Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization
Abstract
1. Introduction
2. Results
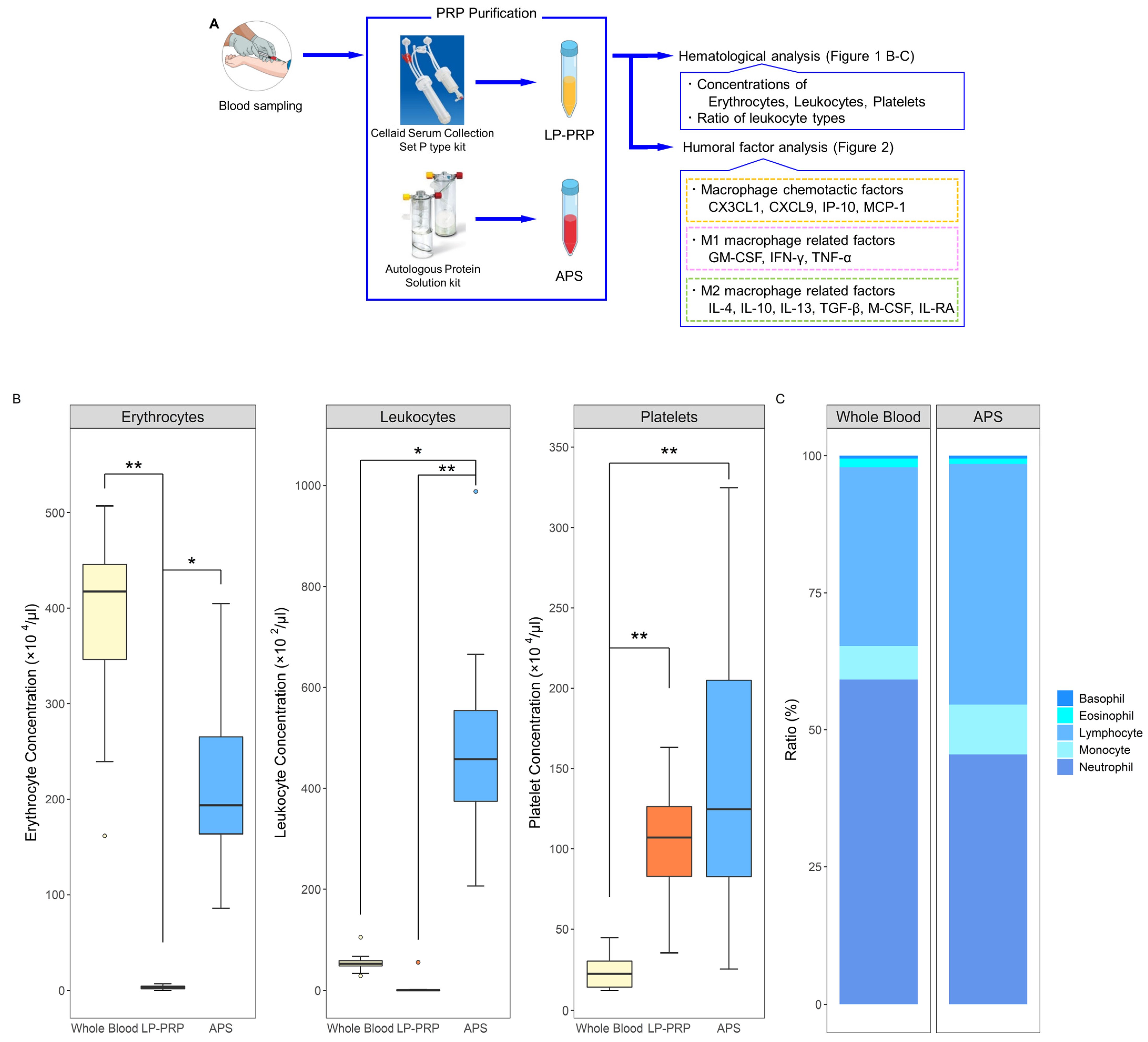
2.1. Hematological Analysis of PRPs
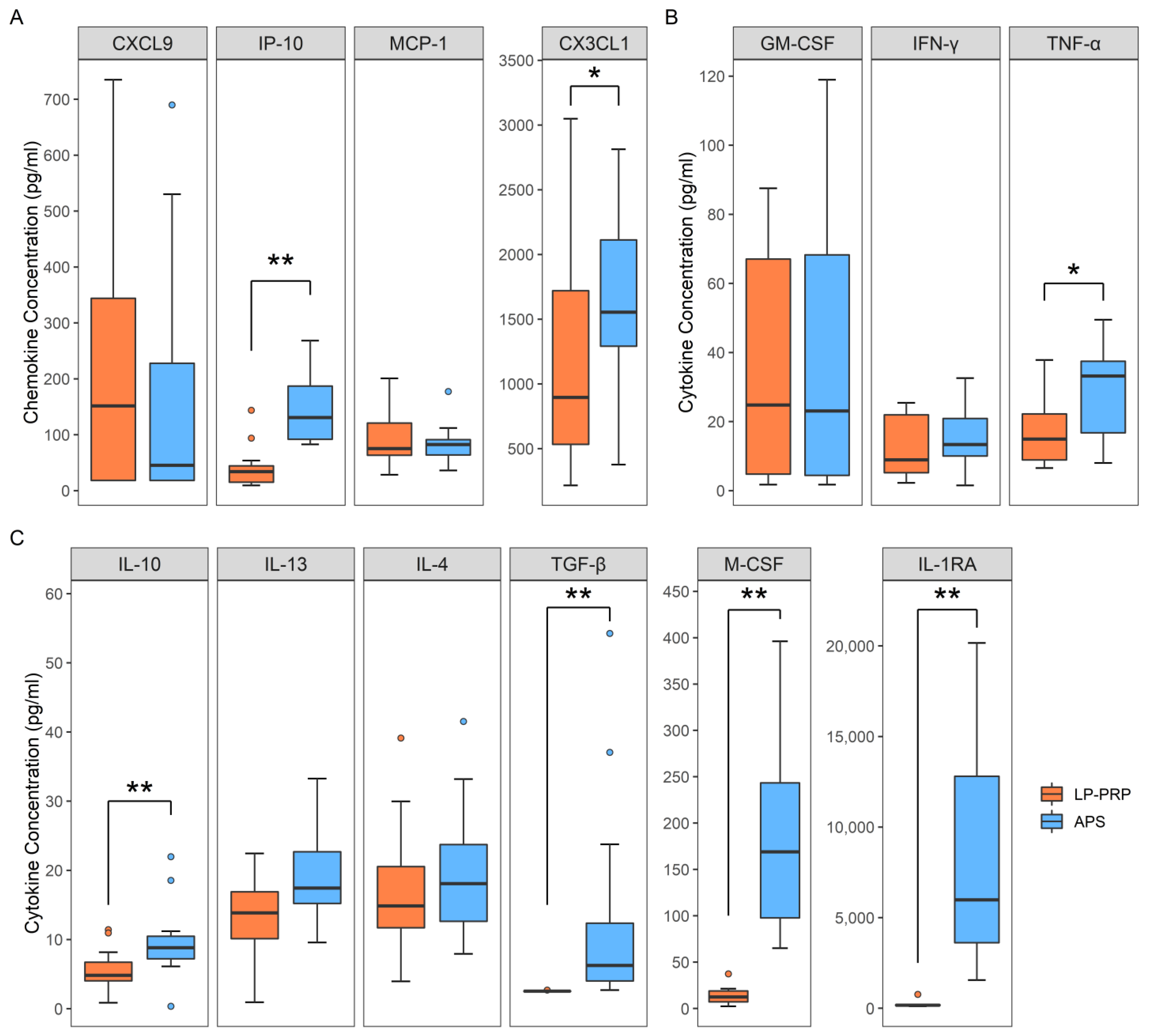
2.2. Analysis of Humoral Factors
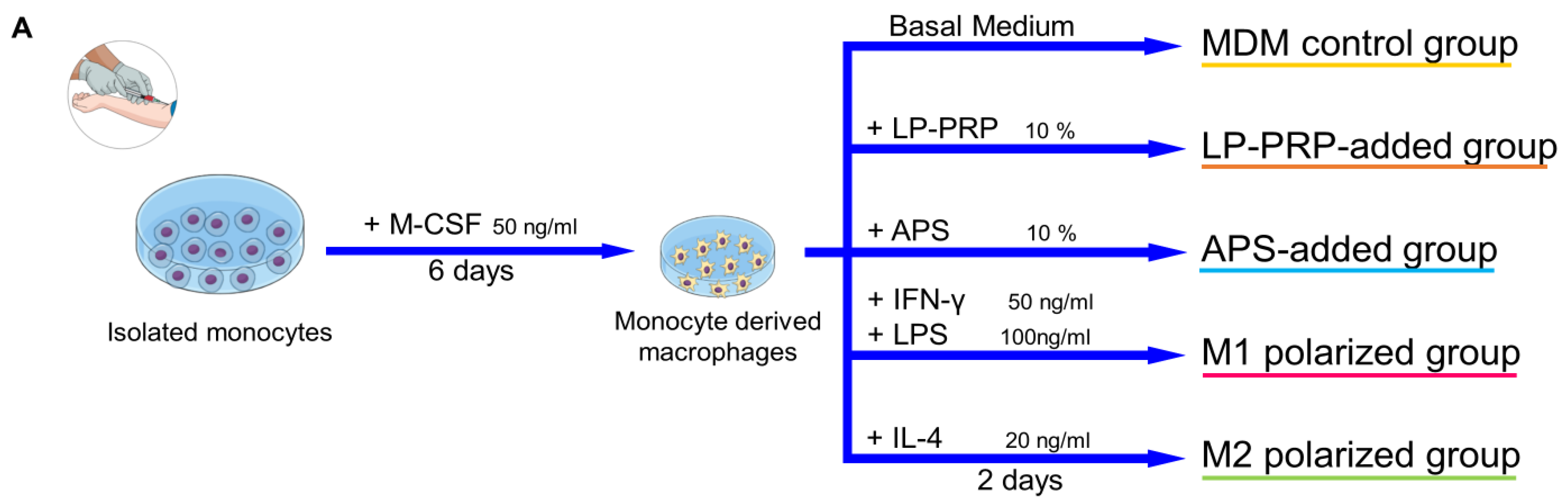
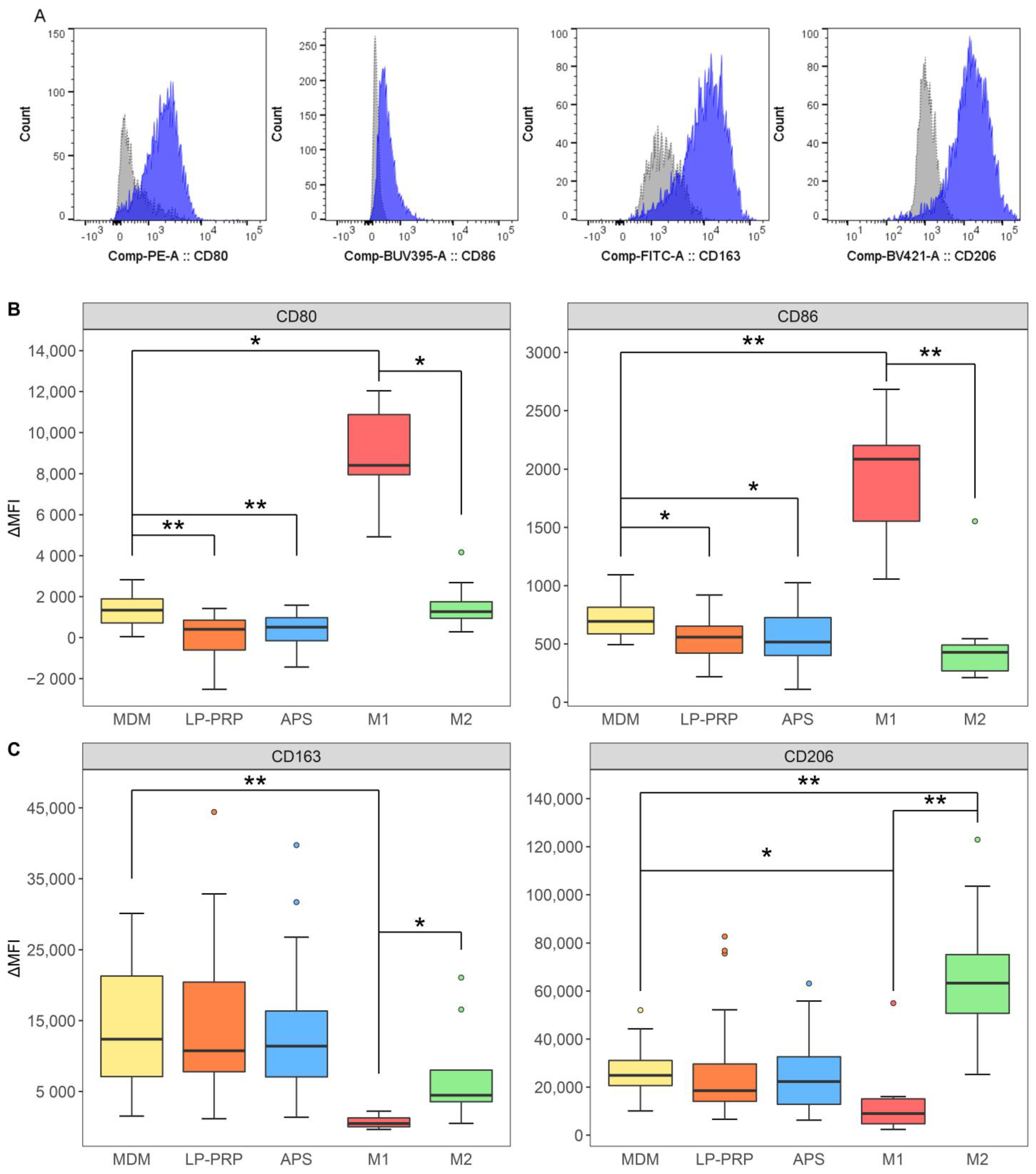
2.3. Effect of the Addition of PRP Supernatants on Macrophage Phenotypes
2.4. Effect of PRPs on M1 Macrophages
3. Discussion
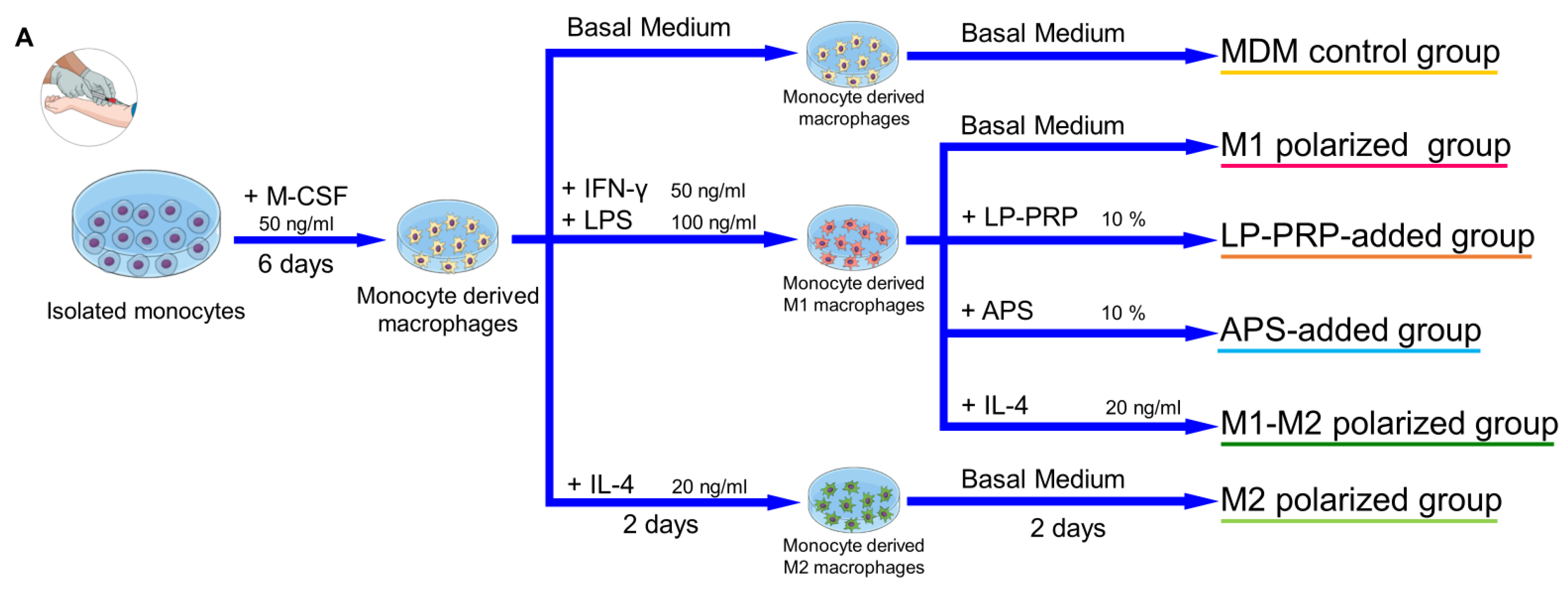
4. Materials and Methods
4.1. Ethics Statement
4.2. PRP Purification
4.3. Hematological Analysis
4.4. Analysis of Humoral Factors
4.5. Isolation of Monocytes
4.6. Cell Culture
4.6.1. Preparation of Monocyte-Derived Macrophages and Addition of PRP Supernatants
4.6.2. Preparation of M1 Macrophages and Addition of PRP Supernatants
4.7. Flow Cytometry
4.8. Gene Expression Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APS | Autologous protein solution |
| CX3CL1 | C-XXX-C motif chemokine ligand 1 |
| CXCL9 | C-X-C motif chemokine ligand 9 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| IFN-γ | Interferon-gamma |
| IL-10 | Interleukin-10 |
| IL-13 | Interleukin-13 |
| IL-1β | Interleukin-1 beta |
| IL-1RA | Interleukin-1 receptor antagonist |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IP-10 | Interferon gamma-induced protein-10 |
