Zika Virus Growth in Human Kidney Cells Is Restricted by an Elevated Glucose Level
Abstract
1. Introduction
2. Results and Discussion
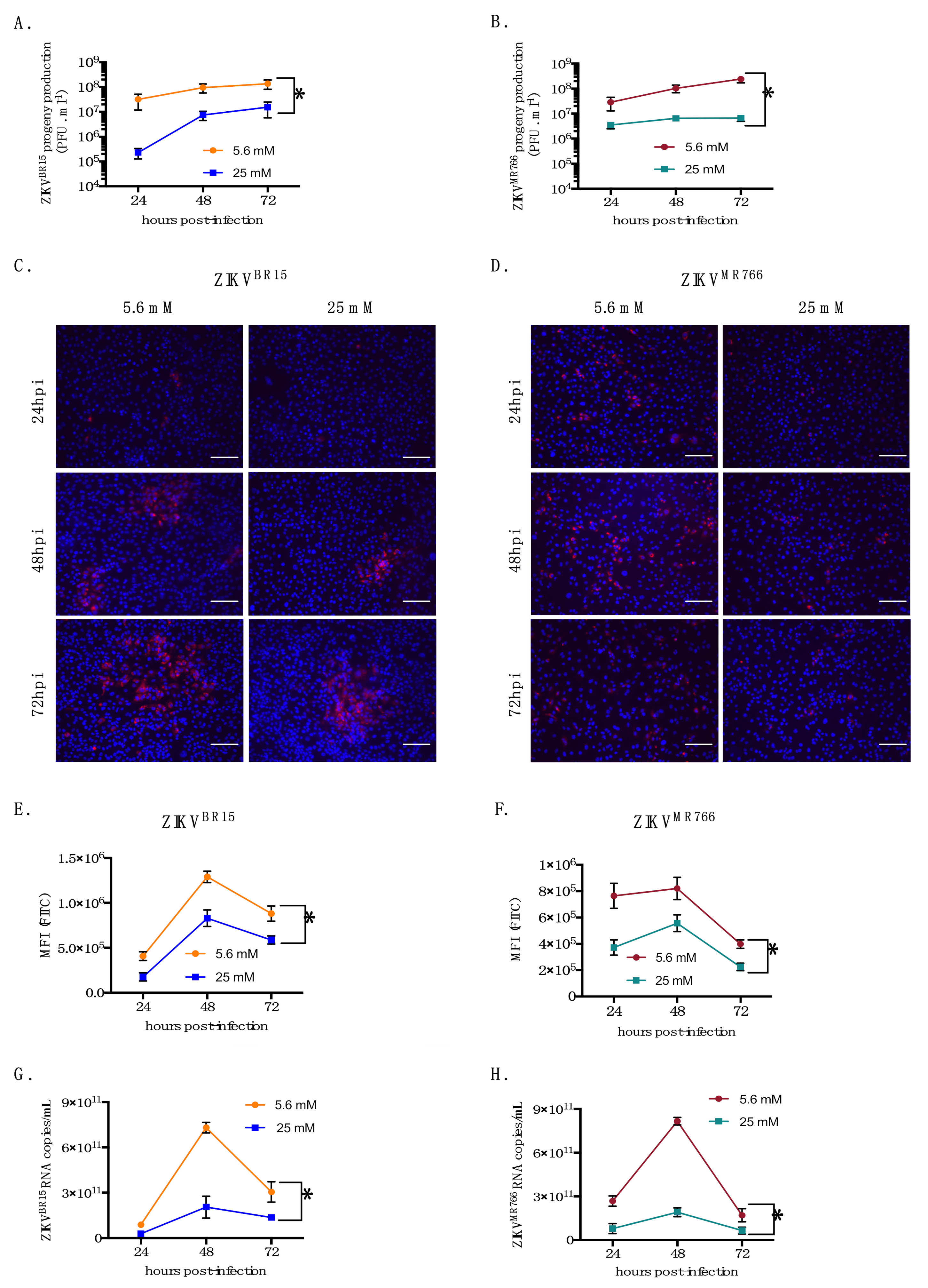
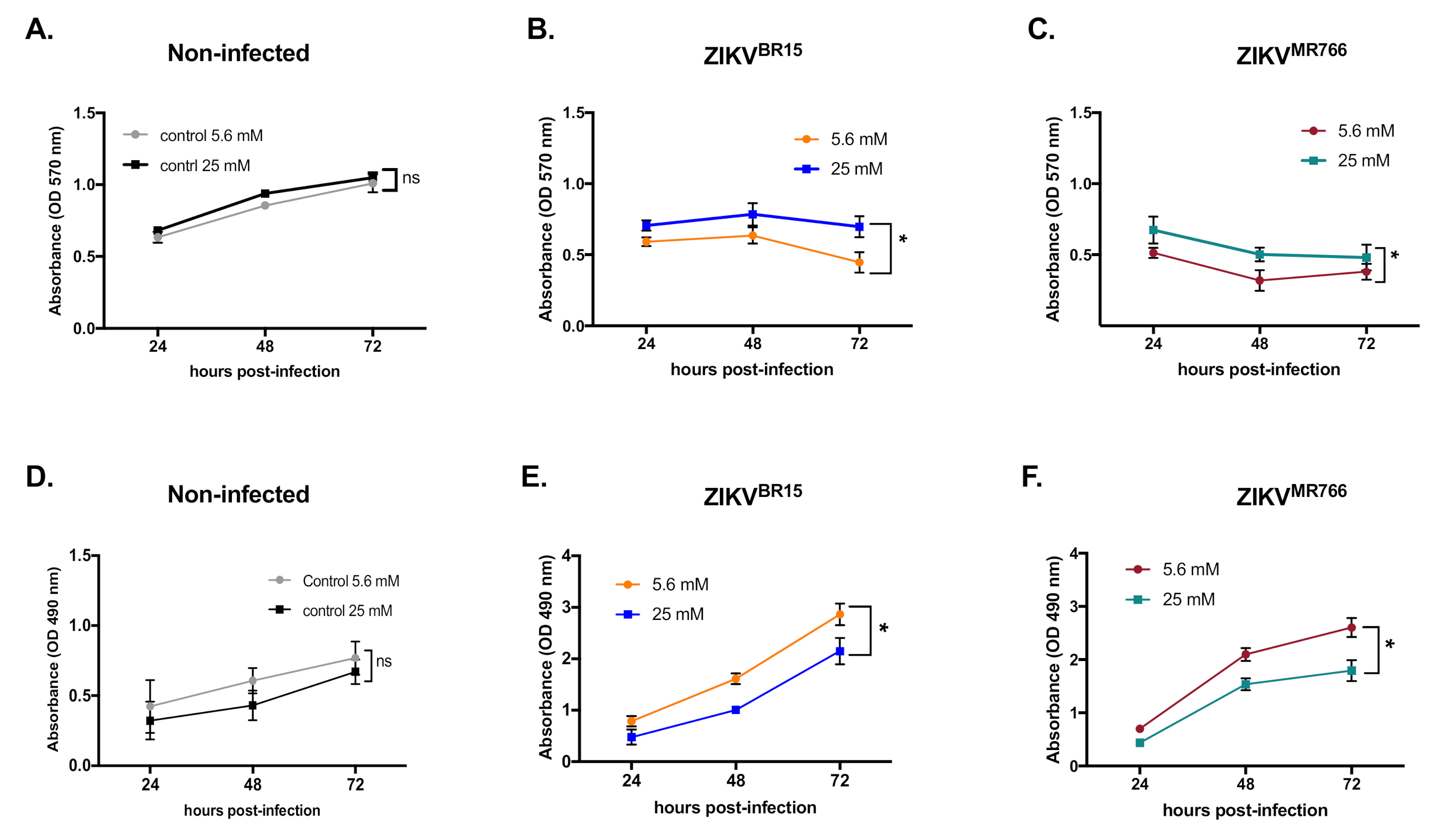
2.1. High-Glucose Condition Restricts ZIKV Growth in HK-2 Cells
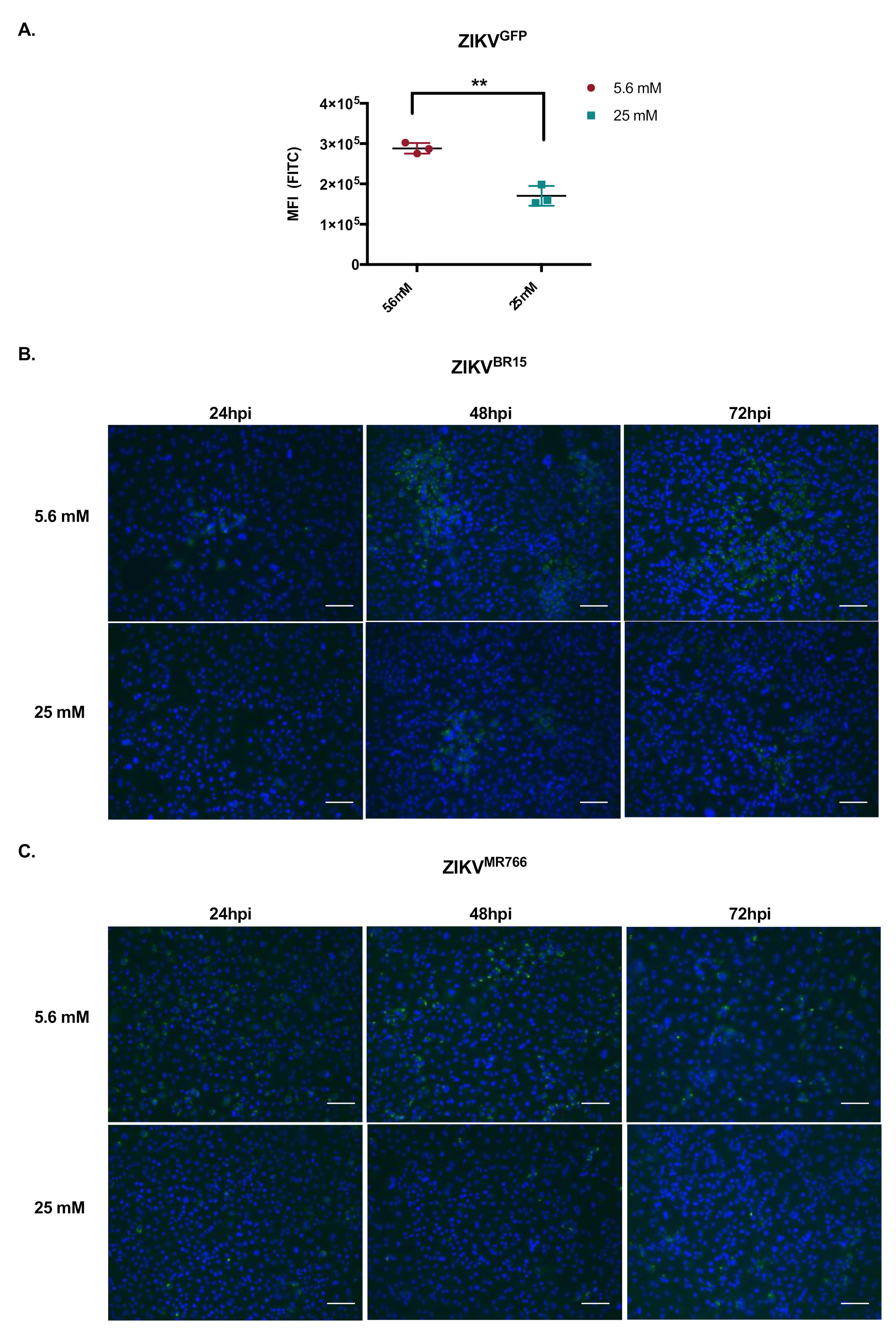
2.2. High-Glucose Condition Does Not Affect Early Steps of ZIKV Infection in HK-2 Cells
2.3. High Glucose Level Impacts ZIKV Replication in HK-2 Cells
2.4. Concluding Remarks and Discussion
3. Materials and Methods
3.1. Cells, Virus, and Reagents
3.2. Plaque-Forming Assay
3.3. Flow Cytometry Assay
3.4. Immunofluorescence Assay
3.5. LDH Assay
3.6. RT-qPCR
3.7. Virus Binding Assay
3.8. Internalisation Assay
3.9. Fusion Assay
3.10. MTT Assay
3.11. Transfection Experiments
3.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BSA | Bovine Serum Albumin |
| DAG | Angstrom (unit of length equal to 10−10 m) |
| DKD | Diabetic Kidney Disease |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | DiMethyl SulfOxide |
| DNA | DeoxyriboNucleic Acid |
| dsRNA | double stranded RNA |
| EGCG | EpiGalloCatechin Gallate |
| ER | Endoplasmic Reticulum |
| FBS | Fetal Bovine Serum |
| GFP | Green Fluorescent Protein |
| HK-2 | Human -Kidney-2 |
| hpi | hours post-infection |
| IgG | Immunoglobulin G |
| LDH | Lactate DeHydrogenase |
| mAb | monoclonal Antibody |
| MEM | Minimum Essential Medium |
| MFI | a Fluorescent Intensity |
| MOI | Multiplicity of Infection |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PBS | Phosphate-Buffered Saline |
| PFA | ParaFormAldehyde |
| PFU | Plaque Forming Unit |
| PKC | Protein Kinase C |
| Q3G | Isoquercitrin |
| RNA | RiboNucleic Acid |
| RT-qPCR | quantitative Reverse Transcription-Polymerase Chain Reaction |
| WNV | West Nile Virus |
| ZIKV | Zika Virus |
| ZIKVBR15 | Zika Virus Brazilian strain |
| ZIKVGFP | Zika Virus GFP |
| ZIKVMR766 | Zika Virus Uganda strain |
References
