The Archaeal Small Heat Shock Protein Hsp17.6 Protects Proteins from Oxidative Inactivation
Abstract
1. Introduction
2. Results
2.1. The Small Heat Shock Proteins of M. psychrophilus Elevated the Stationary Cell Density of E. coli
2.2. Hsp17.6 Overexpressed E. coli Gained Higher Survivability in Oxidants
2.3. Hsp17.6 Mitigated Protein Inactivation by Oxidants
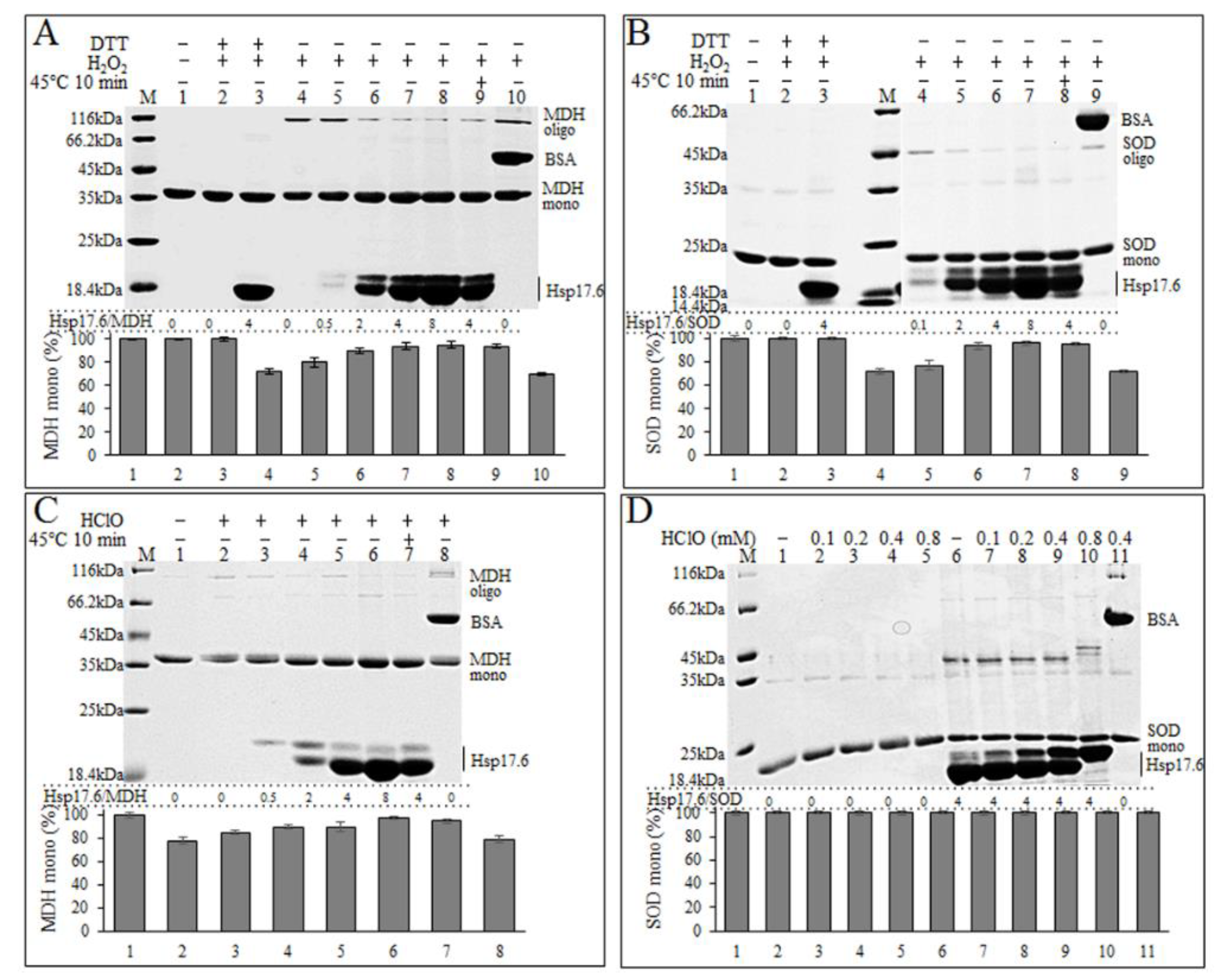
2.4. Hsp17.6 Appeared to Alleviate the H2O2-Oxidized Disulfide Linking and the HClO-Induced Degradation of Proteins
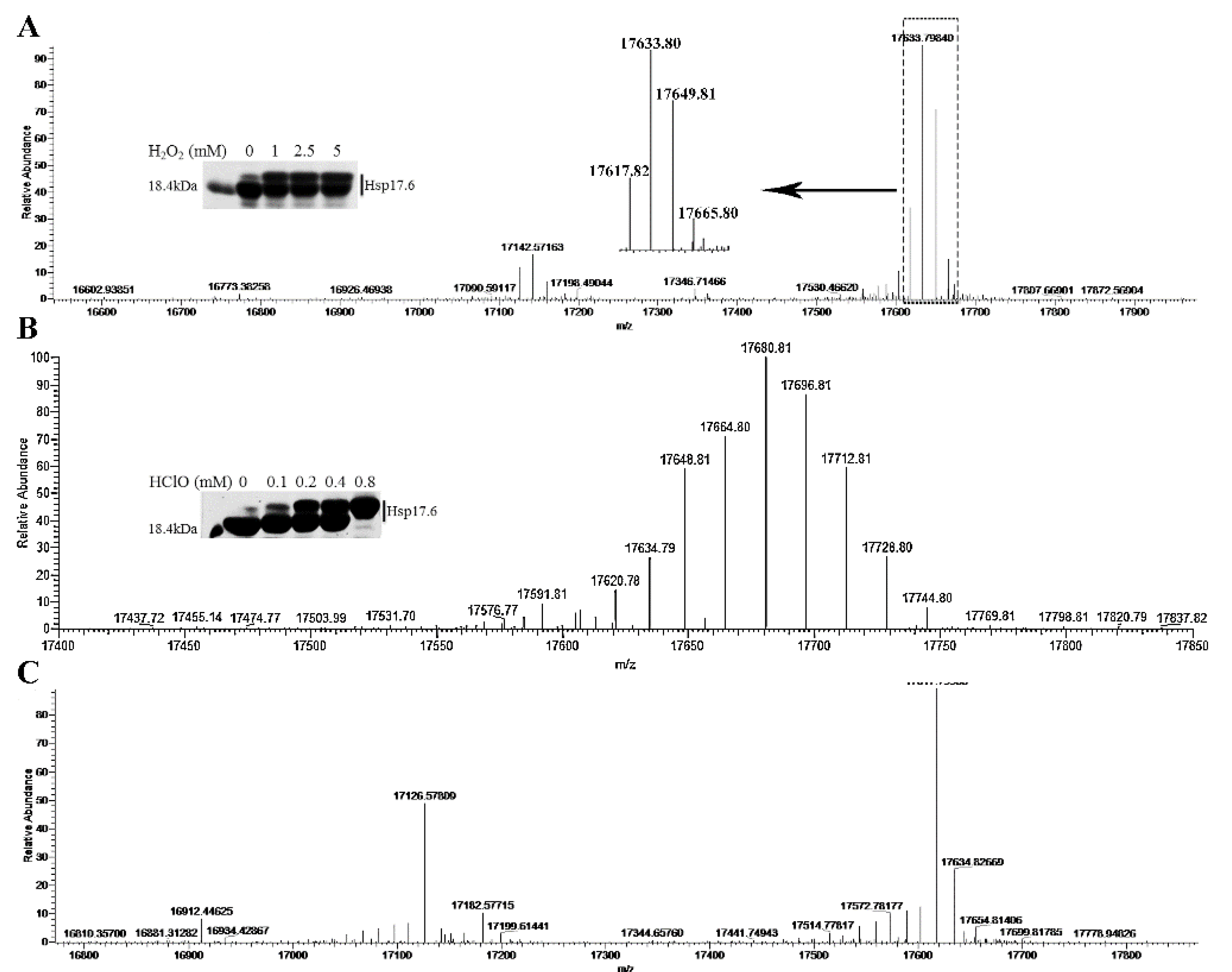
2.5. Mass Spectrometry Detected the Oxidized Amino Acid Residues of Hsp17.6
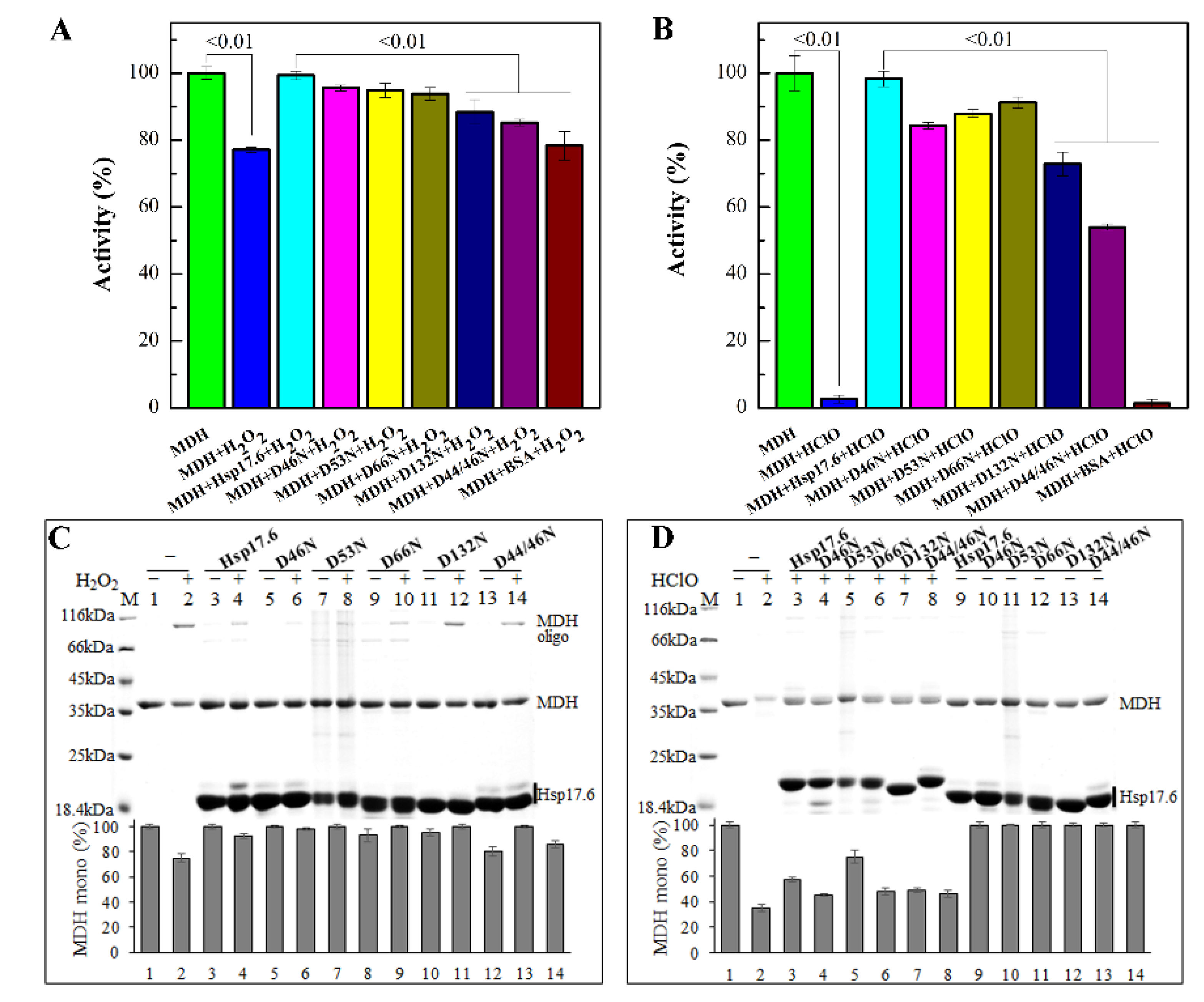
2.6. Substitution of Aspartate Residues Reduced the Protection of Hsp17.6 on Protein Oxidative Inactivation
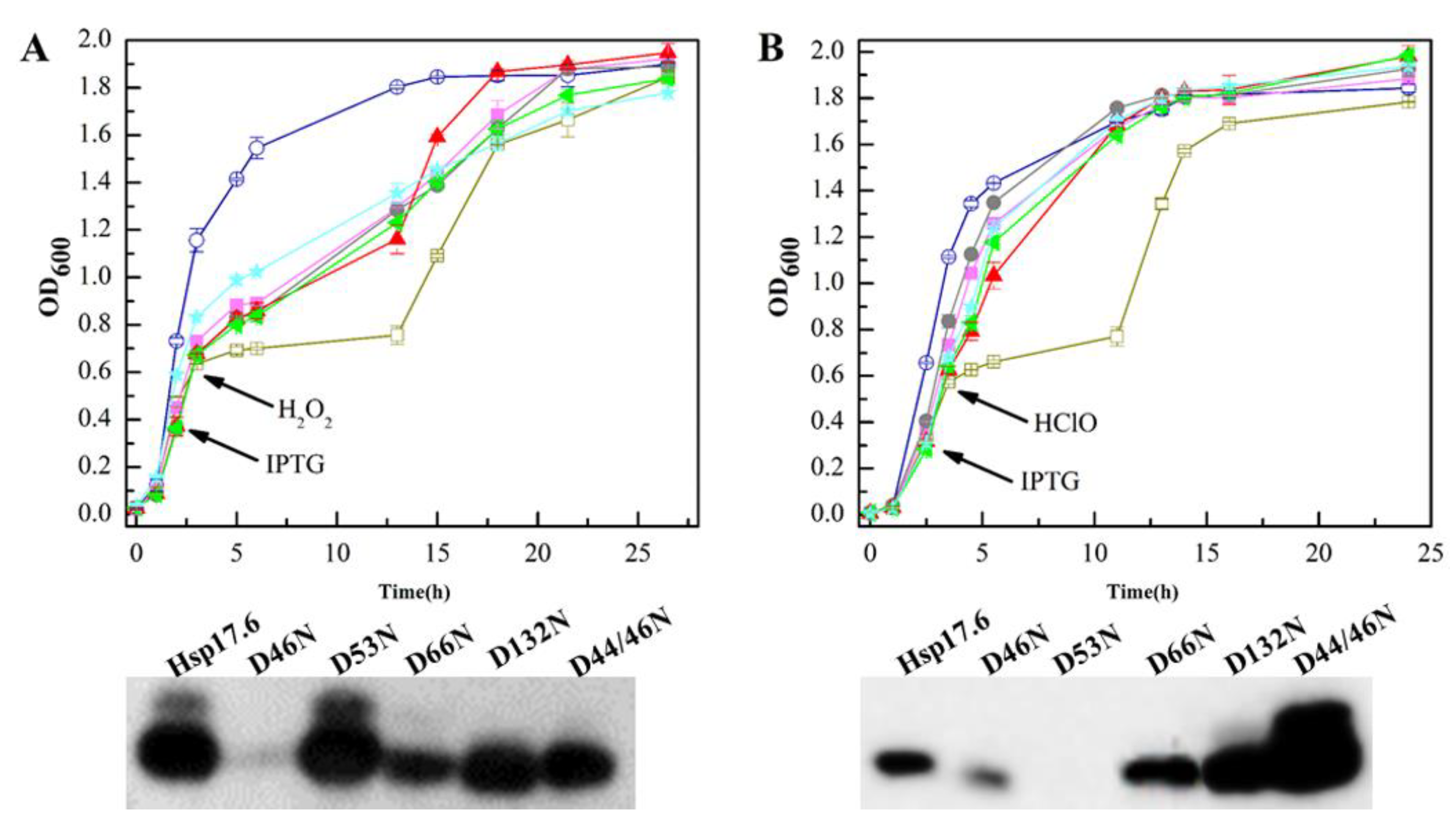
2.7. The Hsp17.6 Aspartate Residue Mutants Reduced Attenuating Oxidant-Suppressed E. coli Growth
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Growth Experiments
4.3. Construction of Hsp17.6 Residue Substitution Mutants
4.4. Protein Expression and Purification
4.5. Enzymatic Assays on Oxidants Treated Proteins
4.6. Light Scattering Assay
4.7. Mass Spectrometry Analysis
4.8. Non-Reducing SDS-PAGE Analysis on Oxidized Proteins
4.9. Western Blot
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jong, W.W.D.; Caspers, G.J.; Leunissen, J.A.M. Genealogy of The α-crystallin-small Heat-shock Protein Superfamily. Int. J. Biol. Macromol. 1998, 22, 162. [Google Scholar] [CrossRef]
