Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents
Abstract
1. Introduction
2. Results
2.1. p-S10H3 Expression is Upregulated in a Subpopulation of Ipsilateral SDH Neurons with Differing Rostrocaudal Density Following Burn Injury
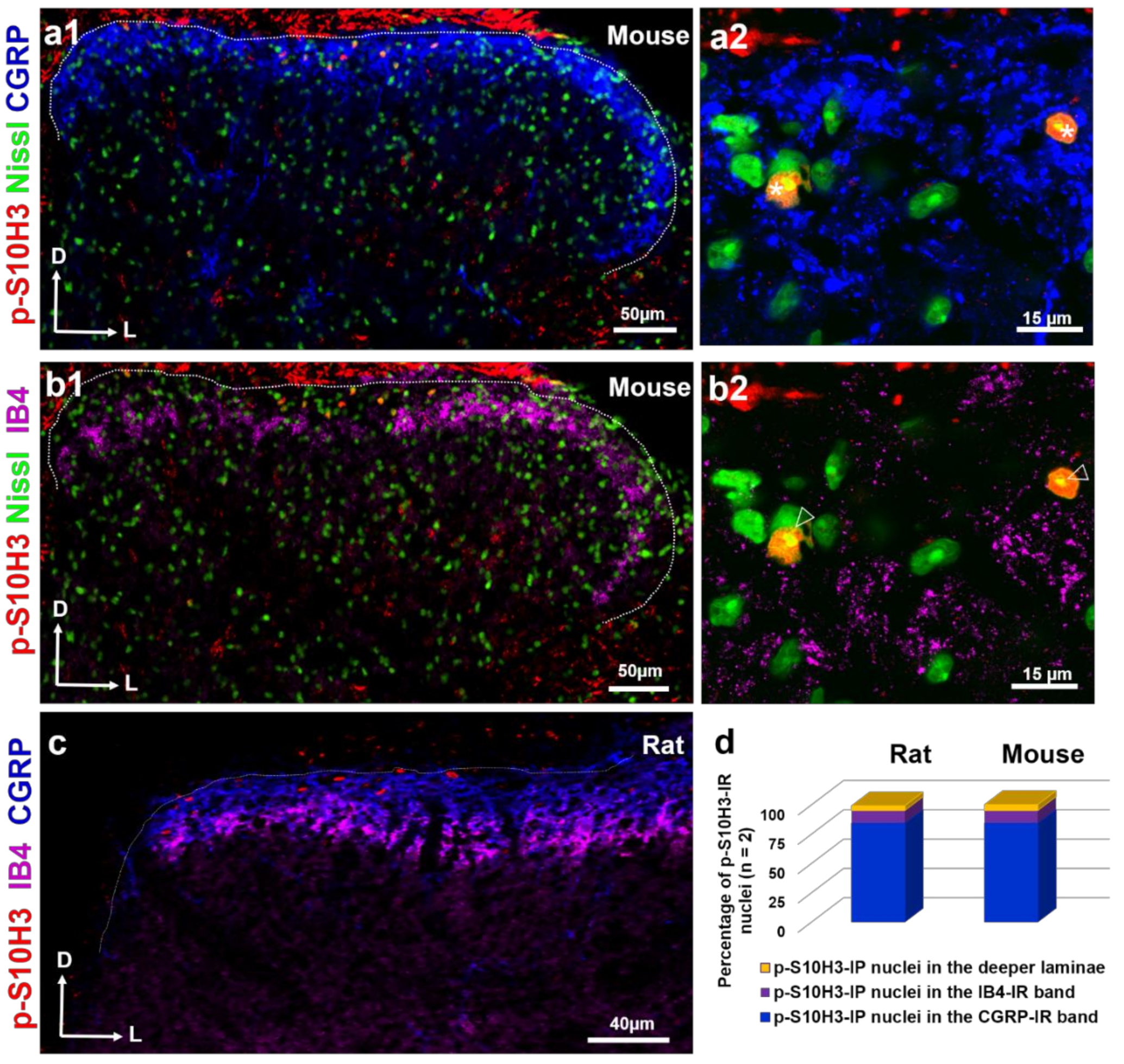
2.2. SDH Neurons Expressing p-S10H3 Following Burn Injury are in Close Apposition Mainly to CGRP-Containing Peptidergic Afferents
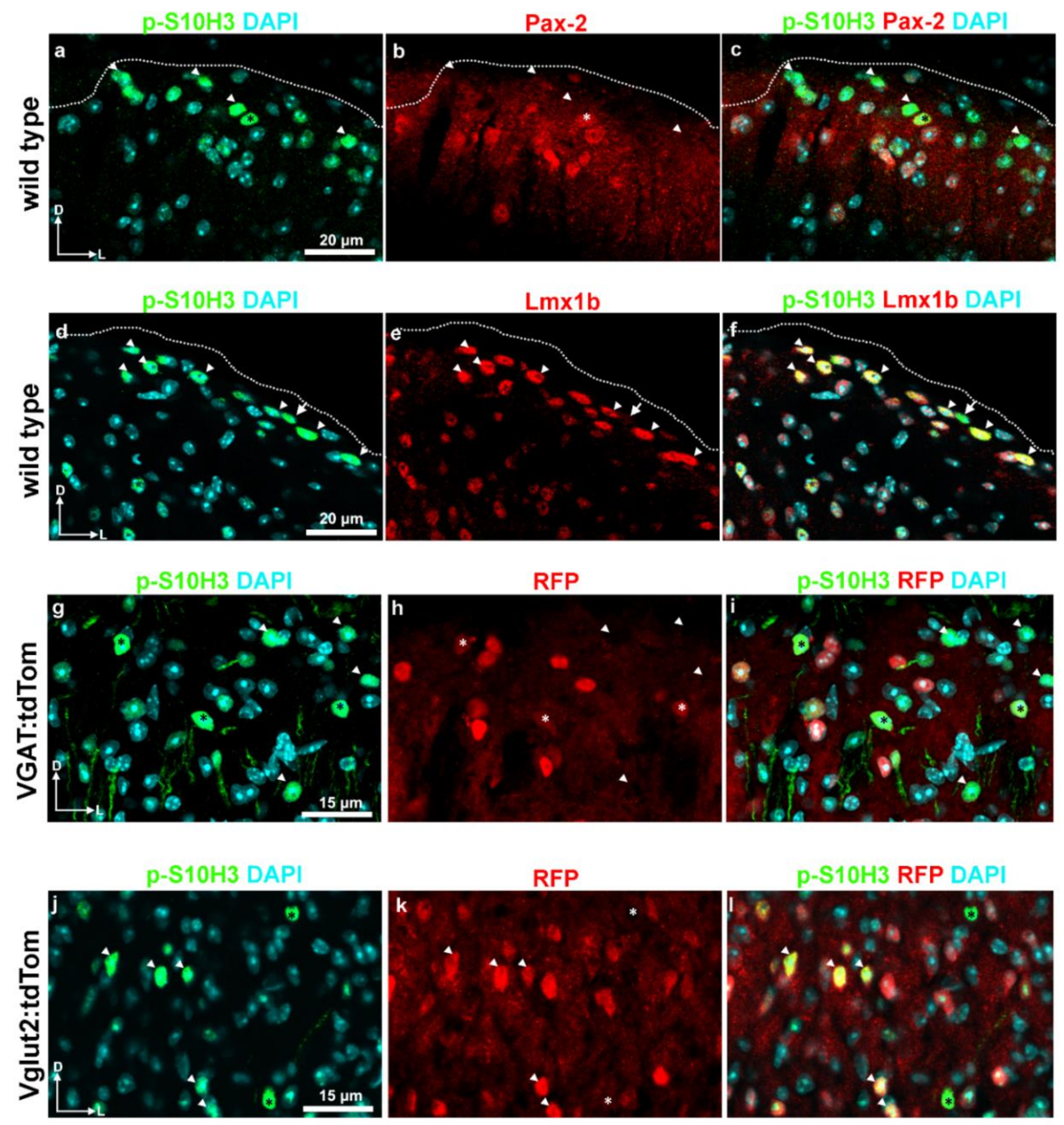
2.3. S10H3 Phosphorylation Occurs Predominantly in SDH Neurons in Mice
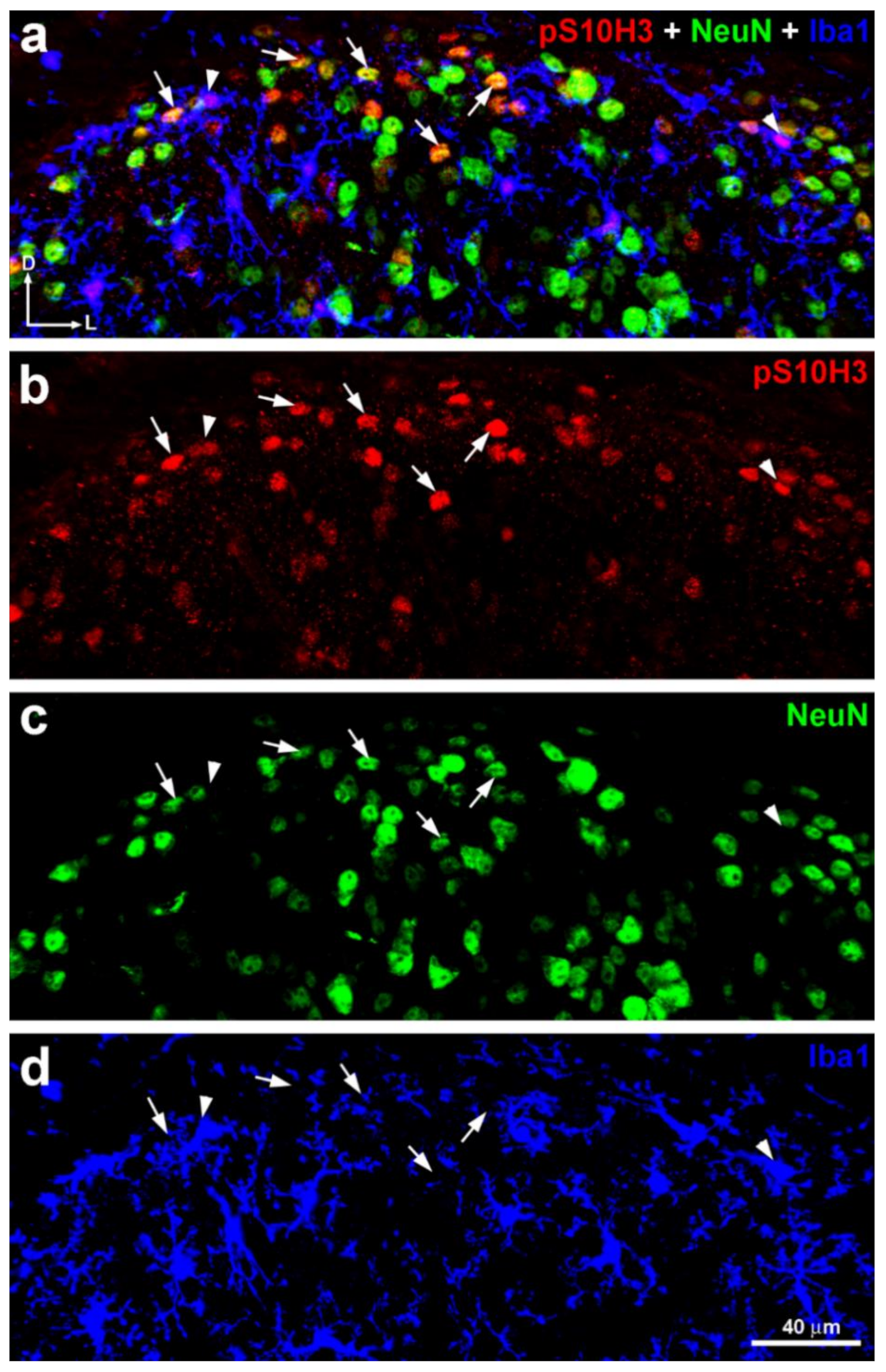
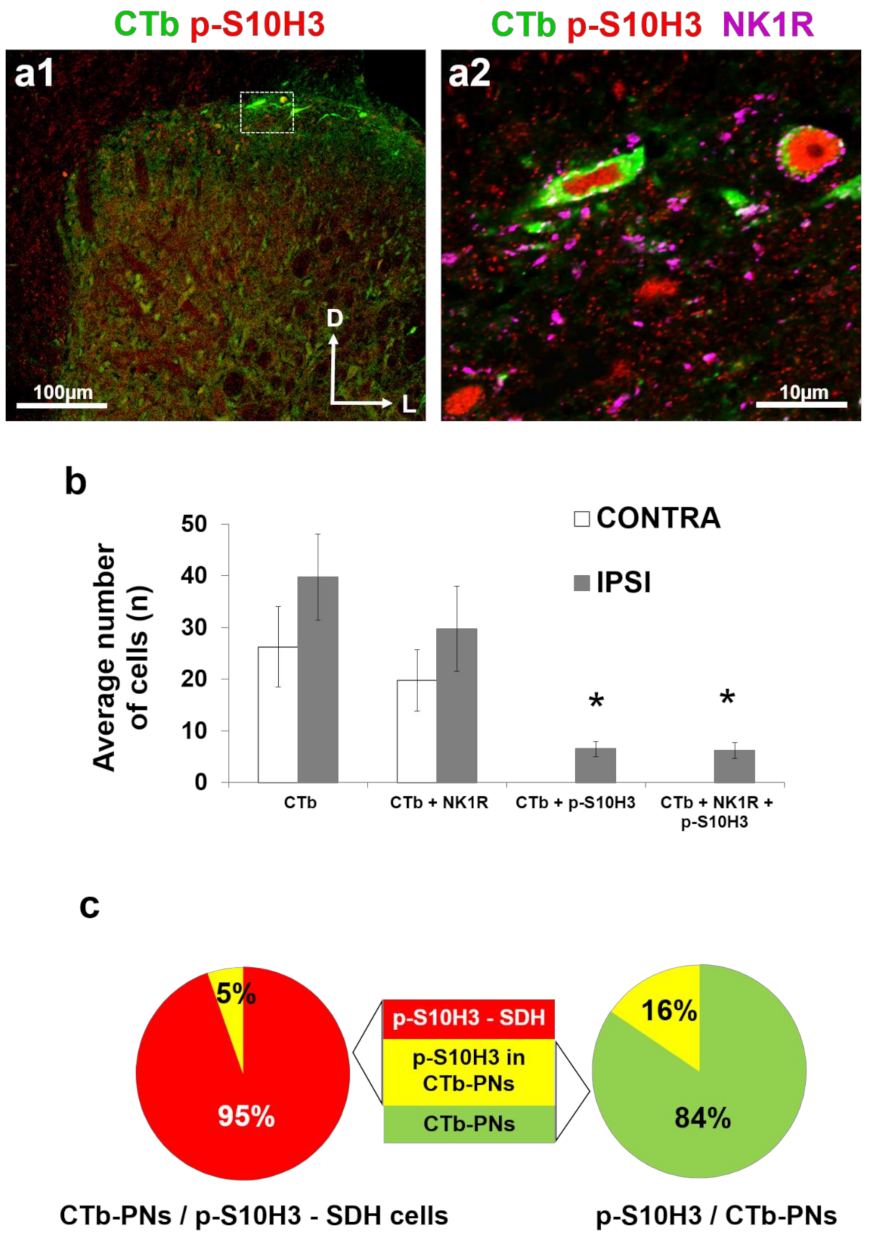
2.4. Only a Small Proportion of Projection Neurons Expresses Nuclear p-S10H3 Following Burn Injury
2.5. S10H3 Phosphorylation Occurs Predominantly in Local Interneurons in Mice
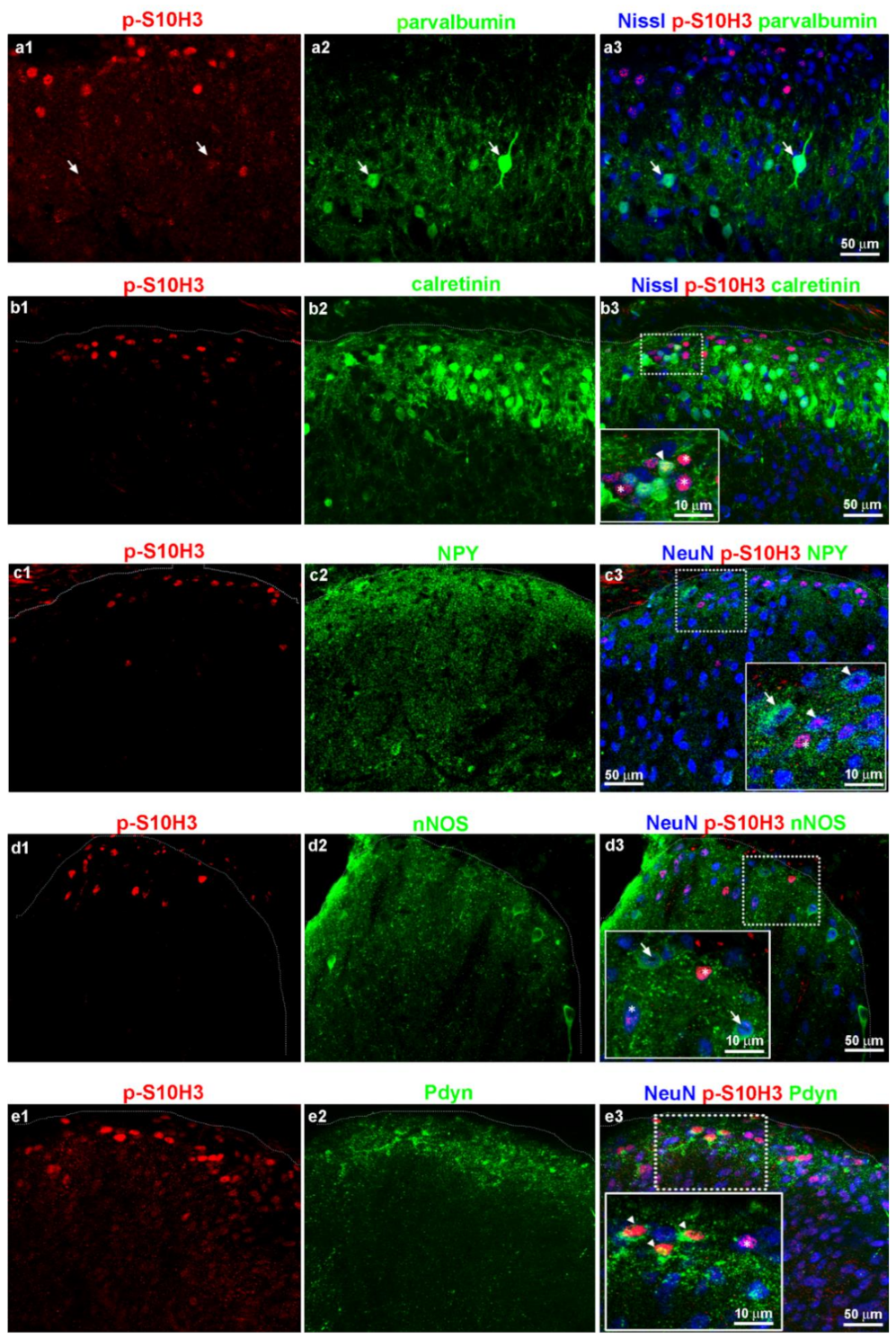
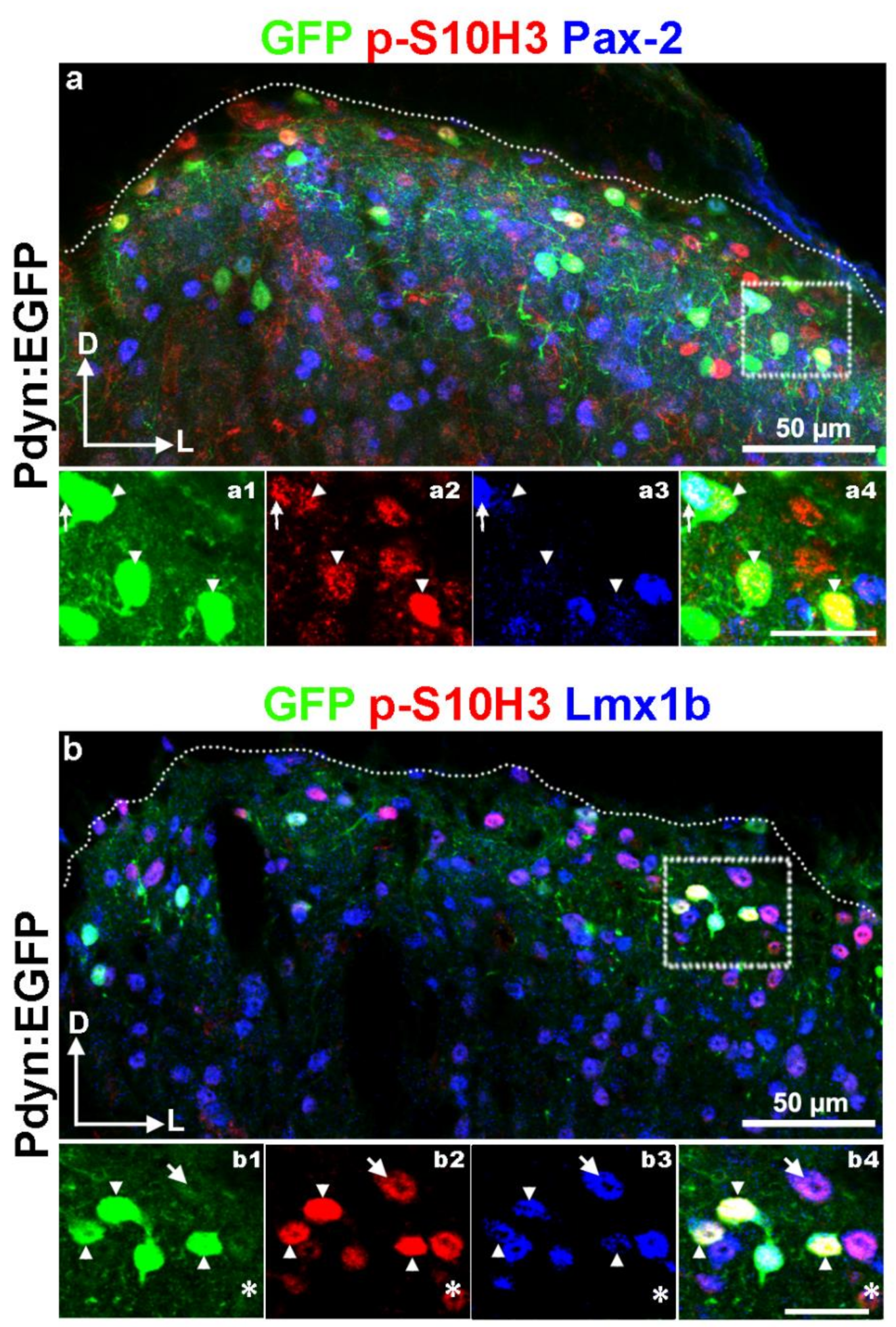
2.6. Burn Injury-Induced S10H3 Phosphorylation Occurs Mainly in a Dynorphinergic Population of SDH Neurons in Mice
2.7. The Highest Proportion of p-S10H3 Nuclei Appears in Excitatory Dynorphinergic Neurons Following Burn Injury
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Considerations
4.2. Burn Injury Model
4.3. Retrograde Labeling of SDH Projection Neurons
4.4. Immunoperoxidase Staining
4.5. Double and Triple Immunofluorescent Staining
4.6. Combination of In Situ Hybridization with Immunofluorescence (ISH/IF)
4.7. Imaging and Quantification
4.8. Antibody Characterization
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAG | a hybrid promoter consisting of the cytomegalovirus enhancer fused to the chicken beta-actin promoter |
| cas9 | CRISPR associated protein 9 endonuclease |
| CCK | cholecystokinin |
| CFA | complete Freund’s adjuvant |
| CGRP | calcitonin gene-related peptide |
| CONTRA | contralateral |
| CR | calretinin |
| cre | cre recombinase derived from the P1 bacteriophage |
| cRNA | complementary RNA |
| CTb | cholera toxin b-subunit |
| DAB | 3,3′-diaminobenzidine |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DEPC | diethyl-pyrocarbonate |
| DIG | digoxigenin |
| Dyn | dynorphinergic |
| EGFP | enhanced green fluorescent protein |
| ERK | extracellular signal-regulated kinase |
| GABA | gamma-amino butyric-acid |
| GENSAT | gene expression nervous system atlas |
| GRP | gastrin releasing peptide |
| H3 | histone 3 |
| HRP | horseradish peroxidase |
| IB4 | isolectin B4 |
| Iba1 | ionized calcium binding adaptor molecule 1 |
| IF | immunofluorescence |
| IHC | immunohistochemistry |
| IPSI | ipsilateral |
| IP | immunopositive |