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Gatherer, D.; Kohl, A. Zika Virus: A Previously Slow Pandemic Spreads Rapidly through the Americas. J. Gen. Virol. 2016, 97, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Merfeld, E.; Ben-Avi, L.; Kennon, M.; Cerveny, K.L. Potential Mechanisms of Zika-Linked Microcephaly: Zika-Linked Microcephaly. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e273. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain–Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef]
- D’Ortenzio, E.; Matheron, S.; de Lamballerie, X.; Hubert, B.; Piorkowski, G.; Maquart, M.; Descamps, D.; Damond, F.; Yazdanpanah, Y.; Leparc-Goffart, I. Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374, 2195–2198. [Google Scholar] [CrossRef]
- Musso, D.; Stramer, S.L.; Busch, M.P. Zika Virus: A New Challenge for Blood Transfusion. Lancet 2016, 387, 1993–1994. [Google Scholar] [CrossRef]
- Motta, I.J.F.; Spencer, B.R.; Cordeiro da Silva, S.G.; Arruda, M.B.; Dobbin, J.A.; Gonzaga, Y.B.M.; Arcuri, I.P.; Tavares, R.C.B.S.; Atta, E.H.; Fernandes, R.F.M.; et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N. Engl. J. Med. 2016, 375, 1101–1103. [Google Scholar] [CrossRef]
- Smit, J.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef]
- Stiasny, K.; Fritz, R.; Pangerl, K.; Heinz, F.X. Molecular Mechanisms of Flavivirus Membrane Fusion. Amino Acids 2011, 41, 1159–1163. [Google Scholar] [CrossRef]
- Murray, K.; Walker, C.; Herrington, E.; Lewis, J.A.; McCormick, J.; Beasley, D.W.C.; Tesh, R.B.; Fisher-Hoch, S. Persistent Infection with West Nile Virus Years after Initial Infection. J. Infect. Dis. 2010, 201, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Burdmann, E.A. Flaviviruses and Kidney Diseases. Adv. Chronic Kidney Dis. 2019, 26, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, D.J. Zika Virus Infection and Implications for Kidney Disease. J. Mol. Med. 2018, 96, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Available online: Www.Who.Int/News-Room/Fact-Sheets/Details/Diabetes (accessed on 6 January 2021).
- Eckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving Importance of Kidney Disease: From Subspecialty to Global Health Burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Collins, A.J.; Foley, R.N.; Chavers, B.; Gilbertson, D.; Herzog, C.; Ishani, A.; Johansen, K.; Kasiske, B.L.; Kutner, N.; Liu, J.; et al. US Renal Data System 2013 Annual Data Report. Am. J. Kidney Dis. 2014, 63, A7. [Google Scholar] [CrossRef]
- Afkarian, M.; Sachs, M.C.; Kestenbaum, B.; Hirsch, I.B.; Tuttle, K.R.; Himmelfarb, J.; de Boer, I.H. Kidney Disease and Increased Mortality Risk in Type 2 Diabetes. J. Am. Soc. Nephrol. 2013, 24, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, D.J. Zika Virus Infection of the Human Glomerular Cells: Implications for Viral Reservoirs and Renal Pathogenesis. J. Infect. Dis. 2017, 216, 162–171. [Google Scholar] [CrossRef]
- Morais, C.; Westhuyzen, J.; Pat, B.; Gobe, G.; Healy, H. High Ambient Glucose Is Effect Neutral on Cell Death and Proliferation in Human Proximal Tubular Epithelial Cells. Am. J. Physiol. Ren. Physiol. 2005, 289, F401–F409. [Google Scholar] [CrossRef]
- Gadea, G.; Bos, S.; Krejbich-Trotot, P.; Clain, E.; Viranaicken, W.; El-Kalamouni, C.; Mavingui, P.; Desprès, P. A Robust Method for the Rapid Generation of Recombinant Zika Virus Expressing the GFP Reporter Gene. Virology 2016, 497, 157–162. [Google Scholar] [CrossRef]
- Bos, S.; Viranaicken, W.; Turpin, J.; El-Kalamouni, C.; Roche, M.; Krejbich-Trotot, P.; Desprès, P.; Gadea, G. The Structural Proteins of Epidemic and Historical Strains of Zika Virus Differ in Their Ability to Initiate Viral Infection in Human Host Cells. Virology 2018, 516, 265–273. [Google Scholar] [CrossRef]
- Bos, S.; Viranaicken, W.; Frumence, E.; Li, G.; Desprès, P.; Zhao, R.Y.; Gadea, G. The Envelope Residues E152/156/158 of Zika Virus Influence the Early Stages of Virus Infection in Human Cells. Cells 2019, 8, 1444. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Rossmann, M.G. Molecular Mechanisms Involved in the Early Steps of Flavivirus Cell Entry. Microbes Infect. 