Phosphodiesterases Expression during Murine Cardiac Development
Abstract
1. Introduction
2. Results
2.1. PDE mRNA Expression Levels Vary between Embryonic and Fetal Cardiac Development
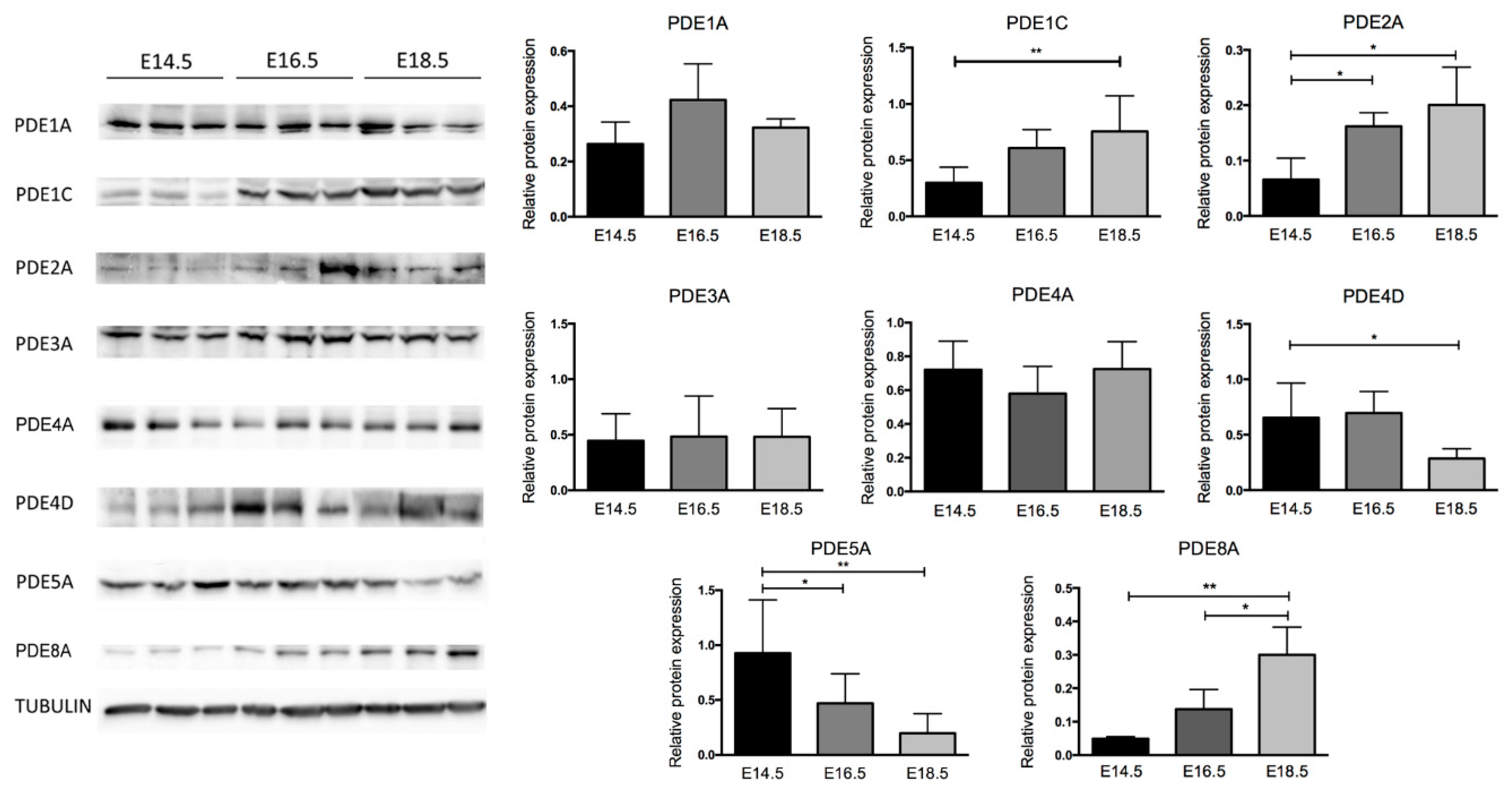
2.2. Variation of PDE Expression Occurs at Protein Levels in Embryonic and Fetal Heart
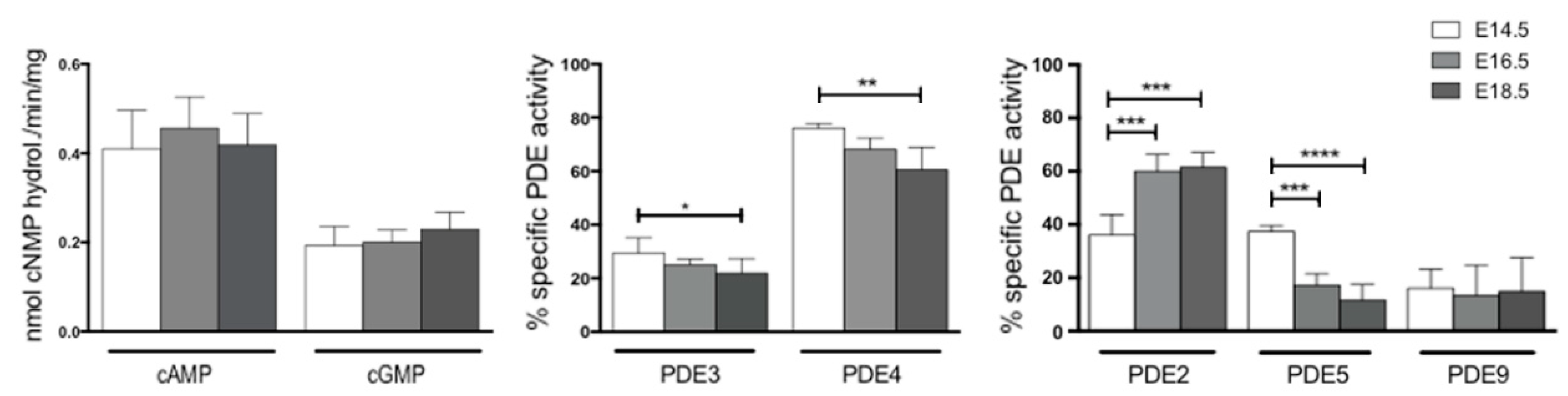
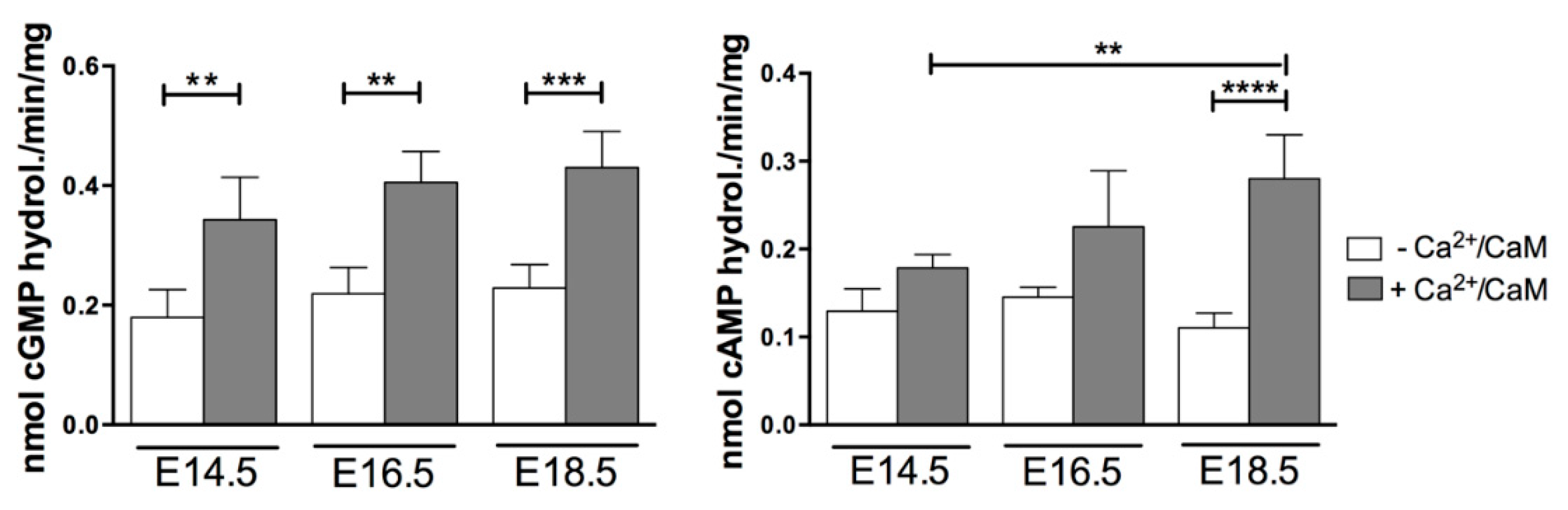
2.3. Few PDEs Showed Enzymatic Activity Modulation at Embryonic and Fetal Life
3. Discussion
4. Materials and Methods
4.1. Mouse Husbandry and Embryos Collection
4.2. qRT-PCR
4.3. Western Blot
4.4. PDEs Activity Assay
4.5. Statistics Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E. | Embryonic day |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| AC | Adenylyl cyclase |
| NO | Nitric oxide |
| PDE | Phosphodiesterase |
| Ca2+ | Calcium |
| CaM | Calmodulin |
| TAC | Transverse aortic constriction |
References
- Boer, A.B.; Berg, G.V.D.; Boer, P.A.J.; Moorman, A.F.M.; Ruijter, J.M. Growth of the developing mouse heart: An interactive qualitative and quantitative 3D atlas. Develop. Biol. 2012, 368, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, S.M.; Foley, J.F.; Elmore, S.A. Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5. Toxicol. Pathol. 2009, 37, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.; Czubryt, M.P.; Wigle, J.T. Molecular mechanisms of cardiac development- (Cardiac adaptations- chapter 2). In Cardiac Adaptations; Advances in Biochemistry in Health and Disease; Ostadal, B., Dhalla, N., Eds.; Springer: New York, NY, USA, 2012; Volume 4. [Google Scholar]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, J.; Chung, J.; Manganiello, V.C. Advances targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug. Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef]
- Knight, W.E.; Yan, C. Cardiac cyclic nucleotide phosphodiesterases: Function, regulation and therapeutic prospects. Horm. Metab. Res. 2012, 44, 766–775. [Google Scholar] [CrossRef]