| IR | immunoreactive |
| IRES | internal ribosome entry site |
| ISH | in situ hybridization |
| Lmx1b | LIM homeobox transcription factor 1-beta |
| LPb | lateral parabrachial nucleus |
| LSL | lox-stop-lox |
| MAPK | mitogen-activated protein kinase |
| NDS or NGS | normal donkey or goat serum |
| NeuN | Fox-3 or Hexaribonucleotide Binding Protein-3 |
| NK1R | neurokinin-1 receptor |
| Nnos | neuronal nitrogen monoxide synthase |
| NPFF | neuropeptide FF |
| NPY | neuropeptide Y |
| Pax-2 | Paired box gene 2 |
| Pdyn | prodynorphin |
| p-ERK1/2 | phospho-ERK |
| PFA | paraformaldehyde |
| PKCγ | protein kinase C gamma |
| PN | projection neuron |
| p-S10H3 | phosphorylation of serine 10 in histone 3 |
| PTM | post-translational modification |
| PV | parvalbumin |
| RFP | red fluorescent protein |
| SDH | superficial dorsal horn |
| SOM | somatostatin |
| SP | substance P |
| SSC | saline–sodium citrate buffer |
| Tac1 | the gene for Preprotachykinin-1 |
| UDP-labeled cRNA | uridine diphosphate-labeled cRNA |
| VGAT | vesicular gamma-amino butyric-acid (GABA) transporter |
| Vglut2 | vesicular glutamate transporter 2 |
References
- Sahbaie, P.; Liang, D.Y.; Shi, X.Y.; Sun, Y.; Clark, J.D. Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure. Mol. Pain 2016, 12, 1744806916641950. [Google Scholar] [CrossRef]
- Khangura, R.K.; Bali, A.; Jaggi, A.S.; Singh, N. Histone acetylation and histone deacetylation in neuropathic pain: An unresolved puzzle? Eur. J. Pharmacol. 2017, 795, 36–42. [Google Scholar] [CrossRef]
- Tochiki, K.K.; Maiaru, M.; Norris, C.; Hunt, S.P.; Geranton, S.M. The mitogen and stress-activated protein kinase 1 regulates the rapid epigenetic tagging of dorsal horn neurons and nocifensive behaviour. Pain 2016, 157, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Torres-Perez, J.V.; Santha, P.; Varga, A.; Szucs, P.; Sousa-Valente, J.; Gaal, B.; Sivado, M.; Andreou, A.P.; Beattie, S.; Nagy, B.; et al. Phosphorylated Histone 3 at Serine 10 Identifies Activated Spinal Neurons and Contributes to the Development of Tissue Injury-Associated Pain. Sci. Rep. 2017, 7, 41221. [Google Scholar] [CrossRef]
- Todd, A.J. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol. Pain 2017, 13, 1744806917693003. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.C.; Acton, D.; Goulding, M. Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol. 2018, 80, 189–217. [Google Scholar] [CrossRef]
