Characterization of Estrogenic Activity and Site-Specific Accumulation of Bisphenol-A in Epididymal Fat Pad: Interfering Effects on the Endocannabinoid System and Temporal Progression of Germ Cells
Abstract
1. Introduction
2. Results
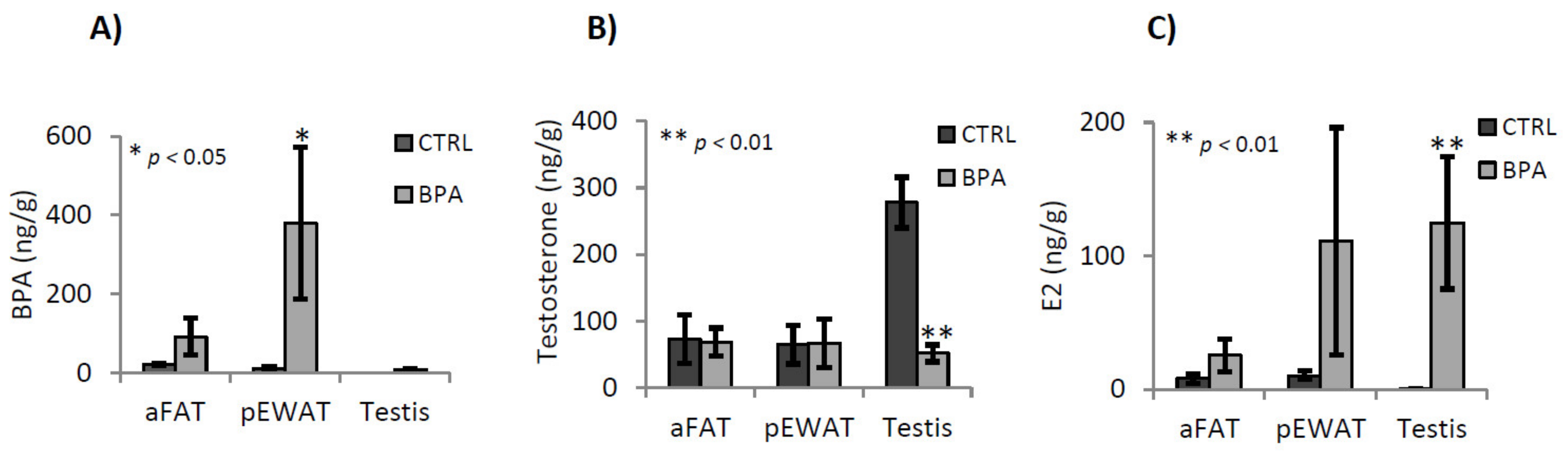
2.1. BPA, Testosterone and 17-β-Estradiol Content in Adipose Tissues and Testis
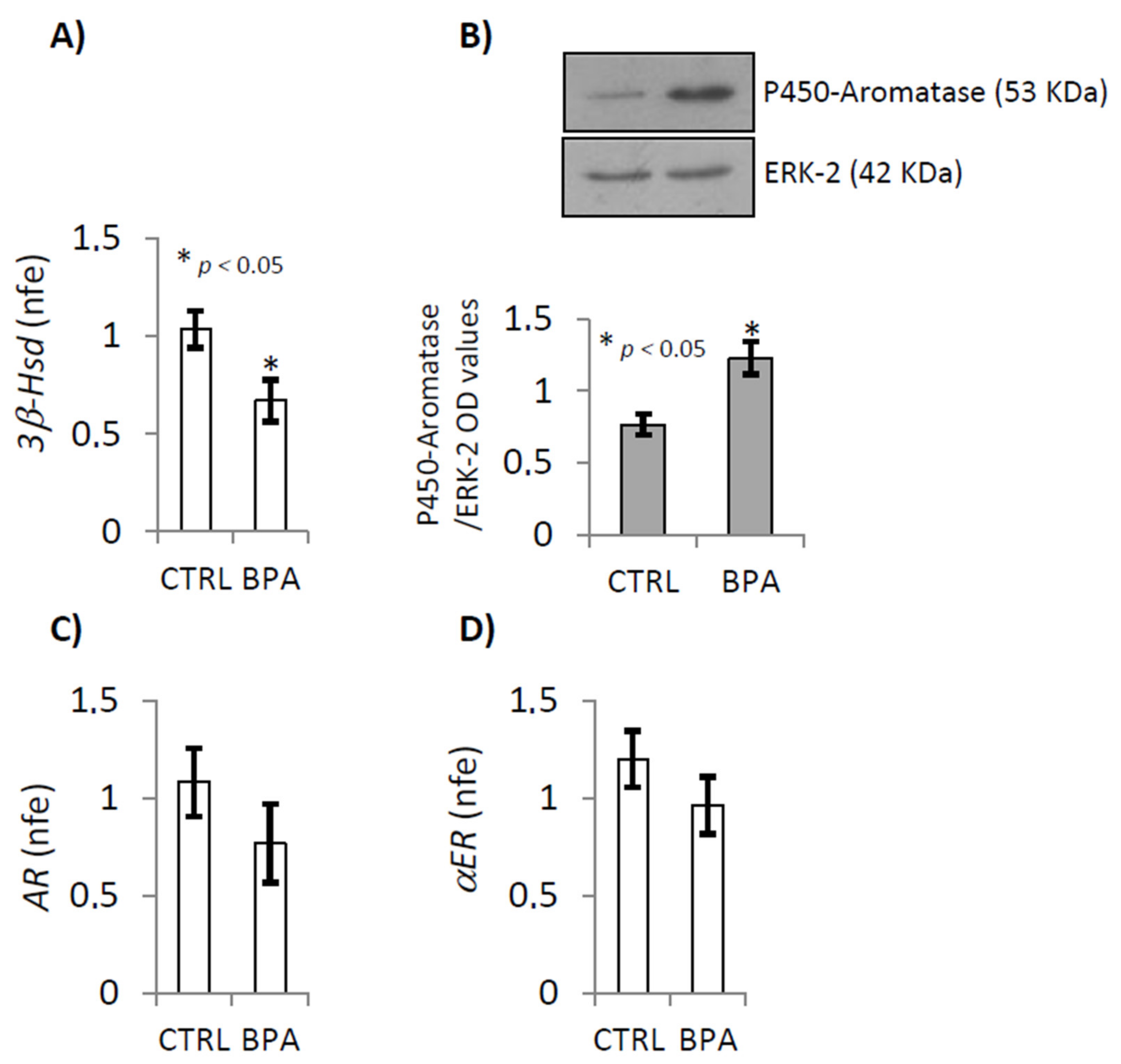
2.2. BPA Effects on Steroidogenesis Enzymes
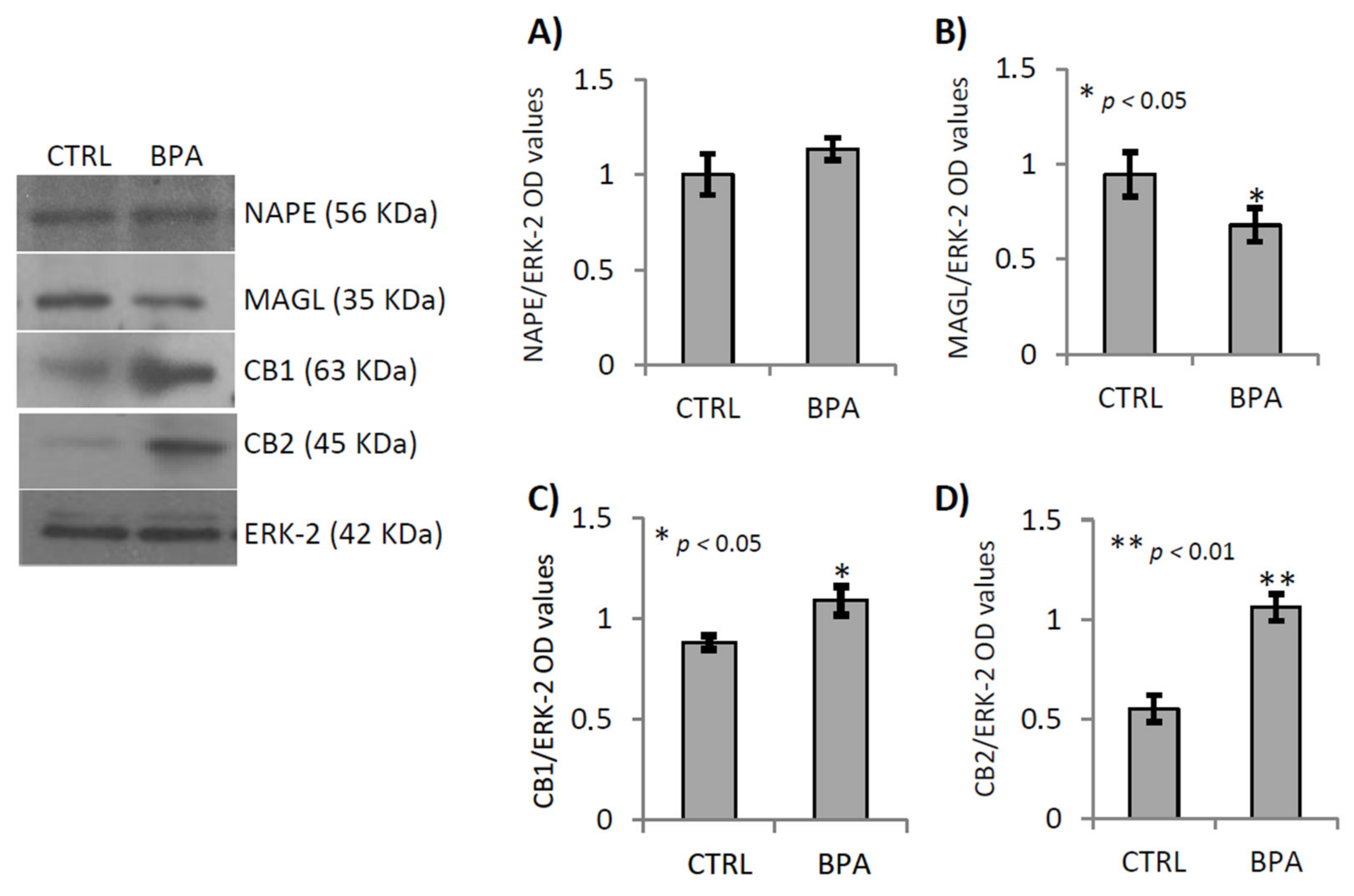
2.3. BPA Effects on Endocannabinoid System Components
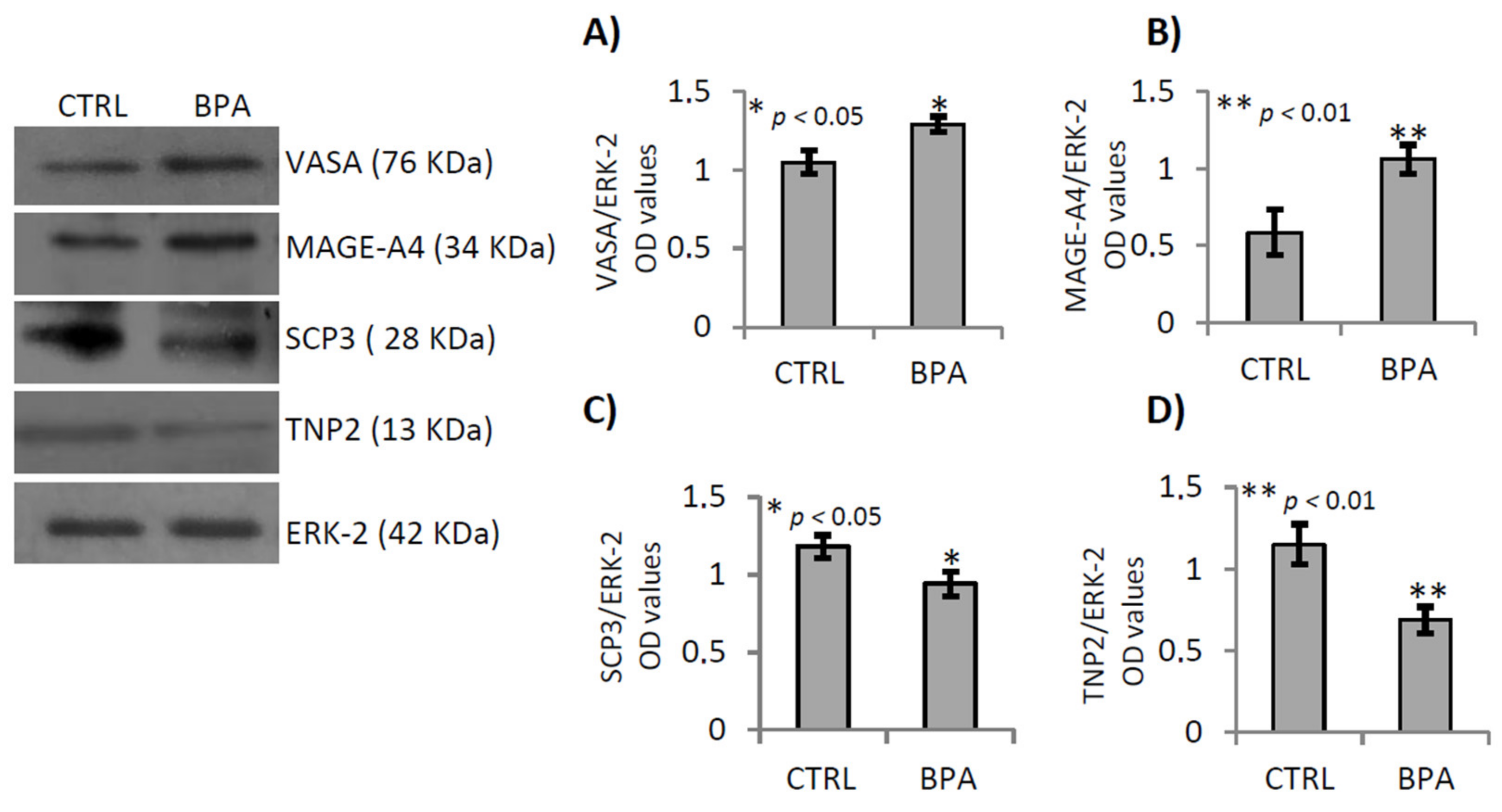
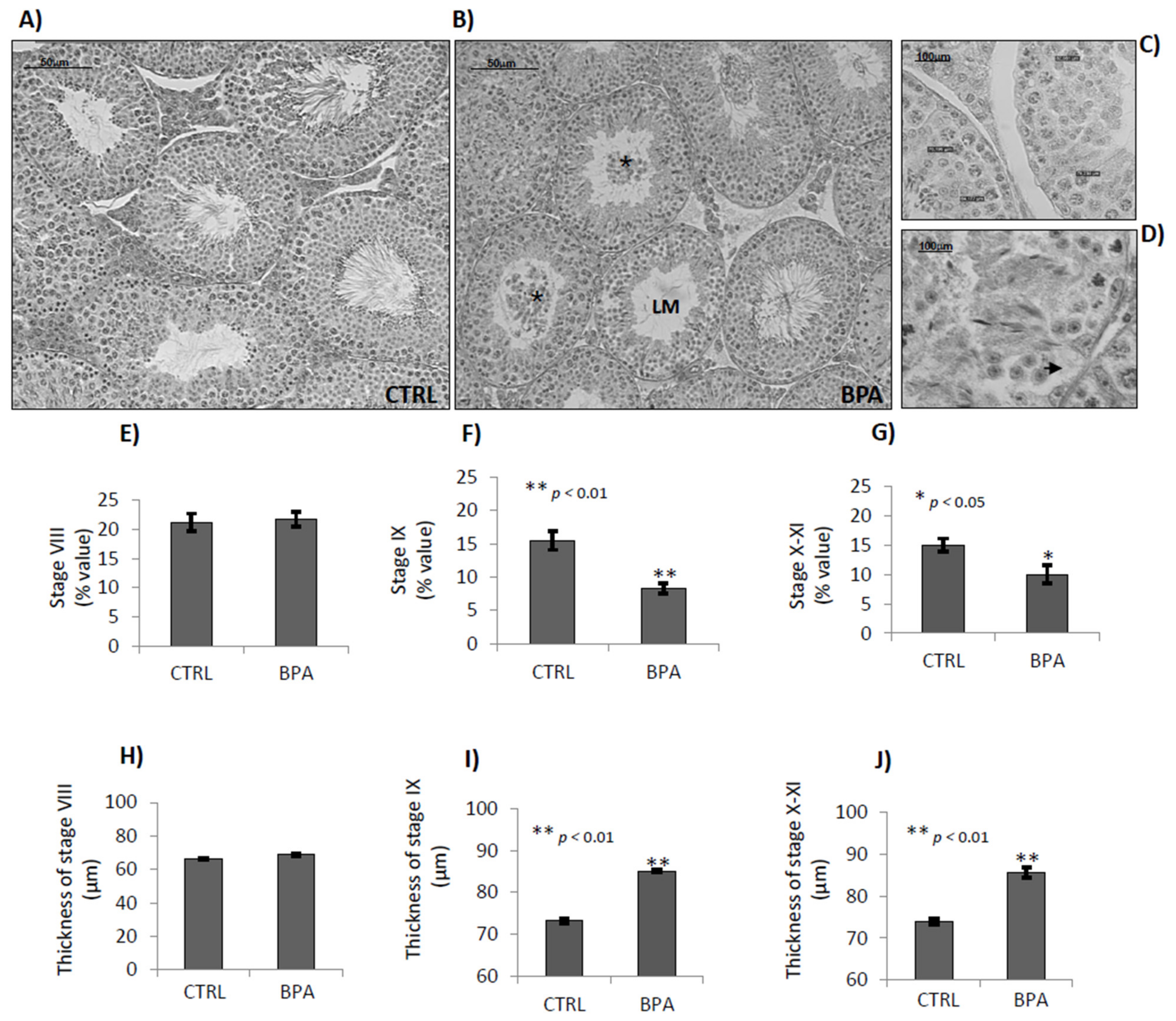
2.4. BPA Effects on Germ Cell Progression
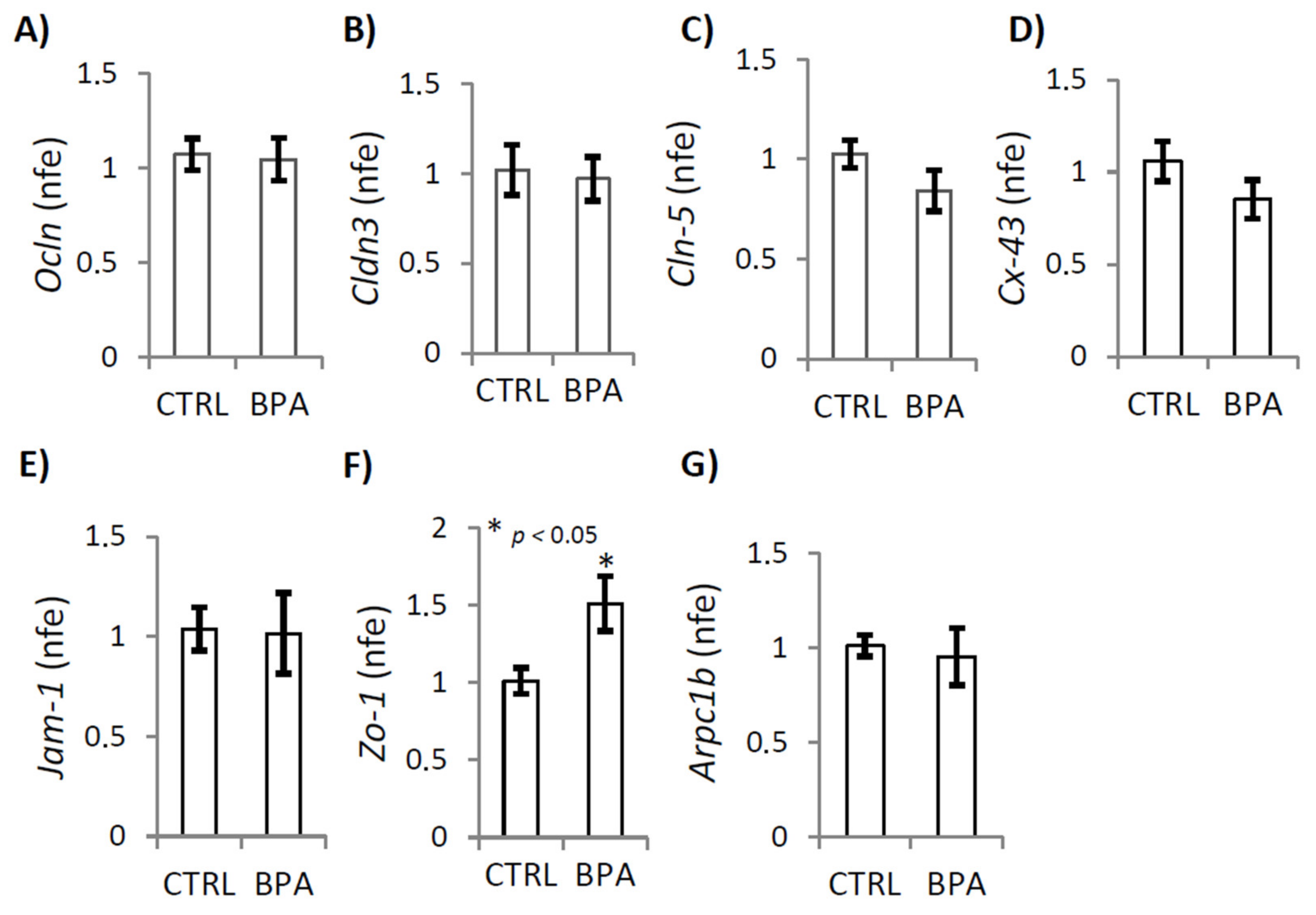
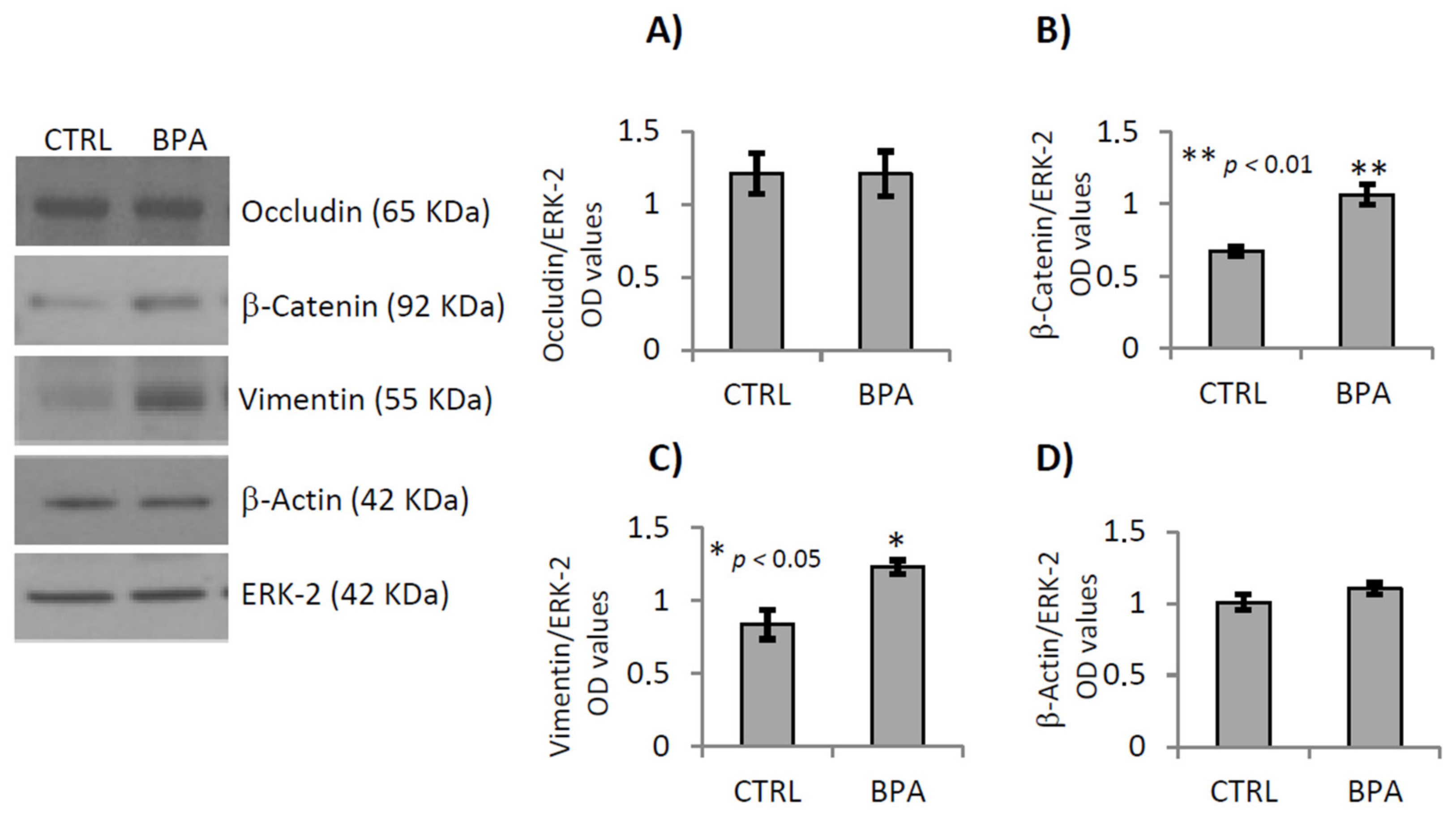
2.5. BPA Effects on Junctional Proteins
3. Discussion
4. Material and Methods
4.1. Experimental Design, BPA Exposure, Parameters and Tissue Collection
4.2. Determination of BPA, 17-β-Estradiol and Testosterone Levels in Adipose and Testicular Tissues
4.3. Total RNA Preparation
4.4. cDNA Synthesis and Quantitative Real Time-PCR (qRT-PCR)
4.5. Protein Extraction and Western Blot Analysis
4.6. Testicular Morphology, Spermatogenetic Stage and Tubular Thickness Analysis
4.7. Statistical Analysis and Data Presentation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grun, F.; Blumberg, B. End ocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009, 304, 19–29. [Google Scholar] [CrossRef]
- Sharpe, R.M. Environmental/lifestyle effects on spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1697–1712. [Google Scholar] [CrossRef]
- Hiroi, H.; Tsutsumi, O.; Momoeda, M.; Takai, Y.; Osuga, Y.; Taketani, Y. Differential interactions of bisphenol A and 17beta-estradiol with estrogen receptor alpha (ERalpha) and ERbeta. Endocr. J. 1999, 46, 773–778. [Google Scholar] [CrossRef]
- Xu, L.C.; Sun, H.; Chen, J.F.; Bian, Q.; Qian, J.; Song, L.; Wang, X.R. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology 2005, 216, 197–203. [Google Scholar] [CrossRef]
- Takayanagi, S.; Tokunaga, T.; Liu, X.; Okada, H.; Matsushima, A.; Shimohigashi, Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-relatedreceptor gamma (ERRgamma) with high constitutive activity. Toxicol. Lett. 2006, 167, 95–105. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Porreca, I.; Rizzo, F.; Ganbaatar, E.; Carchia, E.; Mallardo, M.; Ambrosino, C. Bisphenol A interferes with thyroid specific gene expression. Toxicology 2013, 304, 21–31. [Google Scholar] [CrossRef]
- Porreca, I.; Ulloa Severino, L.; D’Angelo, F.; Cuomo, D.; Ceccarelli, M.; Altucci, L.; Ambrosino, C. Stockpile of slight transcriptomic changes determines the indirect genotoxicity of low-dose BPA in thyroid cells. PLoS ONE 2016, 11, e0151618. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Z.; Shi, Q.M.; Ge, X.; Wang, H.X.; Li, M.X.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.P.; et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef]