- Haslbeck, M. sHsps and Their Role in the Chaperone Network. Cell. Mol. Life Sci. 2002, 59, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.M.; Aquilina, J.A. Evidence for Specific Subunit Distribution and Interactions in the Quaternary Structure of α-crystallin. Proteins 2010, 78, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.R.; Lee, G.J.; Vierling, E. Evolution, Structure and Function of the Small Heat Shock Proteins in Plants. J. Exp. Bot. 1996, 47, 325–338. [Google Scholar] [CrossRef]
- Boston, R.S.; Viitanen, P.V.; Vierling, E. Molecular Chaperones and Protein Folding in Plants. Plant Mol. Biol. 1996, 32, 191–222. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Masternak, M.M.; Kopchick, J.J.; Conover, C.A.; Bartke, A.; Miller, R.A. Endocrine Regulation of Heat Shock Protein mRNA Levels in Long-lived Dwarf Mice. Mech. Ageing Dev. 2009, 130, 393–400. [Google Scholar] [CrossRef]
- Kalioraki, M.A.; Artemaki, P.I.; Sklirou, A.D.; Kontos, C.K.; Adamopoulos, P.G.; Papadopoulos, I.N.; Trougakos, I.P.; Scorilas, A. Heat Shock Protein Beta 3 (HSPB3) is an Unfavorable Molecular Biomarker in Colorectal Adenocarcinoma. Mol. Carcinog. 2020, 59, 116–125. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Seit-Nebi, A.S.; Gusev, N.B. Structure, Properties, and Functions of the Human Small Heat-shock Protein HSP22 (HspB8, H11, E2IG1): A Critical Review. J. Neurosci. Res. 2010, 86, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.J.; Hageman, J.; Carra, S.; Kampinga, H.H. Structural and Functional Diversities between Members of the Human HSPB, HSPH, HSPA, and DnaJ Chaperone Families. Biochemistry 2008, 47, 7001–7011. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P. Small Stress Proteins: Chaperones That Act as Regulators of Intracellular Redox State and Programmed Cell Death. Biol. Chem. 1998, 379, 19–26. [Google Scholar] [PubMed]
- Carra, S.; Alberti, S.; Arrigo, P.A.; Benesch, J.L.; Benjamin, I.J.; Boelens, W.; Bartelt-Kirbach, B.; Brundel, B.J.J.M.; Buchner, J.; Bukau, B.; et al. The Growing World of Small Heat Shock Proteins: From Structure to Functions. Cell Stress Chaperones 2017, 22, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kandror, O.; Goldberg, A.L. Trigger Factor is Induced upon Cold Shock and Enhances Viability of Escherichia coli at Low Temperatures. Proc. Natl. Acad. Sci. USA 1997, 94, 4978–4981. [Google Scholar] [CrossRef]
- Manuel, F.; Chernikova, T.N.; Yakimov, M.M.; Golyshin, P.N.; Timmis, K.N. Chaperonins Govern Growth of Escherichia coli at Low Temperatures. Nat. Biotechnol. 2003, 21, 1266–1267. [Google Scholar]
- Matuszewska, E.; Kwiatkowska, J.; Kuczynska-Wisnik, D.; Laskowska, E. Escherichia coli Heat-shock Proteins IbpA/B are Involved in Resistance to Oxidative Stress Induced by Copper. Microbiology 2008, 154, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.; Watanabe, T.; Nakamoto, H. A Small Heat-shock Protein Confers Stress Tolerance and Stabilizes Thylakoid Membrane Proteins in Cyanobacteria Under Oxidative Stress. Arch. Microbiol. 2009, 191, 319–328. [Google Scholar] [CrossRef]