- Molet, J.; Pohl, M. Gene-based approaches in pain research and exploration of new therapeutic targets and strategies. Eur. J. Pharmacol. 2013, 716, 129–141. [Google Scholar] [CrossRef]
- Haring, M.; Zeisel, A.; Hochgerner, H.; Rinwa, P.; Jakobsson, J.E.T.; Lonnerberg, P.; La Manno, G.; Sharma, N.; Borgius, L.; Kiehn, O.; et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018, 21, 869–880. [Google Scholar] [CrossRef]
- Xie, Y.F.; Wang, J.; Bonin, R.P. Optogenetic exploration and modulation of pain processing. Exp. Neurol. 2018, 306, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.X.; Wang, S.M.; Bai, Y.H.; Luo, T.T.; Wang, J.Q.; Dai, C.Q.; Guo, B.L.; Luo, S.C.; Wang, D.H.; Yang, Y.L.; et al. Lentiviral Vectors and Adeno-Associated Virus Vectors: Useful Tools for Gene Transfer in Pain Research. Anat. Rec. 2018, 301, 825–836. [Google Scholar] [CrossRef]
- Todd, A.J.; Spike, R.C.; Brodbelt, A.R.; Price, R.F.; Shehab, S.A. Some inhibitory neurons in the spinal cord develop c-fos-immunoreactivity after noxious stimulation. Neuroscience 1994, 63, 805–816. [Google Scholar] [CrossRef]
- Todd, A.J.; Spike, R.C.; Young, S.; Puskar, Z. Fos induction in lamina I projection neurons in response to noxious thermal stimuli. Neuroscience 2005, 131, 209–217. [Google Scholar] [CrossRef]
- Polgar, E.; Wright, L.L.; Todd, A.J. A quantitative study of brainstem projections from lamina I neurons in the cervical and lumbar enlargement of the rat. Brain Res. 2010, 1308, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Baseer, N.; Al-Baloushi, A.S.; Watanabe, M.; Shehab, S.A.; Todd, A.J. Selective innervation of NK1 receptor-lacking lamina I spinoparabrachial neurons by presumed nonpeptidergic Adelta nociceptors in the rat. Pain 2014, 155, 2291–2300. [Google Scholar] [CrossRef]
- Baseer, N.; Polgar, E.; Watanabe, M.; Furuta, T.; Kaneko, T.; Todd, A.J. Projection neurons in lamina III of the rat spinal cord are selectively innervated by local dynorphin-containing excitatory neurons. J. Neurosci. 2012, 32, 11854–11863. [Google Scholar] [CrossRef]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef]
- Gutierrez-Mecinas, M.; Bell, A.; Polgar, E.; Watanabe, M.; Todd, A.J. Expression of Neuropeptide FF Defines a Population of Excitatory Interneurons in the Superficial Dorsal Horn of the Mouse Spinal Cord that Respond to Noxious and Pruritic Stimuli. Neuroscience 2019, 416, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Bell, A.M.; Marin, A.; Taylor, R.; Boyle, K.A.; Furuta, T.; Watanabe, M.; Polgar, E.; Todd, A.J. Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli. Pain 2017, 158, 440–456. [Google Scholar] [CrossRef]
