Genetic Profiling of Malignant Melanoma Arising from an Ovarian Mature Cystic Teratoma: A Case Report
Abstract
1. Introduction
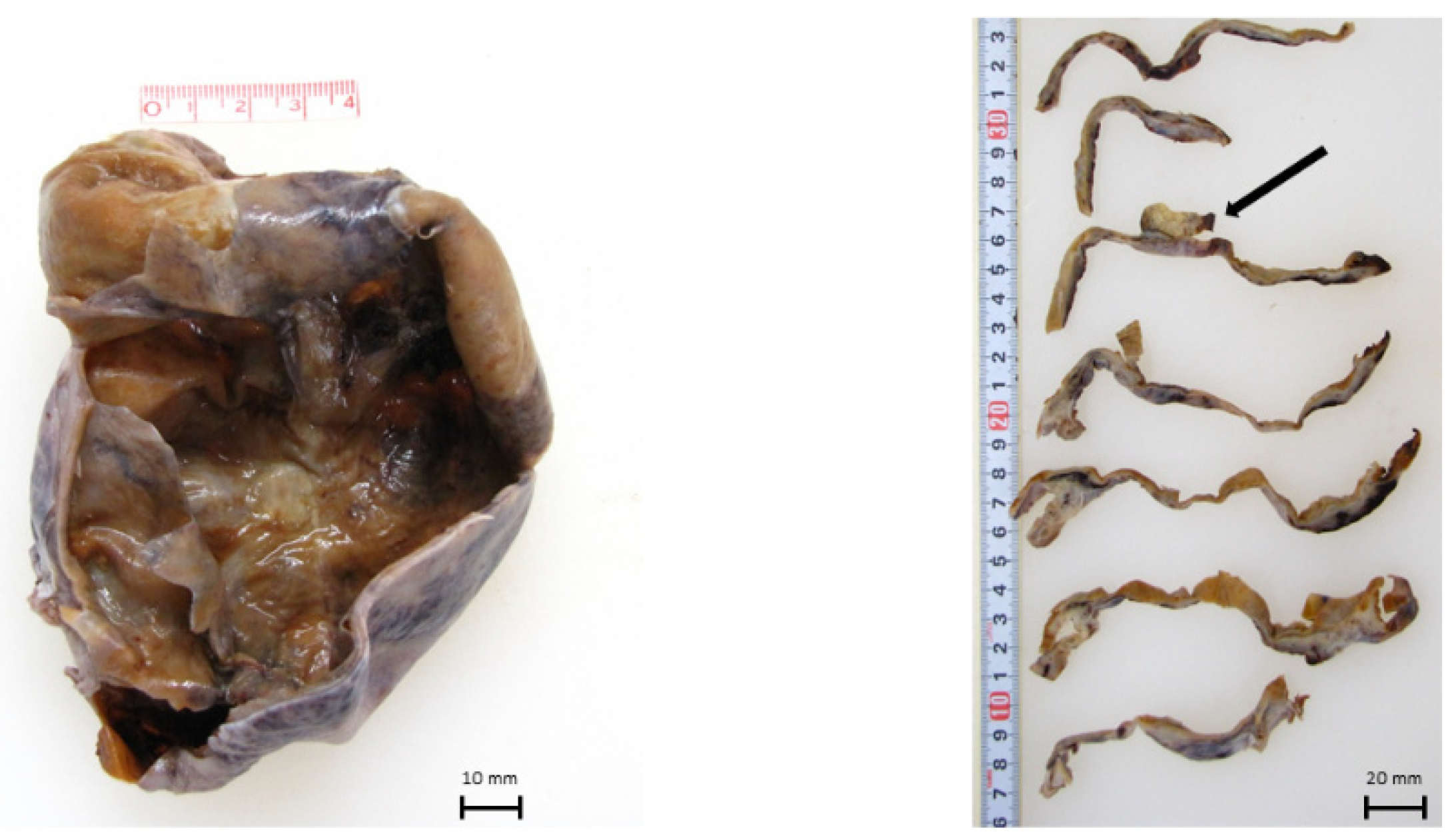
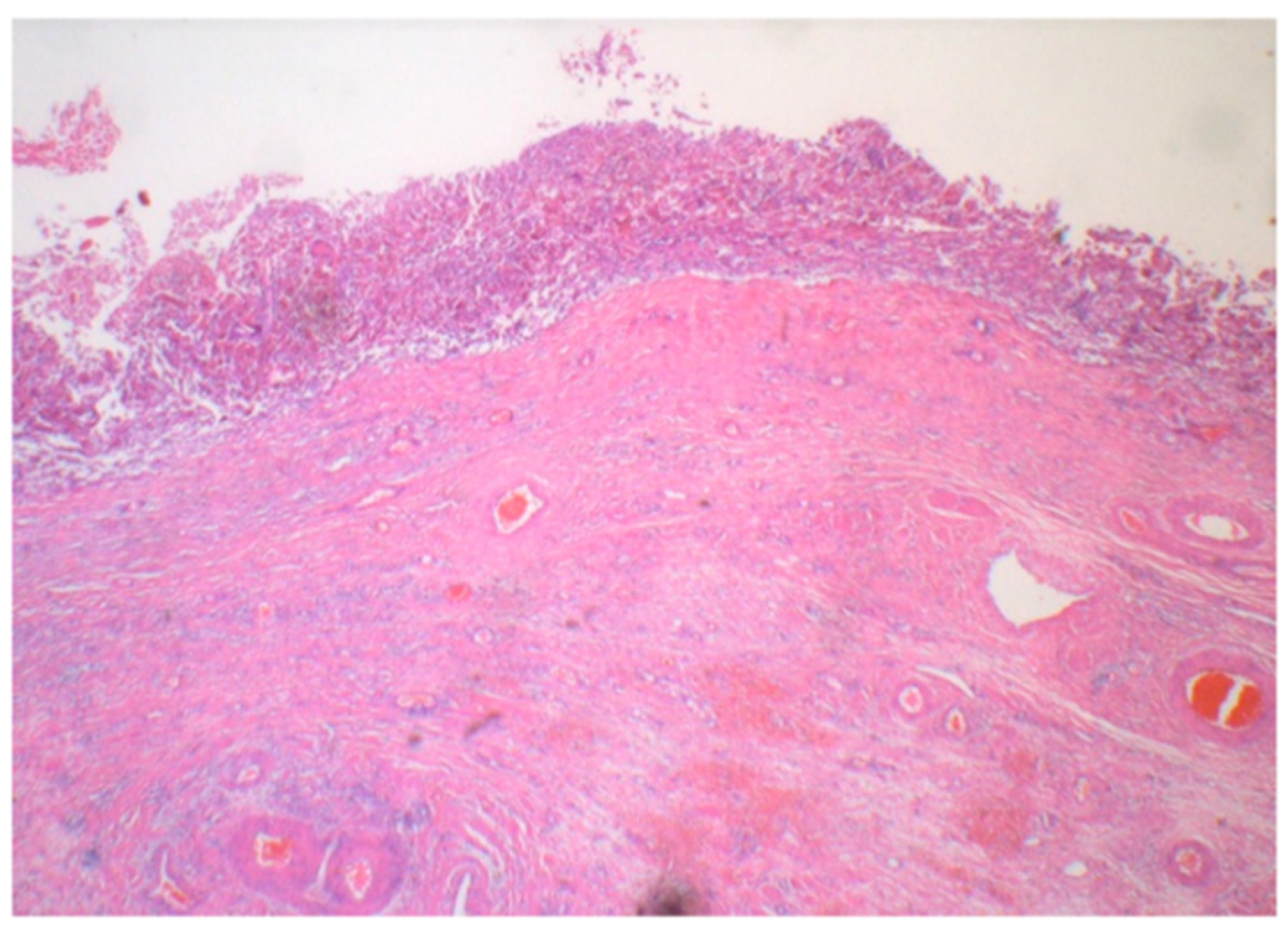
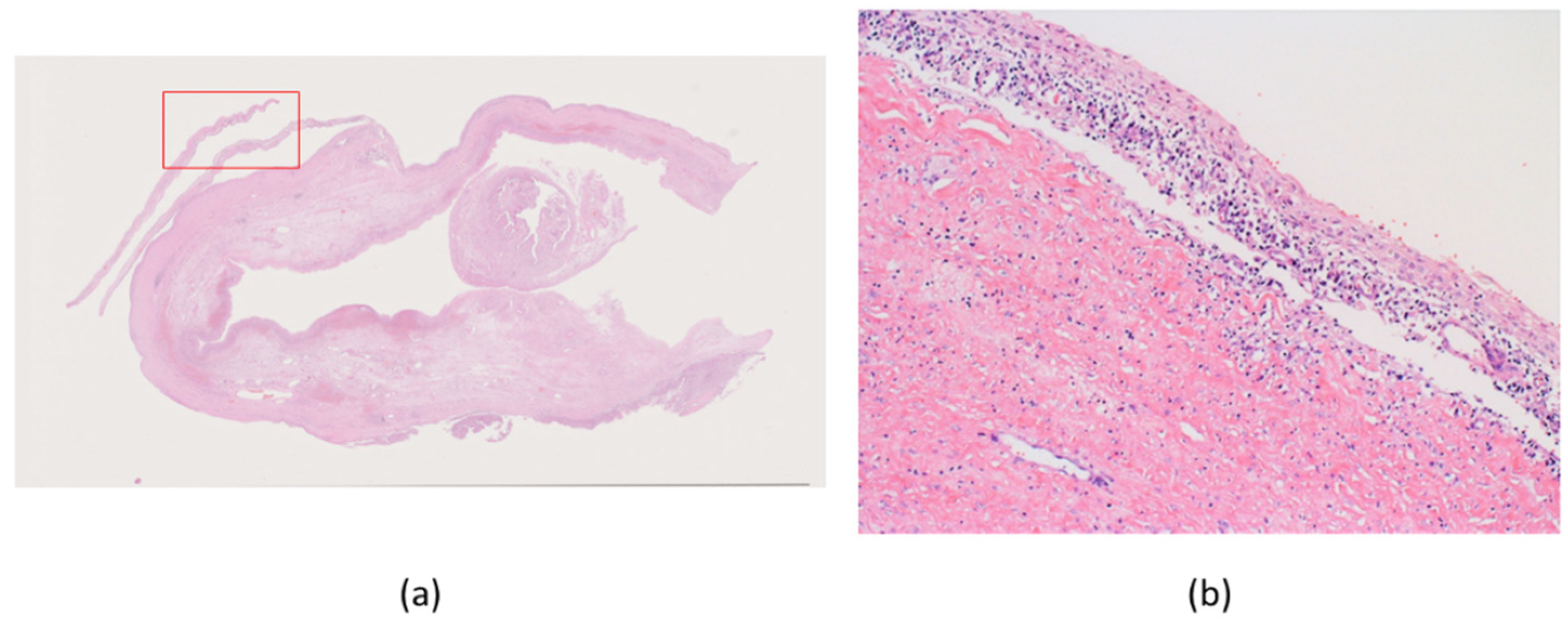
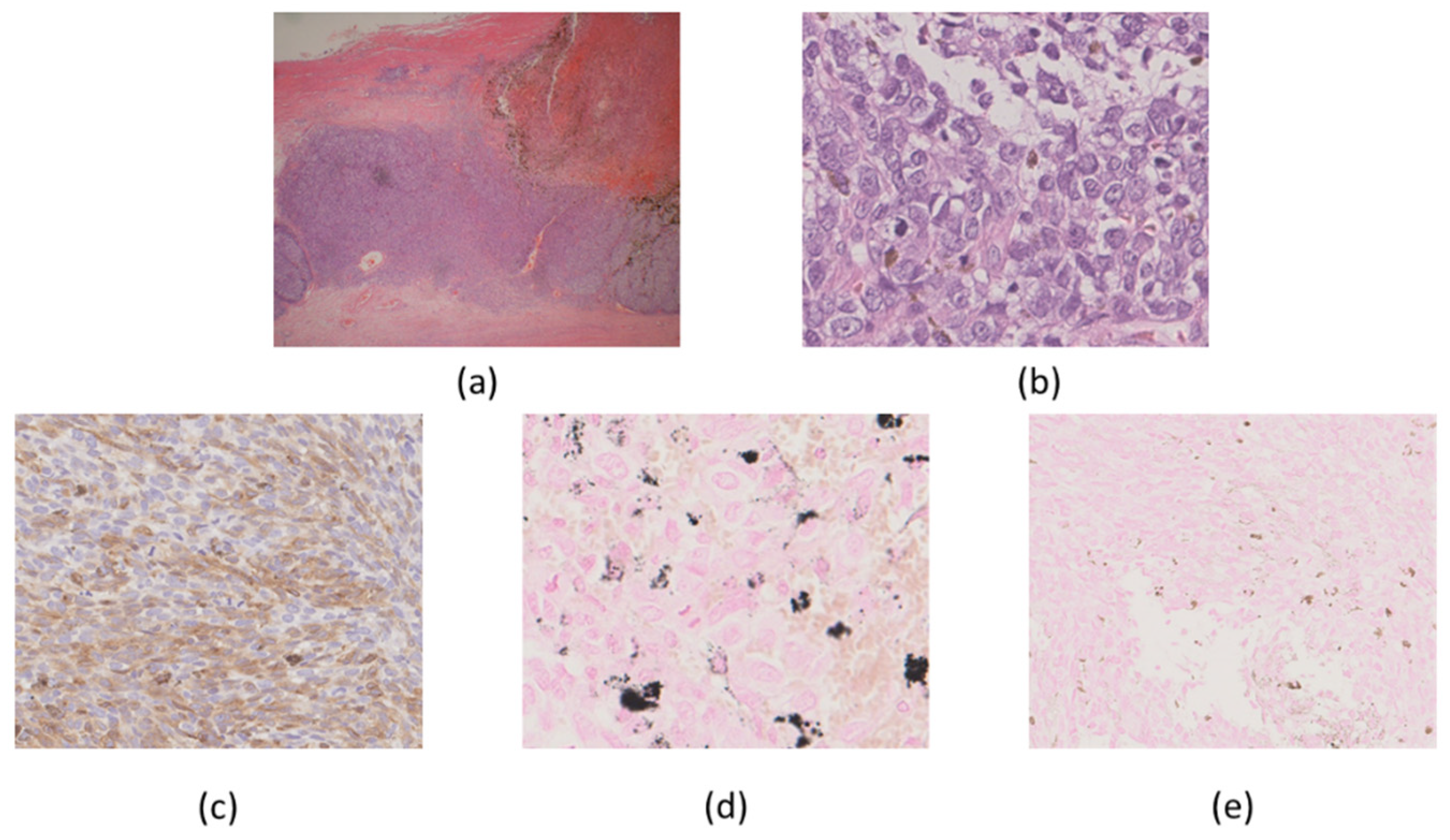
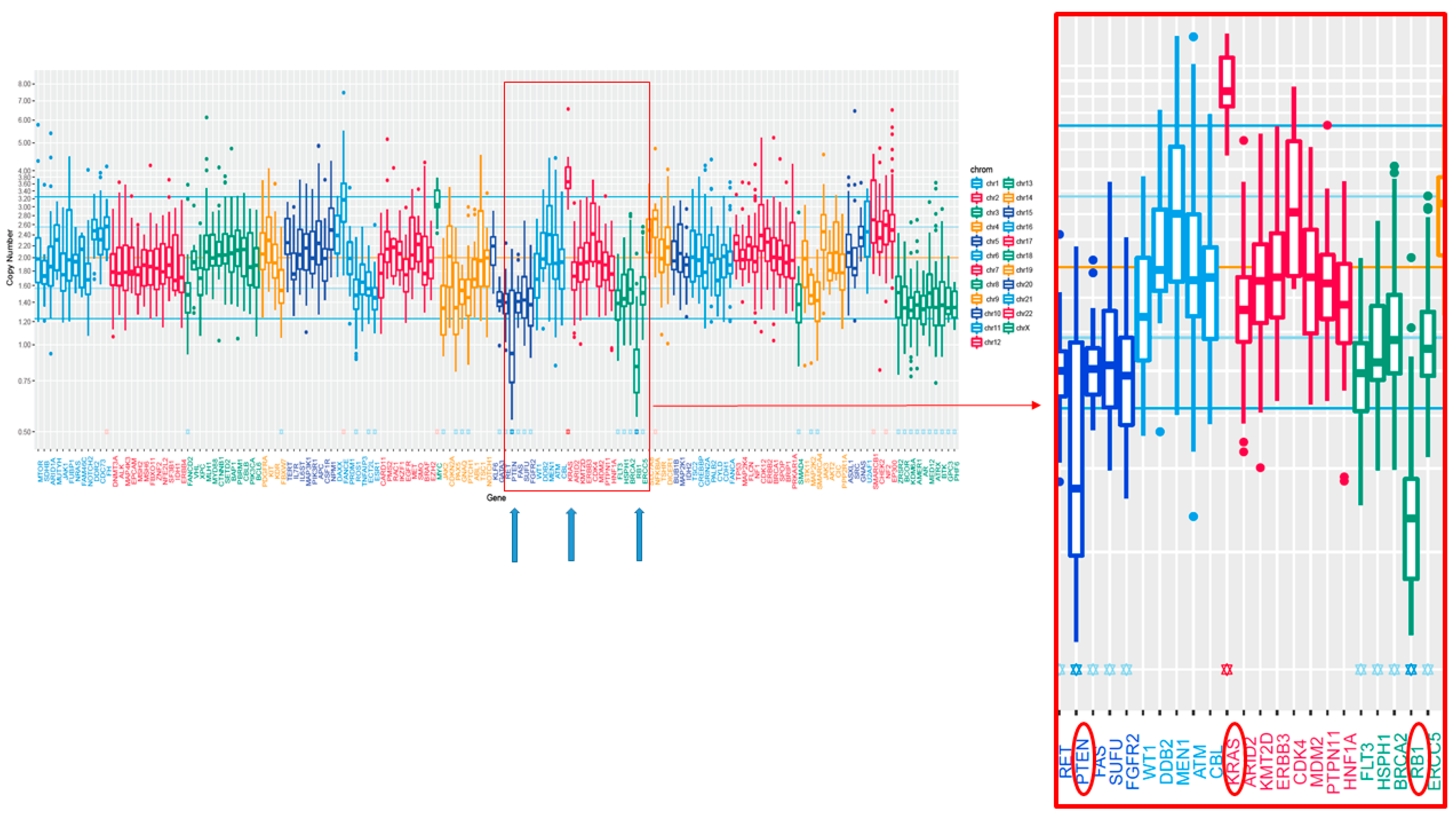
2. Case Presentation
3. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HD | Homozygous deletion |
| PCM | Primary cutaneous melanoma |
| MCT | Mature cystic teratoma |
| MM-MCT | Malignant melanoma arising from ovarian mature cystic teratoma |
References
- Zhang, T.; Dutton-Regester, K.; Brown, K.M.; Hayward, N.K. The genomic landscape of cutaneous melanoma. Pigment Cell Melanoma Res. 2016, 29, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Q.; Behren, A.; Mar, V.J.; Woods, K.; Li, J.; Martin, C.; Sheppard, K.E.; Wolfe, R.; Kelly, J.; Cebon, J.; et al. Whole exome sequencing identifies a recurrent RQCD1 P131L mutation in cutaneous melanoma. Oncotarget 2015, 6, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Garrido, M.; Botton, T.; Talevich, E.; Yeh, I.; Sanborn, J.Z.; Chung, J.; Wang, N.J.; Kakavand, H.; Mann, G.J.; et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat. Genet. 