2011, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Murali, A.; Singh, S.K.; Giri, R. Epigallocatechin Gallate, an Active Green Tea Compound Inhibits the Zika Virus Entry into Host Cells via Binding the Envelope Protein. Int. J. Biol. Macromol. 2017, 104, 1046–1054. [Google Scholar] [CrossRef]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.; Magg, V.; Uch, F.; Mutz, P.; Klein, P.; Haneke, K.; Lohmann, V.; Bartenschlager, R.; Fackler, O.T.; Locker, N.; et al. Flavivirus Infection Uncouples Translation Suppression from Cellular Stress Responses. mBio 2017, 8, e02150-16. [Google Scholar] [CrossRef]
- Mariappan, M.M.; D’Silva, K.; Lee, M.J.; Sataranatarajan, K.; Barnes, J.L.; Choudhury, G.G.; Kasinath, B.S. Ribosomal Biogenesis Induction by High Glucose Requires Activation of Upstream Binding Factor in Kidney Glomerular Epithelial Cells. Am. J. Physiol. Ren. Physiol. 2011, 300, F219–F230. [Google Scholar] [CrossRef]
- Bhuvanakantham, R.; Cheong, Y.K.; Ng, M.-L. West Nile Virus Capsid Protein Interaction with Importin and HDM2 Protein Is Regulated by Protein Kinase C-Mediated Phosphorylation. Microbes Infect. 2010, 12, 615–625. [Google Scholar] [CrossRef]
- Chu, J.J.H.; Leong, P.W.H.; Ng, M.L. Analysis of the Endocytic Pathway Mediating the Infectious Entry of Mosquito-Borne Flavivirus West Nile into Aedes Albopictus Mosquito (C6/36) Cells. Virology 2006, 349, 463–475. [Google Scholar] [CrossRef]
- Ana Blázquez; Ángela Vázquez-Calvo; Miguel Martín-Acebes; Juan-Carlos Saiz Pharmacological Inhibition of Protein Kinase C Reduces West Nile Virus Replication. Viruses 2018, 10, 91. [CrossRef]
- Noppakunmongkolchai, W.; Poyomtip, T.; Jittawuttipoka, T.; Luplertlop, N.; Sakuntabhai, A.; Chimnaronk, S.; Jirawatnotai, S.; Tohtong, R. Inhibition of Protein Kinase C Promotes Dengue Virus Replication. Virol. J. 2016, 13, 35. [Google Scholar] [CrossRef]