- Gutierrez-Mecinas, M.; Bell, A.M.; Shepherd, F.; Polgar, E.; Watanabe, M.; Furuta, T.; Todd, A.J. Expression of cholecystokinin by neurons in mouse spinal dorsal horn. J. Comp. Neurol. 2019, 527, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Davis, O.; Polgar, E.; Shahzad, M.; Navarro-Batista, K.; Furuta, T.; Watanabe, M.; Hughes, D.I.; Todd, A.J. Expression of Calretinin Among Different Neurochemical Classes of Interneuron in the Superficial Dorsal Horn of the Mouse Spinal Cord. Neuroscience 2019, 398, 171–181. [Google Scholar] [CrossRef]
- Gutierrez-Mecinas, M.; Furuta, T.; Watanabe, M.; Todd, A.J. A quantitative study of neurochemically defined excitatory interneuron populations in laminae I-III of the mouse spinal cord. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Polgar, E.; Bell, A.M.; Herau, M.; Todd, A.J. Substance P-expressing excitatory interneurons in the mouse superficial dorsal horn provide a propriospinal input to the lateral spinal nucleus. Brain Struct. Funct. 2018, 223, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Polgar, E.; Sardella, T.C.; Tiong, S.Y.; Locke, S.; Watanabe, M.; Todd, A.J. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain 2013, 154, 2606–2615. [Google Scholar] [CrossRef]
- Tiong, S.Y.; Polgar, E.; Van Kralingen, J.C.; Watanabe, M.; Todd, A.J. Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I-III of the rat spinal cord. Mol. Pain 2011, 7, 36. [Google Scholar] [CrossRef]
- Yasaka, T.; Tiong, S.Y.; Hughes, D.I.; Riddell, J.S.; Todd, A.J. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 2010, 151, 475–488. [Google Scholar] [CrossRef]
- Zeisel, A.; Hochgerner, H.; Lonnerberg, P.; Johnsson, A.; Memic, F.; Van der Zwan, J.; Haring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.; O’Brien, A.; Adams, L.; Cowin, G.; Ruitenberg, M.J.; Sengul, G.; Watson, C. Vertebral landmarks for the identification of spinal cord segments in the mouse. NeuroImage 2013, 68, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.P.; Rossi, J. Peptide- and non-peptide-containing unmyelinated primary afferents: The parallel processing of nociceptive information. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1985, 308, 283–289. [Google Scholar]
- Nagy, J.I.; Hunt, S.P. The termination of primary afferents within the rat dorsal horn: Evidence for rearrangement following capsaicin treatment. J. Comp. Neurol. 1983, 218, 145–158. [Google Scholar] [CrossRef]