- Mita, L.; Baldi, A.; Diano, N.; Viggiano, E.; Portaccio, M.; Nicolucci, C.; Grumiro, L.; Menale, C.; Mita, D.G.; Spugnini, E.P.; et al. Differential accumulation of BPA in some tissues of offspring of Balb-C mice exposed to different BPA doses. Environ. Toxicol. Pharmacol. 2012, 33, 9–15. [Google Scholar] [CrossRef]
- Nishikawa, M.; Iwano, H.; Yanagisawa, R.; Koike, N.; Inoue, H.; Yokota, H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 2010, 118, 1196–1203. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 26, 79–89. [Google Scholar] [CrossRef]
- Inoue, H.; Tsuruta, A.; Kudo, S.; Ishii, T.; Fukushima, Y.; Iwano, H.; Yokota, H.; Kato, S. Bisphenol a glucuronidation and excretion in liver of pregnant and nonpregnant female rats. Drug Metab. Dispos. 2005, 33, 55–59. [Google Scholar] [CrossRef]
- Matsumoto, J.; Yokota, H.; Yuasa, A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ. Health Perspect. 2002, 110, 193–196. [Google Scholar] [CrossRef]
- Errico, S.; Chioccarelli, T.; Moggio, M.; Diano, N.; Cobellis, G. A new LC-MS/MS method for simultaneous and quantitative detection of bisphenol-A and steroids in target tissues: A power tool to characterize the interference of bisphenol-A exposure on steroid levels. Molecules 2019, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Stewart, J.D. Highlight report: The bisphenol A controversy. Arch. Toxicol. 2011, 85, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Tyl, R.W. Basic exploratory research versus guideline-compliant studies used for hazard evaluation and risk assessment: Bisphenol A as a case study. Environ. Health Perspect. 2009, 117, 1644–1651. [Google Scholar] [CrossRef]
- Okada, A.; Kai, O. Effects of estradiol-17beta and bisphenol A administered chronically to mice throughout pregnancy and lactation on the male pups’ reproductive system. Asian J. Androl. 2008, 10, 271–276. [Google Scholar] [CrossRef]
- Salian, S.; Doshi, T.; Vanage, G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009, 85, 742–752. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Vom Saal, F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef]
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef]
- Trasande, L.; Zoeller, R.T.; Hass, U.; Kortenkamp, A.; Grandjean, P.; Myers, J.P.; Di Gangi, J.; Bellanger, M.; Hauser, R.; Legler, J.; et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J. Clin. Endocrinol. Metab. 2015, 100, 1245–1255. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Liu, F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021, 262, 127880. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Yang, Q.; Sui, X.; Cao, J.; Liu, C.; Zheng, S.; Bao, M.; Huang, Y.; Wu, K. Effects of Exposure to Bisphenol A during Pregnancy on the Pup Testis Function. Int. J. Endocrinol. 2019, 2019, 6785289. [Google Scholar] [CrossRef]
- Castro, B.; Sánchez, P.; Torres, J.M.; Preda, O.; Del Moral, R.G.; Ortega, E. Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS ONE 2013, 8, e55905. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, S.C.; Jubendradass, R.; Jayakanthan, M.; Rani, S.J.; Mathur, P.P. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: An in vivo and in silico study. Food Chem. Toxicol. 2012, 50, 1124–1133. [Google Scholar] [CrossRef]