2015, 47, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Krauthammer, M.; Kong, Y.; Bacchiocchi, A.; Evans, P.; Pornputtapong, N.; Wu, C.; McCusker, J.P.; Ma, S.; Cheng, E.; Straub, R.; et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat. Genet. 2015, 47, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Arafeh, R.; Qutob, N.; Emmanuel, R.; Keren-Paz, A.; Madore, J.; Elkahloun, A.; Wilmott, J.S.; Gartner, J.J.; Di Pizio, A.; Winograd-Katz, S.; et al. Recurrent inactivating RASA2 mutations in melanoma. Nat. Genet. 2015, 47, 1408–1410. [Google Scholar] [CrossRef]
- Andrews, H.R. Primary melanotic sarcoma of the ovary. Trans. Obstet. Soc. 1901, 43, 228–231. [Google Scholar]
- Davis, G.L. Malignant melanoma arising in mature ovarian cystic teratoma (dermoid cyst). Report of two cases and literature analysis. Int. J. Gynecol. Pathol. 1996, 15, 356–362. [Google Scholar] [CrossRef]
- Moehrle, M.; Fischbach, H.; Nuessle, B.; Rassner, G. Primary malignant melanoma arising in a cystic necrotic ovarian teratoma. Eur. J Obstet. Gynecol. Reprod. Biol. 2001, 99, 268–271. [Google Scholar] [CrossRef]