- Saeed, A.W.; Ribeiro-da-Silva, A. Non-peptidergic primary afferents are presynaptic to neurokinin-1 receptor immunoreactive lamina I projection neurons in rat spinal cord. Mol. Pain 2012, 8, 64. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Luz, L.L.; Szucs, P.; Pinho, R.; Safronov, B.V. Monosynaptic excitatory inputs to spinal lamina I anterolateral-tract-projecting neurons from neighbouring lamina I neurons. J. Physiol. 2010, 588, 4489–4505. [Google Scholar] [CrossRef]
- Balazs, A.; Meszar, Z.; Hegedus, K.; Kenyeres, A.; Hegyi, Z.; Docs, K.; Antal, M. Development of putative inhibitory neurons in the embryonic and postnatal mouse superficial spinal dorsal horn. Brain Struct. Funct. 2017, 222, 2157–2171. [Google Scholar] [CrossRef]
- Cheng, L.; Arata, A.; Mizuguchi, R.; Qian, Y.; Karunaratne, A.; Gray, P.A.; Arata, S.; Shirasawa, S.; Bouchard, M.; Luo, P.; et al. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat. Neurosci. 2004, 7, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, T.; Xiang, Y.; Xie, Z.; Chen, Y.; Yan, R.; Xu, J.; Cheng, L. Ptf1a, Lbx1 and Pax2 coordinate glycinergic and peptidergic transmitter phenotypes in dorsal spinal inhibitory neurons. Dev. Biol. 2008, 322, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M. Pax2 is persistently expressed by GABAergic neurons throughout the adult rat dorsal horn. Neurosci. Lett. 2017, 638, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lopes, C.; Qian, Y.; Liu, Y.; Cheng, L.; Goulding, M.; Turner, E.E.; Lima, D.; Ma, Q. Tlx1 and Tlx3 coordinate specification of dorsal horn pain-modulatory peptidergic neurons. J. Neurosci. 2008, 28, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Hughes, D.I.; Polgar, E.; Nagy, G.G.; Mackie, M.; Ottersen, O.P.; Maxwell, D.J. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur. J. Neurosci. 2003, 17, 13–27. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef]
- Al Ghamdi, K.S.; Polgar, E.; Todd, A.J. Soma size distinguishes projection neurons from neurokinin 1 receptor-expressing interneurons in lamina I of the rat lumbar spinal dorsal horn. Neuroscience 2009, 164, 1794–1804. [Google Scholar] [CrossRef][Green Version]
- Barry, D.M.; Liu, X.T.; Liu, B.; Liu, X.Y.; Gao, F.; Zeng, X.; Liu, J.; Yang, Q.; Wilhelm, S.; Yin, J.; et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat. Commun. 2020, 11, 1397. [Google Scholar] [CrossRef]
- Boyle, K.A.; Gutierrez-Mecinas, M.; Polgar, E.; Mooney, N.; O’Connor, E.; Furuta, T.; Watanabe, M.; Todd, A.J. A quantitative study of neurochemically defined populations of inhibitory interneurons in the superficial dorsal horn of the mouse spinal cord. Neuroscience 2017, 363, 120–133. [Google Scholar] [CrossRef]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef] [PubMed]