- Craven, P.A.; Davidson, C.M.; DeRubertis, F.R. Increase in Diacylglycerol Mass in Isolated Glomeruli by Glucose From De Novo Synthesis of Glycerolipids. Diabetes 1990, 39, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ayo, S.H.; Radnik, R.; Garoni, J.A.; Troyer, D.A.; Kreisberg, J.I. High Glucose Increases Diacylglycerol Mass and Activates Protein Kinase C in Mesangial Cell Cultures. Am. J. Physiol. Ren. Physiol. 1991, 261, F571–F577. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.K.; Craven, P.A.; DeRubertis, F.R. Activation of Protein Kinase C Reduces Thromboxane Receptors in Glomeruli and Mesangial Cells. Kidney Int. 1993, 44, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lindenmeyer, M.T.; Rastaldi, M.P.; Ikehata, M.; Neusser, M.A.; Kretzler, M.; Cohen, C.D.; Schlöndorff, D. Proteinuria and Hyperglycemia Induce Endoplasmic Reticulum Stress. J. Am. Soc. Nephrol. 2008, 19, 2225–2236. [Google Scholar] [CrossRef]
- Cortese, M.; Goellner, S.; Acosta, E.G.; Neufeldt, C.J.; Oleksiuk, O.; Lampe, M.; Haselmann, U.; Funaya, C.; Schieber, N.; Ronchi, P.; et al. Ultrastructural Characterization of Zika Virus Replication Factories. Cell Rep. 2017, 18, 2113–2123. [Google Scholar] [CrossRef]
- Turpin, J.; Frumence, E.; Harrabi, W.; Haddad, J.G.; El Kalamouni, C.; Desprès, P.; Krejbich-Trotot, P.; Viranaïcken, W. Zika Virus Subversion of Chaperone GRP78/BiP Expression in A549 Cells during UPR Activation. Biochimie 2020, 175, 99–105. [Google Scholar] [CrossRef]
- Negi, C.K.; Jena, G. Nrf2, a Novel Molecular Target to Reduce Type 1 Diabetes Associated Secondary Complications: The Basic Considerations. Eur. J. Pharmacol. 2019, 843, 12–26. [Google Scholar] [CrossRef]
- Yoh, K.; Hirayama, A.; Ishizaki, K.; Yamada, A.; Takeuchi, M.; Yamagishi, S.; Morito, N.; Nakano, T.; Ojima, M.; Shimohata, H.; et al. Hyperglycemia Induces Oxidative and Nitrosative Stress and Increases Renal Functional Impairment in Nrf2-Deficient Mice. Genes Cells 2008. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The Protective Role of Nrf2 in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef]