- Sarnie, K.N.; Haddad, A.; Fortune, D. 31. A malignant melanoma arising in a mature cystic teratoma. Pathology 2014, 46, S116. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Rand, J.V.; Sheehan, C.E.; Jennings, T.A.; Al-Rohil, R.N.; Otto, G.; Curran, J.C.; Palmer, G.; Downing, S.; et al. Comprehensive genomic profiling of relapsed and metastatic adenoid cystic carcinomas by next-generation sequencing reveals potential new routes to targeted therapies. Am. J. Surg. Pathol. 2014, 38, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Aimono, E.; Tanishima, S.; Imai, M.; Nagatsuma, A.K.; Hayashi, H.; Yoshimura, Y.; Nakayama, K.; Kyo, S.; Nishihara, H. Olaparib monotherapy for BRIP1-mutated high-grade serous endometrial cancer. JCO Precis. Oncol. 2020, 4, PO.19.00368. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Aimono, E.; Tanishima, S.; Nomura, H.; Imai, M.; Hayashi, H.; Nishihara, H. Genetic profiling of patients with adenoid cystic carcinoma of the Bartholin’s glands reveals potential new routes for targeted therapies: A case report. Diagn. Pathol. 2020, 15, 64. [Google Scholar] [CrossRef]
- Nakamura, K.; Aimono, E.; Tanishima, S.; Imai, M.; Nagatsuma, A.K.; Hayashi, H.; Yoshimura, Y.; Nakayama, K.; Kyo, S.; Nishihara, H. Intratumoral genomic heterogeneity may hinder precision medicine strategies in patients with serous ovarian carcinoma. Diagnostics 2020, 10, 200. [Google Scholar] [CrossRef]
- Rim, S.; Kim, S.; Choi, H. Malignant transformation of ovarian mature cystic teratoma. Int. J. Gynecol. Cancer 2006, 16, 140–144. [Google Scholar] [CrossRef]
- Cooke, A.L.; Ennis, D.; Evers, L.; Dowson, S.; Chan, M.Y.; Paul, J.; Hirschowitz, L.; Glasspool, R.M.; Singh, N.; Bell, S.; et al. The driver mutational landscape of ovarian squamous cell carcinomas arising in mature cystic teratoma. Clin. Cancer Res. 2017, 23, 7633–7640. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Stambolic, V.; Suzuki, A.; de la Pompa, J.L.; Brothers, G.M.; Mirtsos, C.; Sasaki, T.; Ruland, J.; Penninger, J.M.; Siderovski, D.P.; Mak, T.W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998, 95, 29–39. [Google Scholar] [CrossRef]
- Cantley, L. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262. [Google Scholar] [CrossRef]