- Sardella, T.C.; Polgar, E.; Garzillo, F.; Furuta, T.; Kaneko, T.; Watanabe, M.; Todd, A.J. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol. Pain 2011, 7, 76. [Google Scholar] [CrossRef]
- Chwang, W.B.; O’Riordan, K.J.; Levenson, J.M.; Sweatt, J.D. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn. Mem. 2006, 13, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Crosio, C.; Heitz, E.; Allis, C.D.; Borrelli, E.; Sassone-Corsi, P. Chromatin remodeling and neuronal response: Multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J. Cell Sci. 2003, 116, 4905–4914. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.Y.; Li, X.; Clark, J.D. Epigenetic regulation of opioid-induced hyperalgesia, dependence, and tolerance in mice. J. Pain 2013, 14, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mecinas, M.; Trollope, A.F.; Collins, A.; Morfett, H.; Hesketh, S.A.; Kersante, F.; Reul, J.M. Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13806–13811. [Google Scholar] [CrossRef]
- Lorenzo, L.E.; Ramien, M.; St Louis, M.; De Koninck, Y.; Ribeiro-da-Silva, A. Postnatal changes in the Rexed lamination and markers of nociceptive afferents in the superficial dorsal horn of the rat. J. Comp. Neurol. 2008, 508, 592–604. [Google Scholar] [CrossRef]
- White, J.P.M.; Ko, C.W.; Fidalgo, A.R.; Cibelli, M.; Paule, C.C.; Anderson, P.J.; Cruz, C.; Gomba, S.; Matesz, K.; Veress, G.; et al. Severe burn injury induces a characteristic activation of extracellular signal-regulated kinase 1/2 in spinal dorsal horn neurons. Eur. J. Pain 2011, 15, 683–690. [Google Scholar] [CrossRef]
- Mantyh, P.W.; DeMaster, E.; Malhotra, A.; Ghilardi, J.R.; Rogers, S.D.; Mantyh, C.R.; Liu, H.; Basbaum, A.I.; Vigna, S.R.; Maggio, J.E.; et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 1995, 268, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; McGill, M.M.; Shehab, S.A. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur. J. Neurosci. 2000, 12, 689–700. [Google Scholar] [CrossRef]
- Spike, R.C.; Puskar, Z.; Andrew, D.; Todd, A.J. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur. J. Neurosci. 2003, 18, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Bester, H.; De Felipe, C.; Hunt, S.P. The NK1 receptor is essential for the full expression of noxious inhibitory controls in the mouse. J. Neurosci. 2001, 21, 1039–1046. [Google Scholar] [CrossRef]