- Jin, P.; Wang, X.; Chang, F.; Bai, Y.; Li, Y.; Zhou, R.; Chen, L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J. Biomed. Res. 2013, 27, 135–144. [Google Scholar] [PubMed]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.Y.; et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 2010, 194, 16–25. [Google Scholar] [CrossRef]
- Wu, J.H.; Jiang, X.R.; Liu, G.M.; Liu, X.Y.; He, G.L.; Sun, Z.Y. Oral exposure to low-dose bisphenol A aggravates testosterone-induced benign hyperplasia prostate in rats. Toxicol. Ind. Health 2011, 27, 810–819. [Google Scholar] [CrossRef]
- Fiorini, C.; Tilloy-Ellul, A.; Chevalier, S.; Charuel, C.; Pointis, G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod. Toxicol. 2004, 18, 413–421. [Google Scholar] [CrossRef]
- Li, M.W.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Disruption of the blood-testis barrier integrity by bisphenol a in vitro: Is this a suitable model for studying blood testis barrier dynamics? Int. J. Biochem. Cell Biol. 2009, 41, 2302–2314. [Google Scholar] [CrossRef]
- Toyama, Y.; Suzuki-Toyota, F.; Maekawa, M.; Ito, C.; Toshimori, K. Adverse effects of bisphenol A to spermiogenesis in mice and rats. Arch. Histol. Cytol. 2004, 67, 373–381. [Google Scholar] [CrossRef]
- Chioccarelli, T.; Manfrevola, F.; Migliaccio, M.; Altucci, L.; Porreca, V.; Fasano, S.; Cobellis, G. Fetal-Perinatal Exposure to Bisphenol-A Affects Quality of Spermatozoa in Adulthood Mouse. Int. J. Endocrinol. 2020, 2020, 2750501. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Yoon, S.J.; Park, Y.J.; Ryu, B.Y.; Pang, M.G. A novel approach to assessing bisphenol-A hazards using an in vitro model system. BMC Genom. 2016, 17, 577. [Google Scholar] [CrossRef]
- Dobrzyńska, M.M.; Radzikowska, J. Genotoxicity and reproductive toxicity of bisphenol A and X-ray/bisphenol A combination in male mice. Drug Chem. Toxicol. 2013, 36, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hansel, W. The essentiality of the epididymal fat pad for spermatogenesis. Endocrinology 2010, 151, 5565–5567. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Huddleston, G.G.; Clancy, A.N.; Harris, R.B.; Bartness, T.J. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology 2010, 151, 5669–5679. [Google Scholar] [CrossRef] [PubMed]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and gonadal hormones: Bidirectional interactions in physiology and behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef]