- Zaccolo, M.; Movsesian, M.A. cAMP and cGMP signaling cross-talk: Role of phosphodiesterases and implications for cardiac pathophysiology. Circ. Res. 2007, 100, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.H.; Palmer, D.; Tilley, D.G.; Dunkerley, H.A.; Netherton, S.J.; Raymond, D.R.; Elbatarny, H.S.; Jimmo, S.L. Cyclic nucleotide phosphodiesterase activity, expression and targeting in cells of the cardiovascular systems. Mol. Pharmacol. 2003, 64, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Cornacchione, M.; Barbagallo, F.; Grazia, A.; Barrios, F.; Fassina, L.; Monaco, L.; Giannetta, E.; Gianfrilli, D.; Garofalo, S.; et al. Inhibition of type 5 phosphodiesterase counteracts β2-adrenergic signaling in beating cardiomyocytes. Circ. Res. 2015, 106, 408–420. [Google Scholar]
- Assenza, M.R.; Barbagallo, F.; Barios, F.; Cornacchione, M.; Campolo, F.; Vivarelli, E.; Gianfrilli, D.; Auletta, L.; Soricelli, A.; Isidori, A.M.; et al. Critical role of phosphodiesterase 2A in mouse congenital heart defects. Cardiovasc. Res. 2018, 114, 830–845. [Google Scholar] [CrossRef]
- Wang, X.; Yamada, S.; LaRiviere, W.B.; Ye, H.; Bakeberg, J.L.; Irazabal, M.V.; Chebib, F.T.; Van Deursen, J.; Harris, P.C.; Sussman, C.R.; et al. Generation and phenotypic characterization of PDE1a mutant mice. PLoS ONE 2017, 12, e0181087. [Google Scholar] [CrossRef] [PubMed]
- Knight, W.E.; Chen, S.; Zhang, Y.; Oikawa, M.; Wu, M.; Zhou, Q.; Miller, C.L.; Cai, Y.; Mickelsen, D.M.; Moravec, C.; et al. PDE1C deficiency antagonizes pathological cardiac remodeling and dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, E7116–E7125. [Google Scholar] [CrossRef]
- Oikawa, M.; Wu, M.; Lim, S.; Knight, W.E.; Miller, C.L.; Cai, Y.; Lu, Y.; Blaxall, B.C.; Takeishi, Y.; Abe, J.; et al. Cyclic nucleotide phosphodiesterase 3A1 protects the heart against ischemia reperfusion injury. J. Mol. Cell. Cardiol. 2014, 64, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.; Richter, W.; Mika, D.; Castro, L.R.V.; Abi-Gerges, A.; Xie, M.; Scheitrum, C.; Lefebvre, F.; Schittl, J.; Mateo, P.; et al. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias in mice. J. Clin. Investig. 2011, 121, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Lehnart, S.E.; Wehrens, X.H.T.; Reiken, S.; Warrier, S.; Belevych, A.E.; Harvey, R.D.; Richter, W.; Jin, S.