- Bester, H.; Matsumoto, N.; Besson, J.M.; Bernard, J.F. Further evidence for the involvement of the spinoparabrachial pathway in nociceptive processes: A c-Fos study in the rat. J. Comp. Neurol. 1997, 383, 439–458. [Google Scholar] [CrossRef]
- Cameron, D.; Polgar, E.; Gutierrez-Mecinas, M.; Gomez-Lima, M.; Watanabe, M.; Todd, A.J. The organisation of spinoparabrachial neurons in the mouse. Pain 2015, 156, 2061–2071. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Eberhart, D.; Urban, R.; Meda, K.; Solorzano, C.; Yamanaka, H.; Rice, D.; Basbaum, A.I. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 2013, 78, 312–324. [Google Scholar] [CrossRef]
- Vong, L.; Ye, C.; Yang, Z.; Choi, B.; Chua, S., Jr.; Lowell, B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011, 71, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, H.; Xu, Z.; Ishii-Watabe, A.; Uchida, E.; Hayakawa, T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 2000, 1, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kardon, A.P.; Polgar, E.; Hachisuka, J.; Snyder, L.M.; Cameron, D.; Savage, S.; Cai, X.; Karnup, S.; Fan, C.R.; Hemenway, G.M.; et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014, 82, 573–586. [Google Scholar] [CrossRef]
- Smith, K.M.; Boyle, K.A.; Madden, J.F.; Dickinson, S.A.; Jobling, P.; Callister, R.J.; Hughes, D.I.; Graham, B.A. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: Implications for spinal pain processing. J. Physiol. 2015, 593, 4319–4339. [Google Scholar] [CrossRef]
- Ji, R.R.; Baba, H.; Brenner, G.J.; Woolf, C.J. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 1999, 2, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Befort, K.; Brenner, G.J.; Woolf, C.J. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J. Neurosci. 2002, 22, 478–485. [Google Scholar] [CrossRef]
- Naranjo, J.R.; Mellstrom, B.; Achaval, M.; Sassone-Corsi, P. Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron 1991, 6, 607–617. [Google Scholar] [CrossRef]
- Noguchi, K.; Kowalski, K.; Traub, R.; Solodkin, A.; Iadarola, M.J.; Ruda, M.A. Dynorphin expression and Fos-like immunoreactivity following inflammation induced hyperalgesia are colocalized in spinal cord neurons. Mol. Brain Res. 1991, 10, 227–233. [Google Scholar] [CrossRef]