- Saji, H.; Iizuka, R.; Yoshida, T.; Abe, T.; Kidokoro, S.I.; Ishii, N.; Yohda, M. Role of the IXI/V Motif in Oligomer Assembly and Function of StHsp14.0, a Small Heat Shock Protein from the Acidother-mophilic archaeon, Sulfolobus tokodaii Strain 7. Proteins 2008, 71, 771–782. [Google Scholar] [CrossRef]
- Abe, T.; Oka, T.; Nakagome, A.; Tsukada, Y.; Yasunaga, T.; Yohda, M. StHsp14.0, a Small Heat Shock Protein of Sulfolobus tokodaii Strain 7, Protects Denatured Proteins from Aggregation in the Partially Dissociated Conformation. J. Biochem. 2011, 150, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Kim, K.K.; Yokota, H.; Kim, S.-H. Small Heat Shock Protein of Methanococcus jannaschii, A Hyperthermophile. Proc. Natl. Acad. Sci. USA 1998, 95, 9129–9133. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, M.; Kastenmüller, A.; Buchner, J.; Weinkauf, S.; Braun, N. Structural Dynamics of Archaeal Small Heat Shock Proteins. J. Mol. Biol. 2008, 378, 362–374. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, N.; Liu, X.; Dong, X. Methanogenesis from Methanol at Low Temperatures by a Novel Psychrophilic Methanogen, “Methanolobus psychrophilus” sp. nov., Prevalent in Zoige Wetland of the Tibetan Plateau. Appl. Environ. Microbiol. 2008, 74, 6114–6120. [Google Scholar] [PubMed]
- Chen, Z.; Yu, H.; Li, L.; Hu, S.; Dong, X. The Genome and Transcriptome of a Newly Described Psychrophilic Archaeon, Methanolobus psychrophilus R15, Reveal its Cold Adaptive Characteristics. Environ. Microbiol. Rep. 2012, 4, 633–641. [Google Scholar] [PubMed]