-L.C.; Conti, M.; Marks, A.R. Phosphodiesterase 4d deficiency in the Ryanodine-Receptor complex promotes heart failure and arrhythmias. Cell 2005, 123, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Pokreisz, P.; Vandenwijngaert, S.; Bito, V.; Van den Bergh, A.; Lenaerts, I.; Busch, C.; Marsboom, G.; Gheysens, O.; Vermeersch, P.; Biesmans, L.; et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 2009, 119, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Patrucco, E.; Albergine, M.S.; Santana, L.F.; Beavo, J.A. Phosphodiesterase 8A (PDE8A) regulates excitation-contraction coupling in ventricular myocytes. J. Mol. Cell. Cardiol. 2010, 49, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.I.; Zhu, G.; Sasaki, T.; Cho, G.S.; Hamdani, N.; Holewinski, R.; Jo, S.-H.; Danner, T.; Zhang, M.; Rainer, P.P.; et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 2015, 519, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Knight, W.E.; Yan, C. Roles of PDE1 in Pathological cardiac remodeling and dysfunction. J. Cardiovasc. Dev. Dis. 2018, 5, 22. [Google Scholar] [CrossRef]
- Abi-Gerges, A.; Richter, W.; Lefebvre, F.; Mateo, P.; Varin, A.; Heymes, C.; Samuel, J.-L.; Lugnier, C.; Conti, M.; Fischmeister, R.; et al. Decreased Expression and Activity of cAMP Phosphodiesterases in Cardiac Hypertrophy and Its Impact on β-Adrenergic cAMP Signals. Circ. Res. 2009, 105, 784–792. [Google Scholar] [CrossRef]
- Catalano, S.; Campana, A.; Giordano, C.; Győrffy, B.; Tarallo, R.; Rinaldi, A.; Bruno, G.; Ferraro, A.; Romeo, F.; Lanzino, M.; et al. Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: Implications for targeted therapy. Clin. Cancer Res. 2015, 22, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Dorner-Ciossek, C.; Kroker, K.S.; Rosenbrock, H. Role of PDE9 in cognition. Adv. Neurobiol. 2017, 17, 231–254. [Google Scholar]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef]
- Thakkar, J.K.; Sperelakis, N. Changes in cyclic nucleotide levels during embryonic development in chick hearts. J. Dev. Physiol. 1987, 9, 497–505. [Google Scholar] [PubMed]
