Licursi, V.; Negri, R.; Ioio, R.D.; Sabatini, S.; Tusscher, K.T. A Self-Organized PLT/Auxin/ARR-B Network Controls the Dynamics of Root Zonation Development in Arabidopsis thaliana. Dev. Cell 2020, 53, 431–443.e23. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.A.; Kelly, S.; Clayton, R.; Weeks, Z.; Emms, D.M.; Langdale, J.A. Article NO GAMETOPHORES 2 Is a Novel Regulator of the 2D to 3D Growth Transition in the Moss Physcomitrella patens. Curr. Biol. 2021, 31, 555–563.e4. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Keijzer, J.; Freire Rios, A.; Willemsen, V. Physcomitrium patens: A Single Model to Study Oriented Cell Divisions in 1D to 3D Patterning. Int. J. Mol. Sci. 2021, 22, 2626. https://doi.org/10.3390/ijms22052626
de Keijzer J, Freire Rios A, Willemsen V. Physcomitrium patens: A Single Model to Study Oriented Cell Divisions in 1D to 3D Patterning. International Journal of Molecular Sciences. 2021; 22(5):2626. https://doi.org/10.3390/ijms22052626
Chicago/Turabian Stylede Keijzer, Jeroen, Alejandra Freire Rios, and Viola Willemsen. 2021. "Physcomitrium patens: A Single Model to Study Oriented Cell Divisions in 1D to 3D Patterning" International Journal of Molecular Sciences 22, no. 5: 2626. https://doi.org/10.3390/ijms22052626
APA Stylede Keijzer, J., Freire Rios, A., & Willemsen, V. (2021). Physcomitrium patens: A Single Model to Study Oriented Cell Divisions in 1D to 3D Patterning. International Journal of Molecular Sciences, 22(5), 2626. https://doi.org/10.3390/ijms22052626