- Suglia, A.; Chianese, R.; Migliaccio, M.; Ambrosino, C.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Chioccarelli, T. Bisphenol A induces hypothalamic down-regulation of the the cannabinoid receptor 1 and anorexigenic effects in male mice. Pharmacol. Res. 2016, 113, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Chioccarelli, T.; Cacciola, G.; Altucci, L.; Lewis, S.E.; Simon, L.; Ricci, G.; Ledent, C.; Meccariello, R.; Fasano, S.; Pierantoni, R.; et al. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology 2010, 151, 5017–5029. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Chioccarelli, T.; Altucci, L.; Ledent, C.; Mason, J.I.; Fasano, S.; Pierantoni, R.; Cobellis, G. Low 17beta-estradiol levels in Cnr1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biol. Reprod. 2013, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Chioccarelli, T.; Fasano, S.; Pierantoni, R.; Cobellis, G. Estrogens and spermiogenesis: New insights from type 1 cannabinoid receptor knockout mice. Int. J. Endocrinol. 2013, 2013, 501350. [Google Scholar] [CrossRef]
- Nunez, A.A.; Kannan, K.; Giesy, J.P.; Fang, J.; Clemens, L.G. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 2001, 42, 917–922. [Google Scholar] [CrossRef]
- Rivas, A.M.; Fernandez, M.F.; Cerrillo, I.; Ibarluzea, J.; Olea-Serrano, M.F.; Pedraza, V.; Olea, N. Human exposure to endocrine disruptors: Standarization of a marker of estrogenic exposure in adipose tissue. APMIS 2001, 109, 185–197. [Google Scholar] [CrossRef]
- Fernandez, M.F. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 2007, 24, 259–264. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Martella, A.; Silvestri, C.; Maradonna, F.; Gioacchini, G.; Allarà, M.; Radaelli, G.; Overby, D.R.; Di Marzo, V.; Carnevali, O. Bisphenol A Induces Fatty Liver by an Endocannabinoid-Mediated Positive Feedback Loop. Endocrinology 2016, 157, 1751–1763. [Google Scholar] [CrossRef]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.C.; Dalla Valle, L.; Carnevali, O. BPA-Induced Deregulation Of Epigenetic Patterns: Effects On Female Zebrafish Reproduction. Sci. Rep. 2016, 6, 21982. [Google Scholar] [CrossRef] [PubMed]
- Carchia, E.; Porreca, I.; Almeida, P.J.; D’Angelo, F.; Cuomo, D.; Ceccarelli, M.; De Felice, M.; Mallardo, M.; Ambrosino, C. Evaluation of low doses BPA-inducedperturbation of glycemia by toxicogenomics points to a primary role ofpancreatic islets and to the mechanism of toxicity. Cell Death Dis. 2015, 6, e1959. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Champagne, F.A. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav. Immun. 2011, 25, 1084–1093. [Google Scholar] [CrossRef]
- Lee, H.K.; Lee, J.K.; Cho, B. The Role of Androgen in the Adipose Tissue of Males. World J. Mens. Health 2013, 31, 136–140. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Grimaldi, P.; Orlando, P.; Di Siena, S.; Lolicato, F.; Petrosino, S.; Bisogno, T.; Geremia, R.; De Petrocellis, L.; Di Marzo, V. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 11131–11136. [Google Scholar] [CrossRef]
- Bennetzen, M.F.; Wellner, N.; Ahmed, S.S.; Ahmed, S.M.; Diep, T.A.; Hansen, H.S.; Richelsen, B.; Pedersen, S.B. Investigations of the human endocannabinoid system in two subcutaneous adipose tissue depots in lean subjects and in obese subjects before and after weight loss. Int. J. Obes. 2011, 35, 1377–1384. [Google Scholar] [CrossRef]