- Li, J.; Qi, L.; Guo, Y.; Yue, L.; Li, Y.; Ge, W.; Wu, J.; Shi, W.; Dong, X. Global Mapping Transcriptional Start Sites Revealed Both Transcriptional and Post-transcriptional Regulation of Cold Adaptation in the Methanogenic Archaeon Methanolobus psychrophilus. Sci. Rep. 2015, 5, 9209. [Google Scholar] [CrossRef]
- Nyström, T. Stationary-phase Physiology. Annu. Rev. Microbiol. 2004, 58, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Terman, A. Garbage Catastrophe Theory of Aging: Imperfect Removal of Oxidative Damage? Redox Rep. 2001, 6, 15–26. [Google Scholar] [CrossRef]
- Dahl, J.-U.; Gray, M.J.; Jakob, U. Protein Quality Control under Oxidative Stress Conditions. J. Mol. Biol. 2015, 427, 1549–1563. [Google Scholar] [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically Encoded Fluorescent Indicator for Intracellular Hydrogen Peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.F. The Solubility of Nitrogen, Oxygen and Argon in Water and Seawater. Deep Sea Res. 1970, 17, 721–735. [Google Scholar] [CrossRef]
- Winter, J.; Ilbert, M.; Graf, P.; Özcelik, D.; Jakob, U. Bleach Activates a Redox-regulated Chaperone by Oxidative Protein Unfolding. Cell 2008, 135, 691–701. [Google Scholar] [CrossRef]
- Jakob, U. Redox Switch of Hsp33 Has a Novel Zinc-binding Motif. J. Biol. Chem. 2000, 275, 38302–38310. [Google Scholar] [CrossRef] [PubMed]