- Gutierrez, G.M.; Kong, E.; Sabbagh, Y.; Brown, N.E.; Lee, J.S.; Demay, M.B.; Thomas, D.M.; Hinds, P.W. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1-/- mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18402–18407. [Google Scholar] [CrossRef] [PubMed]
- Calo, E.; Quintero-Estades, J.A.; Danielian, P.S.; Nedelcu, S.; Berman, S.D.; Lees, J.A. Rb regulates fate choice and lineage commitment in vivo. Nature 2010, 466, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L.; Landsberg, R.L.; Huss-Garcia, Y.; Sardet, C.; Lees, J.A.; Auwerx, J. E2Fs regulate adipocyte differentiation. Dev. Cell 2002, 3, 39–49. [Google Scholar] [CrossRef]
- Draper, G.J.; Sanders, B.M.; Kingston, J.E. Second primary neoplasms in patients with retinoblastoma. Br. J. Cancer 1986, 53, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Pollina, L.; Stasi, I.; Ruzzo, A.; Scartozzi, M.; Santini, S.; Masi, G.; Graziano, F.; Cremolini, C.; Rulli, E.; et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Aimono, E.; Takamatsu, R.; Tanishima, S.; Tohyama, T.; Sasano, K.; Sakuma, H.; Nishihara, H. Genetic Profiling of Malignant Melanoma Arising from an Ovarian Mature Cystic Teratoma: A Case Report. Int. J. Mol. Sci. 2021, 22, 2436. https://doi.org/10.3390/ijms22052436
Nakamura K, Aimono E, Takamatsu R, Tanishima S, Tohyama T, Sasano K, Sakuma H, Nishihara H. Genetic Profiling of Malignant Melanoma Arising from an Ovarian Mature Cystic Teratoma: A Case Report. International Journal of Molecular Sciences. 2021; 22(5):2436. https://doi.org/10.3390/ijms22052436
Chicago/Turabian StyleNakamura, Kohei, Eriko Aimono, Reika Takamatsu, Shigeki Tanishima, Tomonari Tohyama, Katsutoshi Sasano, Hiroshi Sakuma, and Hiroshi Nishihara. 2021. "Genetic Profiling of Malignant Melanoma Arising from an Ovarian Mature Cystic Teratoma: A Case Report" International Journal of Molecular Sciences 22, no. 5: 2436. https://doi.org/10.3390/ijms22052436
APA StyleNakamura, K., Aimono, E., Takamatsu, R., Tanishima, S., Tohyama, T., Sasano, K., Sakuma, H., & Nishihara, H. (2021). Genetic Profiling of Malignant Melanoma Arising from an Ovarian Mature Cystic Teratoma: A Case Report. International Journal of Molecular Sciences, 22(5), 2436. https://doi.org/10.3390/ijms22052436