- Matias, I.; Di Marzo, V. Endocannabinoid system and its role in energy regulation. Expert Rev. Endocrinol. Metab. 2006, 1, 557–569. [Google Scholar] [CrossRef]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell. Biol. 2003, 63, 463–468. [Google Scholar] [CrossRef]
- Dinh, T.P.; Freund, T.F.; Piomelli, D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem. Phys. Lipids 2002, 121, 149–158. [Google Scholar] [CrossRef]
- McKinney, M.K.; Cravatt, B.F. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005, 74, 411–432. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Di Giacomo, D.; De Domenico, E.; Sette, C.; Geremia, R.; Grimaldi, P. Type 2 cannabinoid receptor contributes to the physiological regulation of spermatogenesis. FASEB J. 2016, 30, 1453–1463. [Google Scholar] [CrossRef]
- Migliaccio, M.; Ricci, G.; Suglia, A.; Manfrevola, F.; Mackie, K.; Fasano, S.; Pierantoni, R.; Chioccarelli, T.; Cobellis, G. Analysis of Endocannabinoid System in Rat Testis During the First Spermatogenetic Wave. Front. Endocrinol. 2018, 9, 269. [Google Scholar] [CrossRef]
- Chioccarelli, T.; Manfrevola, F.; Porreca, V.; Fasano, S.; Altucci, L.; Pierantoni, R.; Cobellis, G. The Cannabinoid Receptor CB1 Stabilizes Sperm Chromatin Condensation Status During Epididymal Transit by Promoting Disulphide Bond Formation. Int. J. Mol. Sci. 2020, 21, 3117. [Google Scholar] [CrossRef]
- Chioccarelli, T.; Pierantoni, R.; Manfrevola, F.; Porreca, V.; Fasano, S.; Chianese, R.; Cobellis, G. Histone Post-Translational Modifications and CircRNAs in Mouse and Human Spermatozoa: Potential Epigenetic Marks to Assess Human Sperm Quality. J. Clin. Med. 2020, 9, 640. [Google Scholar] [CrossRef]
- Wenger, T.; Ledent, C.; Csernus, V.; Gerendai, I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem. Biophys. Res. Commun. 2001, 284, 363–368. [Google Scholar] [CrossRef]
- Cacciola, G.; Chioccarelli, T.; Mackie, K.; Meccariello, R.; Ledent, C.; Fasano, S.; Pierantoni, R.; Cobellis, G. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: Possible involvement in adult Leydig cell differentiation. Biol. Reprod. 2008, 79, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Cacciola, G.; Chianese, R.; Chioccarelli, T.; Fasano, S. CB1 activity in male reproduction: Mammalian and nonmammalian animal models. Vitam. Horm. 2009, 81, 367–387. [Google Scholar] [PubMed]
- Meccariello, R.; Chianese, R.; Chioccarelli, T.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Cobellis, G. Intra-testicular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front. Endocrinol. 2014, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Meccariello, R.; Chianese, R.; Chioccarelli, T.; Fasano, S.; Pierantoni, R. Effects of Neuroendocrine CB1 Activity on Adult Leydig Cells. Front. Endocrinol. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Ricci, G.; Cacciola, G.; Orlando, P.; Petrosino, S.; Cascio, M.G.; Bisogno, T.; De Petrocellis, L.; Chioccarelli, T.; Altucci, L.; et al. A gradient of 2-arachidonoylglycerol regulates mouse epididymal sperm cell start-up. Biol. Reprod. 2010, 82, 451–458. [Google Scholar] [CrossRef]