- Sun, Y.; Xiu, C.; Liu, W.; Tao, Y.; Wang, J.; Qu, Y. Grape Seed Proanthocyanidin Extract Protects the Retina against Early Diabetic Injury by Activating the Nrf2 Pathway. Exp. Ther. Med. 2016, 11, 1253–1258. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-Mediated Suppression of NRF2-Signaling Reveals Potent Antiviral and Anti-Inflammatory Activity of 4-Octyl-Itaconate and Dimethyl Fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef] [PubMed]
- Baty, S.A.; Gibney, K.B.; Staples, J.E.; Patterson, A.B.; Levy, C.; Lehman, J.; Wadleigh, T.; Feld, J.; Lanciotti, R.; Nugent, C.T.; et al. Evaluation for West Nile Virus (WNV) RNA in Urine of Patients Within 5 Months of WNV Infection. J. Infect. Dis. 2012, 205, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Y.; Liu, S.-Q.; Deng, C.-L.; Zhang, Q.-Y.; Zhang, B. Detection of Zika Virus by SYBR Green One-Step Real-Time RT-PCR. J. Virol. Methods 2016, 236, 93–97. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reslan, A.; Haddad, J.G.; Moukendza Koundi, L.; Desprès, P.; Bascands, J.-L.; Gadea, G. Zika Virus Growth in Human Kidney Cells Is Restricted by an Elevated Glucose Level. Int. J. Mol. Sci. 2021, 22, 2495. https://doi.org/10.3390/ijms22052495
Reslan A, Haddad JG, Moukendza Koundi L, Desprès P, Bascands J-L, Gadea G. Zika Virus Growth in Human Kidney Cells Is Restricted by an Elevated Glucose Level. International Journal of Molecular Sciences. 2021; 22(5):2495. https://doi.org/10.3390/ijms22052495
Chicago/Turabian StyleReslan, Alawiya, Juliano G. Haddad, Liadrine Moukendza Koundi, Philippe Desprès, Jean-Loup Bascands, and Gilles Gadea. 2021. "Zika Virus Growth in Human Kidney Cells Is Restricted by an Elevated Glucose Level" International Journal of Molecular Sciences 22, no. 5: 2495. https://doi.org/10.3390/ijms22052495
APA StyleReslan, A., Haddad, J. G., Moukendza Koundi, L., Desprès, P., Bascands, J.-L., & Gadea, G. (2021). Zika Virus Growth in Human Kidney Cells Is Restricted by an Elevated Glucose Level. International Journal of Molecular Sciences, 22(5), 2495. https://doi.org/10.3390/ijms22052495