| LP-PRP | Leukocyte-poor platelet rich plasma |
| LR-PRP | Leukocyte-rich platelet rich plasma |
| MCP-1 | Macrophage chemoattractant protein-1 |
| M-CSF | Macrophage colony-stimulating factor |
| MDM | Monocyte derived macrophage |
| MRC1 | Mannose receptor 1 |
| OAK | Osteoarthritis of the knee |
| PRP | Platelet rich plasma |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor-alpha |
References
- Hügle, T.; Geurts, J. What drives osteoarthritis?—Synovialversussubchondral bone pathology. Rheumatology 2016, 56, 1461–1471. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. Response to Letter to the Editor entitled “Comments on ‘OARSI guidelines for the non-surgical management of knee osteoarthritis’”. Osteoarthr. Cartil. 2014, 22, 890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Moskowitz, R.; Nuki, G.; Abramson, S.; Altman, R.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O’Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-Rich Plasma: Growth Factors and Pro- and Anti-Inflammatory Properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Clinical Outcomes of Knee Osteoarthritis Treated with an Autologous Protein Solution Injection: A 1-Year Pilot Double-Blinded Randomized Controlled Trial. Am. J. Sports Med. 2018, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.F.D.A.B.; De Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte- and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Cranio-Maxillofac. Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.E.; Gordon, P.; Barker, R.N.; Wilson, H.M. The activation status of human macrophages presenting antigen determines the efficiency of Th17 responses. Immunobiology 2015, 220, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Utomo, L.; Bastiaansen-Jenniskens, Y.; Verhaar, J.; Van Osch, G. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthr. Cartil. 2016, 24, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Choi, H.-J.; Ha, S.-J.; Lee, K.-T.; Kwon, Y.-G. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. et Biophys. Acta (BBA) Bioenerg. 2013, 1835, 170–179. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.R.; Panchal, S.K.; Friis, T.; Sekar, S.; Crawford, R.; Brown, L.; Xiao, Y.; Prasadam, I. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE 2017, 12, e0183693. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Gambari, L.; Piacentini, A.; Filardo, G.; Fleury-Cappellesso, S.; Barbero, A.; Murphy, M.; Lisignoli, G. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: In vitro evaluation. Osteoarthr. Cartil. 2017, 25, 1161–1171. [Google Scholar] [CrossRef]