- Hosey, M.M.; Green, R.D. Effects of isoproterenol on cyclic AMP and cyclic AMP dependent protein kinase in developing chick myocardium. Biochim. Biophys. Acta 1977, 500, 152–161. [Google Scholar] [CrossRef]
- Feridooni, T.; Hotchkiss, A.; Baguma-Nibasheka, M.; Zhang, F.; Allen, B.; Chinni, S.; Pasumarthi, K.B.S. Effects of β-adrenergic receptor drugs on embryonic ventricular cell proliferation and differentiation and their impact on donor cell transplantation. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H919–H931. [Google Scholar] [CrossRef]
- Galdos, F.X.; Guo, Y.; Paige, S.L.; VanDusen, N.J.; Wu, S.M.; Pu, W.T. Cardiac Regeneration: Lessons from Development. Circ. Res. 2017, 120, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Nagel, D.J.; Zhou, Q.; Cygnar, K.D.; Zhao, H.; Li, F.; Pi, X.; Knight, P.A.; Yan, C. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ. Res. 2015, 116, 1120–1132. [Google Scholar] [CrossRef]
- Pavlaki, N.; Nikolaev, V.O. Imaging of PDE2 and PDE3 mediated cGMP-to cAMP cross-talk in cardiomyocytes. J. Cardiovasc. Dev. Dis. 2018, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Acin-Perez, R.; Russwurm, M.; Günnewig, K.; Gertz, M.; Zoidl, G.; Ramos, L.; Buck, J.; Levin, L.R.; Rassow, J.; Manfredi, G.; et al. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J. Biol. Chem. 2011, 286, 30423–30432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, Q.; Zhou, L.; Liu, K.; Jiao, K. Complex Regulation of Mitochondrial Function During Cardiac Development. J. Am. Heart Assoc. 2019, 8, e012731. [Google Scholar] [CrossRef]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler. Thromb. 2017, 24, 884–894. [Google Scholar] [CrossRef]
- Conti, M. Subcellular targeting of PDE4 in cardiac myocytes and generation of signaling compartments. In Microdomains in the Cardiovascular System; Cardiac and Vascular Biology; Nikolaev, V., Zaccolo, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Xiang, Y.; Naro, F.; Zoudilova, M.; Jin, S.L.C.; Conti, M.; Kobilka, B. Phosphodiesterase 4D is required for B2 adrenoreceptor subtype-specific signaling in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 909–914. [Google Scholar] [CrossRef]