- Forner-Piquer, I.; Santangeli, S.; Maradonna, F.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Habibi, H.R.; Carnevali, O. Role of Bisphenol A on the Endocannabinoid System at central and peripheral levels: Effects on adult female zebrafish. Chemosphere 2018, 205, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, H.J.; Yoon, M.J. VASA (DDX4) is a Putative Marker for Spermatogonia, Spermatocytes and Round Spermatids in Stallions. Reprod. Domest. Anim. 2015, 50, 1032–1038. [Google Scholar] [CrossRef]
- Takahashi, K.; Shichijo, S.; Noguchi, M.; Hirohata, M.; Itoh, K. Identification of MAGE-1 and MAGE-4 proteins in spermatogonia and primary spermatocytes of testis. Cancer Res. 1995, 55, 3478–3482. [Google Scholar]
- Yuan, L.; Liu, J.G.; Zhao, J.; Brundell, E.; Daneholt, B.; Höög, C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell 2000, 5, 73–83. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burrello, N.; Barone, N.; Palermo, I.; Grasso, U.; D’Agata, R. Effects of progesterone on sperm function: Mechanisms of action. Hum. Reprod. 2000, 15, 28–45. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Yang, C.; Zhao, S. Effect of monoacylglycerol lipase inhibition on intestinal permeability in chronic stress model. Biochem. Biophys. Res. Commun. 2020, 525, 962–967. [Google Scholar] [CrossRef]
- Forner-Piquer, I.; Beato, S.; Piscitelli, F.; Santangeli, S.; Di Marzo, V.; Habibi, H.R.; Maradonna, F.; Carnevali, O. Effects of BPA on zebrafish gonads: Focus on the endocannabinoid system. Environ. Pollut. 2020, 264, 114710. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.G.; Zhu, B.Z. Low Concentrations of Bisphenol A Induce Mouse Spermatogonial Cell Proliferation by G Protein-Coupled Receptor 30 and Estrogen Receptor-α. Environ. Health Perspect. 2011, 119, 1775–1780. [Google Scholar] [CrossRef]
- Rossi, G.; Dufrusine, B.; Lizzi, A.R.; Luzi, C.; Piccoli, A.; Fezza, F.; Iorio, R.; D’Andrea, G.; Dainese, E.; Cecconi, S.; et al. Bisphenol A Deranges the Endocannabinoid System of Primary Sertoli Cells with an Impact on Inhibin B Production. Int. J. Mol. Sci. 2020, 21, 8986. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; Bouraima-Lelong, H.; Delalande, C. Estrogens: New players in spermatogenesis. Reprod. Biol. 2011, 11, 174–193. [Google Scholar] [CrossRef]
- Pinto, M.E.; Vilamaior, P.S.; Taboga, S.R.; Góes, R.M. Exposure of young rats to high estrogen doses leads to degeneration of elongated spermatids. Tissue Cell 2008, 40, 31–42. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.M. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar]
- Cheng, C.Y.; Mruk, D.M. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In Sertoli Cell Biology; Griswold, M.D., Ed.; Elsevier: Pullman, WA, USA, 2015; pp. 333–383. [Google Scholar]