- Woodell-May, J.E.W.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Saita, Y.; Kobayashi, Y.; Takaku, T.; Fukusato, S.; Uchino, S.; Wakayama, T.; Ikeda, H.; Kaneko, K. Platelet-rich plasma promotes recruitment of macrophages in the process of tendon healing. Regen. Ther. 2020, 14, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.N.; Rashed, R.R.; El-Hazek, R.M.; El-Sabbagh, W.A.; Rashed, E.R.; El-Ghazaly, M.A. Platelet-rich plasma-induced feedback inhibition of activin A/follistatin signaling: A mechanism for tumor-low risk skin rejuvenation in irradiated rats. J. Photochem. Photobiol. B: Biol. 2018, 180, 17–24. [Google Scholar] [CrossRef]
- Escobar, G.; Escobar, A.; Ascui, G.; Tempio, F.I.; Ortiz, M.C.; Pérez, C.A.; López, M.N. Pure platelet-rich plasma and supernatant of calcium-activated P-PRP induce different phenotypes of human macrophages. Regen. Med. 2018, 13, 427–441. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages Sequentially Change Their Functional Phenotype in Response to Changes in Microenvironmental Influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Gawaz, M.P.; Vogel, S. Platelets in tissue repair: Control of apoptosis and interactions with regenerative cells. Blood 2013, 122, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Knop, E.; De Paula, L.E.; Fuller, R. Platelet-rich plasma for osteoarthritis treatment. Rev. Bras. de Reum. (Engl. Ed.) 2016, 56, 152–164. [Google Scholar] [CrossRef]