- Hildreth, V.; Webb, S.; Bradshaw, L.; Brown, N.A.; Anderson, R.H.; Henderson, D.J. Cells migrating from the neural crest contribute to the innervation of the venous pole of the heart. J. Anat. 2008, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Shibata, S.; Okamoto, Y.; Sato, S.; Fujisawa, S.; Ohba, T.; Ono, K. The mechanism of increased postnatal heart rate and sinoatrial node pacemaker activity in mice. J. Physiol. Sci. 2013, 63, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Sato, S. Quantitative evaluation of ontogenetic change in heart rate and its autonomic regulation in newborn mice with the use of a noninvasive piezoelectric sensor. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1708–H1715. [Google Scholar] [CrossRef]
- Bolger, G.B. RACK1 and β-arrestin2 attenuate dimerization of PDE4 cAMP phosphodiesterase PDE4D5. Cell Signal. 2016, 28, 706–712. [Google Scholar] [CrossRef]
- Conti, M.; Mika, D.; Richter, W. Cyclic AMP compartments and signaling specificity: Role of cyclic nucleotide phosphodiesterases. J. Gen. Physiol. 2014, 143, 29–38. [Google Scholar] [CrossRef]
- Thompson, W.J.; Appleman, M.M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry 1971, 10, 311–316. [Google Scholar] [PubMed]

| Phosphodiesterase (PDE) Family | Gene | Substrate/ Regulation | Transgenic Model |
|---|---|---|---|
| PDE1 | 1A, 1B, 1C | cAMP/cGMP Ca2+/CaM stimulated | Pde1a knockout: lowest blood pressure, increased heart rate, elevated ejection fraction [10]. Pde1c knockout: protected from TAC-induced cardiac dysfunction; antagonize cardiac myocyte hypertrophy and death [11]. |
| PDE2 | 2A | cAMP/cGMP cGMP-stimulated | Pde2a knockout: embryonal death, congenital heart defect [9]. |
| PDE3 | 3A, 3B | cAMP/cGMP cGMP-inhibited | Pde3a overexpression: decreased heart rate and cardiac contractile function; antiapoptotic effect in adult cardiomyocytes [12]. |
| PDE4 | 4A, 4B, 4C, 4D | cAMP | Pde4a knockout: no cardiac alteration [13]; Pde4d knockout: age-related cardiomyopathy; arrhythmia [14]. |