- Varrone, S.; Consiglio, E.; Covelli, I. The Nature of Inhibition of Mitochondrial Malate Dehydrogenase by Thyroxine, Iodine Cyanide and Molecular iodine. Eur. J. Biochem. 2010, 13, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Xican, L. Improved Pyrogallol Autoxidation Method: A Reliable and Cheap Superoxide-scavenging Assay Suitable for all Antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar]
- Chen, Z.; Wang, X.; Yang, F.; Hu, Q.; Tong, H.; Dong, X. Molecular Insights into Hydrogen Peroxide-sensing Mechanism of the Metalloregulator MntR in Controlling Bacterial Resistance to Oxidative Stresses. J. Biol. Chem. 2017, 292, 5519–5531. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-source Platform for Biological-image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, P.; Li, J.; Qi, L.; Dong, X. The Archaeal Small Heat Shock Protein Hsp17.6 Protects Proteins from Oxidative Inactivation. Int. J. Mol. Sci. 2021, 22, 2591. https://doi.org/10.3390/ijms22052591
Ma P, Li J, Qi L, Dong X. The Archaeal Small Heat Shock Protein Hsp17.6 Protects Proteins from Oxidative Inactivation. International Journal of Molecular Sciences. 2021; 22(5):2591. https://doi.org/10.3390/ijms22052591
Chicago/Turabian StyleMa, Pengfei, Jie Li, Lei Qi, and Xiuzhu Dong. 2021. "The Archaeal Small Heat Shock Protein Hsp17.6 Protects Proteins from Oxidative Inactivation" International Journal of Molecular Sciences 22, no. 5: 2591. https://doi.org/10.3390/ijms22052591
APA StyleMa, P., Li, J., Qi, L., & Dong, X. (2021). The Archaeal Small Heat Shock Protein Hsp17.6 Protects Proteins from Oxidative Inactivation. International Journal of Molecular Sciences, 22(5), 2591. https://doi.org/10.3390/ijms22052591