- Wasai, S.; Sato, M.; Maehara, M.; Toyoda, E.; Uchiyama, R.; Takahashi, T.; Okada, E.; Iwasaki, Y.; Suzuki, S.; Watanabe, M. Characteristics of autologous protein solution and leucocyte-poor platelet-rich plasma for the treatment of osteoarthritis of the knee. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of Growth Factor and Platelet Concentration From Commercial Platelet-Rich Plasma Separation Systems. Am. J. Sports Med. 2010, 39, 266–271. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Novak, M.L.; Koh, T.J. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013, 93, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage Polarization in Bacterial Infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Pessler, F.; Dai, L.; Diaz-Torne, C.; Gomez-Vaquero, C.; Paessler, M.E.; Zheng, D.-H.; Einhorn, E.; Range, U.; Scanzello, C.; Schumacher, H.R. The synovitis of “non-inflammatory” orthopaedic arthropathies: A quantitative histological and immunohistochemical analysis. Ann. Rheum. Dis. 2008, 67, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessey, K.; Matuska, A.; Hoeppner, J.; Farr, J.; Klaassen, M.; Kaeding, C.; Lattermann, C.; King, W.; Woodell-May, J. Autologous protein solution prepared from the blood of osteoarthritic patients contains an enhanced profile of anti-inflammatory cytokines and anabolic growth factors. J. Orthop. Res. 2014, 32, 1349–1355. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Bertani, F.R.; Mozetic, P.; Fioramonti, M.; Iuliani, M.; Ribelli, G.; Pantano, F.; Santini, D.; Tonini, G.; Trombetta, M.; Businaro, L.; et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tjiu, J.-W.; Chen, J.-S.; Shun, C.-T.; Lin, S.-J.; Liao, Y.-H.; Chu, C.-Y.; Tsai, T.-F.; Chiu, H.-C.; Dai, Y.-S.; Inoue, H.; et al. Tumor-Associated Macrophage-Induced Invasion and Angiogenesis of Human Basal Cell Carcinoma Cells by Cyclooxygenase-2 Induction. J. Investig. Dermatol. 2009, 129, 1016–1025. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 2336. https://doi.org/10.3390/ijms22052336
Uchiyama R, Toyoda E, Maehara M, Wasai S, Omura H, Watanabe M, Sato M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. International Journal of Molecular Sciences. 2021; 22(5):2336. https://doi.org/10.3390/ijms22052336
Chicago/Turabian StyleUchiyama, Ryoka, Eriko Toyoda, Miki Maehara, Shiho Wasai, Haruka Omura, Masahiko Watanabe, and Masato Sato. 2021. "Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization" International Journal of Molecular Sciences 22, no. 5: 2336. https://doi.org/10.3390/ijms22052336
APA StyleUchiyama, R., Toyoda, E., Maehara, M., Wasai, S., Omura, H., Watanabe, M., & Sato, M. (2021). Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. International Journal of Molecular Sciences, 22(5), 2336. https://doi.org/10.3390/ijms22052336