- Chang, Y.F.; Lee-Chang, J.S.; Harris, K.Y.; Sinha-Hikim, A.P.; Rao, M.K. Role of β-catenin in post-meiotic male germ cell differentiation. PLoS ONE 2011, 6, e28039. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Yang, X.; Song, X.; Chen, X. Effects of Wnt/beta-catenin signaling on bisphenol A exposure in male mouse reproductive cells. Mol. Med. Rep. 2015, 12, 5561–5567. [Google Scholar] [CrossRef]
- Migrenne, S.; Moreau, E.; Pakarinen, P.; Dierich, A.; Merlet, J.; Habert, R.; Racine, C. Mouse testis development and function are differently regulated by follicle-stimulating hormone receptors signaling during fetal and prepubertal life. PLoS ONE 2012, 7, e53257. [Google Scholar] [CrossRef]
- Oakberg, E.F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 1956, 99, 507–516. [Google Scholar] [CrossRef]
- Acone, G.; Trabucco, E.; Colacurci, N.; Cobellis, L.; Mackie, K.; Meccariello, R.; Cacciola, G.; Chioccarelli, T.; Fasano, S.; Pierantoni, R.; et al. Low type I cannabinoid receptor levels characterize placental villous in labou-ring delivery. Placenta 2009, 30, 203–205. [Google Scholar] [CrossRef]
- Trabucco, E.; Acone, G.; Marenna, A.; Pierantoni, R.; Cacciola, G.; Chioccarelli, T.; Mackie, K.; Fasano, S.; Colacurci, N.; Meccariello, R.; et al. Endocannabinoid system in first trimester placenta: Low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta 2009, 30, 516–522. [Google Scholar] [CrossRef]

| Gene Primers | Sequenses 5′–3′ | Tm (°C) | Product Size (bp) |
|---|---|---|---|
| AR S | gacctgcctgatctgtgg | 58 | 206 |
| AR AS | gagtcatccctgcttcataa | ||
| aER S | tgccctactacctggagaa | 58 | 170 |
| aER AS | gtagcgagtctccttggc | ||
| 3b-Hsd S | agtattccgaccagaaaccaag | 60 | 75 |
| 3b-Hsd AS | atccagaatgtctccttccaac | ||
| Ocln S | cctactcctccaatggcaaa | 55 | 208 |
| Ocln AS | ctcttgccctttcctgcttt | ||
| Cldn-3 S | gcacccaccaagatcctcta | 57 | 205 |
| Cldn-3 AS | tcgtctgtcaccatctggaa | ||
| Cldn-5 S | agagcagaggcaccagaatc | 57 | 143 |
| Cldn-5 AS | acacagcaccagacccagaa | ||
| Cx-43 S | ctttgacttcagcctccaag | 54 | 176 |
| Cx-43 AS | gaaaatgaagagcaccgaca | ||
| Jam-1 S | cactgattctccttggactctt | 56 | 157 |
| Jam-1 AS | gaacgacgaggtctgtttgaa | ||
| Zo-1 S | gcaccatgcctaaagctgtc | 57 | 122 |
| Zo-1 AS | actcaacacaccaccattgc | ||
| Arpc1b S | agctgatgtttgaatcgagc | 56 | 188 |
| Arpc1b AS | tttctgtgatgaaggtgacg | ||
| Nape-pld S | tggtttatgaaataccagca | 56 | 159 |
| Nape-pld AS | atctcttcaaaagcggg | ||
| Faah S | agattgagatgtatcgccag | 56 | 260 |
| Faah AS | cttcagaatgttgtcccac | ||
| Dagl S | atcactgtcctctgcgtctt | 54 | 202 |
| Dagl AS | tttctgagtaggcatctgact | ||
| Magl S | ggccctcatctttgtgtcc | 60 | 168 |
| Magl AS | ctgacgaaaacgtggaagtc | ||
| Cb1 S | ctgatcctggtggtgttgat | 60 | 162 |
| Cb1 AS | cctcagagcatagatgatgg | ||
| Cb2 S | aacggtggcttggagttcaa | 57 | 177 |
| Cb2 AS | gaacaggtacgagggctttct | ||
| Rps18 S | gagactctggatgctaactag | 56 | 172 |
| Rps18 AS | ggacatctaagggcatcacag |
| Primary Antibody | µg of Protein | Antibody Dilution | Secondary Antibody |
|---|---|---|---|