| PDE5 | 5A | cGMP | Pde5a overexpression: adverse cardiac remodeling after myocardial infarction; enhanced hypertrophy, reduced contractile function [15]. |
| PDE8 | 8A, 8B | cAMP | Pde8a knockout: increased Ca2+ transient and current during β AR stimulation [16]. |
| PDE9 | 9A | cGMP high affinity | Pde9a knockout: cardio-protected after sustained pressure overload [17]. |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Pde1a | 5′AGGTATCATGCACTGGCTCA 3′ | 5′GAGCGGTCGTTGTACAGAAT 3′ |
| Pde1c | 5′ATGGGGATGATGCTTAGGAG 3′ | 5′ CAATGCTTCGATTACAGCCG 3′ |
| Pde2a | 5′ ACCGAAAGATCCTGCAACTG 3′ | 5′ TTCTCCCAGCACTTTGTCTC 3′ |
| Pde3a | 5′ AGAATCCATGCCACCGATGT 3′ | 5′ CCCATGTGTCCGTGTGTAAA 3′ |
| Pde4a | 5′ TGCTGCAAGAGAACTGC 3′ | 5′ AGGGTCATGTGCTTGGACAT 3′ |
| Pde4d | 5′GCCTCTGACTGTTATCATGCAC3′ | 5′ GCAGCATGGATGTTGTTGTG 3′ |
| Pde5a | 5′ ATCCATGGACTCATCTCTGC 3′ | 5′ GCTTCCTCCAATGTTGAACC 3′ |
| Pde8a | 5′ TCAGAGTGTGCAATGGCAAC 3′ | 5′GTCCATCGAATGTTTCCTCC 3′ |
| Pde9 | 5′ CTACGAGGAGCTGAAGCAGC 3′ | 5′ AGTTTGGAGGAGAATGGCCT 3′ |
| Gapdh | 5′ GTGAAGGTCGGTGTGAACG 3′ | 5′ ATTTGATGTTAGTGGGGTCTCG3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, T.M.d.C.S.; Cardarelli, S.; Giorgi, M.; Lenzi, A.; Isidori, A.M.; Naro, F. Phosphodiesterases Expression during Murine Cardiac Development. Int. J. Mol. Sci. 2021, 22, 2593. https://doi.org/10.3390/ijms22052593
Carvalho TMdCS, Cardarelli S, Giorgi M, Lenzi A, Isidori AM, Naro F. Phosphodiesterases Expression during Murine Cardiac Development. International Journal of Molecular Sciences. 2021; 22(5):2593. https://doi.org/10.3390/ijms22052593
Chicago/Turabian StyleCarvalho, Thays Maria da Conceição Silva, Silvia Cardarelli, Mauro Giorgi, Andrea Lenzi, Andrea M. Isidori, and Fabio Naro. 2021. "Phosphodiesterases Expression during Murine Cardiac Development" International Journal of Molecular Sciences 22, no. 5: 2593. https://doi.org/10.3390/ijms22052593
APA StyleCarvalho, T. M. d. C. S., Cardarelli, S., Giorgi, M., Lenzi, A., Isidori, A. M., & Naro, F. (2021). Phosphodiesterases Expression during Murine Cardiac Development. International Journal of Molecular Sciences, 22(5), 2593. https://doi.org/10.3390/ijms22052593