| AROMATASE (Elabscience 31086) | 50 | 1:250 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| OCCLUDIN (Thermo Fisher 40-4700) | 70 | 1:500 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| b-CATENIN (Santa Cruz sc-7199) | 70 | 1:1000 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| VIMENTIN (Elabscience 27405) | 70 | 1:2000 | HRP-conjugated mouse IgG (Dako Corp., Milan, Italy) |
| b-ACTIN (Elabscience 20031) | 70 | 1:2000 | HRP-conjugated mouse IgG (Dako Corp., Milan, Italy) |
| VASA (Abcam ab13840) | 20 | 1:1000 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| MAGE-A4 (Abcam ab139297) | 70 | 1:1000 | HRP-conjugated mouse IgG (Dako Corp., Milan, Italy) |
| SCP3 (Invitrogen PA1-16766) | 30 | 1:1000 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| TNP2 (Santa Cruz sc-21106) | 70 | 1:500 | HRP-conjugated goat IgG (Dako Corp., Milan, Italy) |
| CB1 (produced by Prof. Ken Mackie) | 20 | 1:1000 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| CB2 (Abcam ab45942) | 30 | 1:1000 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| NAPE-PLD (Cayman 101600) | 50 | 1:500 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| MAGL (Abcam ab24701) | 80 | 1:500 | HRP-conjugated rabbit IgG (Dako Corp., Milan, Italy) |
| ERK-2 (Santa Cruz sc-1647) | / | 1:1000 | HRP-conjugated mouse IgG (Dako Corp., Milan, Italy) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioccarelli, T.; Migliaccio, M.; Suglia, A.; Manfrevola, F.; Porreca, V.; Diano, N.; Errico, S.; Fasano, S.; Cobellis, G. Characterization of Estrogenic Activity and Site-Specific Accumulation of Bisphenol-A in Epididymal Fat Pad: Interfering Effects on the Endocannabinoid System and Temporal Progression of Germ Cells. Int. J. Mol. Sci. 2021, 22, 2540. https://doi.org/10.3390/ijms22052540
Chioccarelli T, Migliaccio M, Suglia A, Manfrevola F, Porreca V, Diano N, Errico S, Fasano S, Cobellis G. Characterization of Estrogenic Activity and Site-Specific Accumulation of Bisphenol-A in Epididymal Fat Pad: Interfering Effects on the Endocannabinoid System and Temporal Progression of Germ Cells. International Journal of Molecular Sciences. 2021; 22(5):2540. https://doi.org/10.3390/ijms22052540
Chicago/Turabian StyleChioccarelli, Teresa, Marina Migliaccio, Antonio Suglia, Francesco Manfrevola, Veronica Porreca, Nadia Diano, Sonia Errico, Silvia Fasano, and Gilda Cobellis. 2021. "Characterization of Estrogenic Activity and Site-Specific Accumulation of Bisphenol-A in Epididymal Fat Pad: Interfering Effects on the Endocannabinoid System and Temporal Progression of Germ Cells" International Journal of Molecular Sciences 22, no. 5: 2540. https://doi.org/10.3390/ijms22052540
APA StyleChioccarelli, T., Migliaccio, M., Suglia, A., Manfrevola, F., Porreca, V., Diano, N., Errico, S., Fasano, S., & Cobellis, G. (2021). Characterization of Estrogenic Activity and Site-Specific Accumulation of Bisphenol-A in Epididymal Fat Pad: Interfering Effects on the Endocannabinoid System and Temporal Progression of Germ Cells. International Journal of Molecular Sciences, 22(5), 2540. https://doi.org/10.3390/ijms22052540