- Ruda, M.A.; Iadarola, M.J.; Cohen, L.V.; Young, W.S., 3rd. In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc. Natl. Acad. Sci. USA 1988, 85, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.D.; Barde, S.; Yang, T.; Lei, B.L.; Eriksson, L.I.; Mathew, J.P.; Andreska, T.; Akassoglou, K.; Harkany, T.; Hokfelt, T.G.M.; et al. Orthopedic surgery modulates neuropeptides and BDNF expression at the spinal and hippocampal levels. Proc. Natl. Acad. Sci. USA 2016, 113, E6686–E6695. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, H.; Pawlowski, S.A.; Fraine, S.L.; Sharif, B.; Hamad, D.; Fatima, T.; Berg, J.; Brown, C.M.; Jan, L.Y.; Ribeiro-da-Silva, A.; et al. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015, 13, 1246–1257. [Google Scholar] [CrossRef]
- Nahin, R.L.; Hylden, J.L.K.; Iadarola, M.J.; Dubner, R. Peripheral Inflammation Is Associated with Increased Dynorphin Immunoreactivity in Both Projection and Local Circuit Neurons in the Superficial Dorsal Horn of the Rat Lumbar Spinal-Cord. Neurosci. Lett. 1989, 96, 247–252. [Google Scholar] [CrossRef]
- Standaert, D.G.; Watson, S.J.; Houghten, R.A.; Saper, C.B. Opioid Peptide Immunoreactivity in Spinal and Trigeminal Dorsal Horn Neurons Projecting to the Parabrachial Nucleus in the Rat. J. Neurosci. 1986, 6, 1220–1226. [Google Scholar] [CrossRef]
- Noguchi, K.; Morita, Y.; Kiyama, H.; Sato, M.; Ono, K.; Tohyama, M. Preproenkephalin gene expression in the rat spinal cord after noxious stimuli. Mol. Brain Res. 1989, 5, 227–234. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Shah, B.P.; Madara, J.C.; Olson, D.P.; Strochlic, D.E.; Garfield, A.S.; Vong, L.; Pei, H.J.; Watabe-Uchida, M.; Uchida, N.; et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 2014, 507, 238. [Google Scholar] [CrossRef] [PubMed]
- Platt, R.J.; Chen, S.D.; Zhou, Y.; Yim, M.J.; Swiech, L.; Kempton, H.R.; Dahlman, J.E.; Parnas, O.; Eisenhaure, T.M.; Jovanovic, M.; et al. CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Sydney, Australia; Orlando, FL, USA, 1986; p. 237. [Google Scholar]
- Yu, Y.J.; Arttamangkul, S.; Evans, C.J.; Williams, J.T.; Von Zastrow, M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J. Neurosci. 2009, 29, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Veress, G.; Meszar, Z.; Muszil, D.; Avelino, A.; Matesz, K.; Mackie, K.; Nagy, I. Characterisation of cannabinoid 1 receptor expression in the perikarya, and peripheral and spinal processes of primary sensory neurons. Brain Struct. Funct. 2013, 218, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Molgaard, S.; Ulrichsen, M.; Boggild, S.; Holm, M.L.; Vaegter, C.; Nyengaard, J.; Glerup, S. Immunofluorescent visualization of mouse interneuron subtypes. F1000Research 2014, 3, 242. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
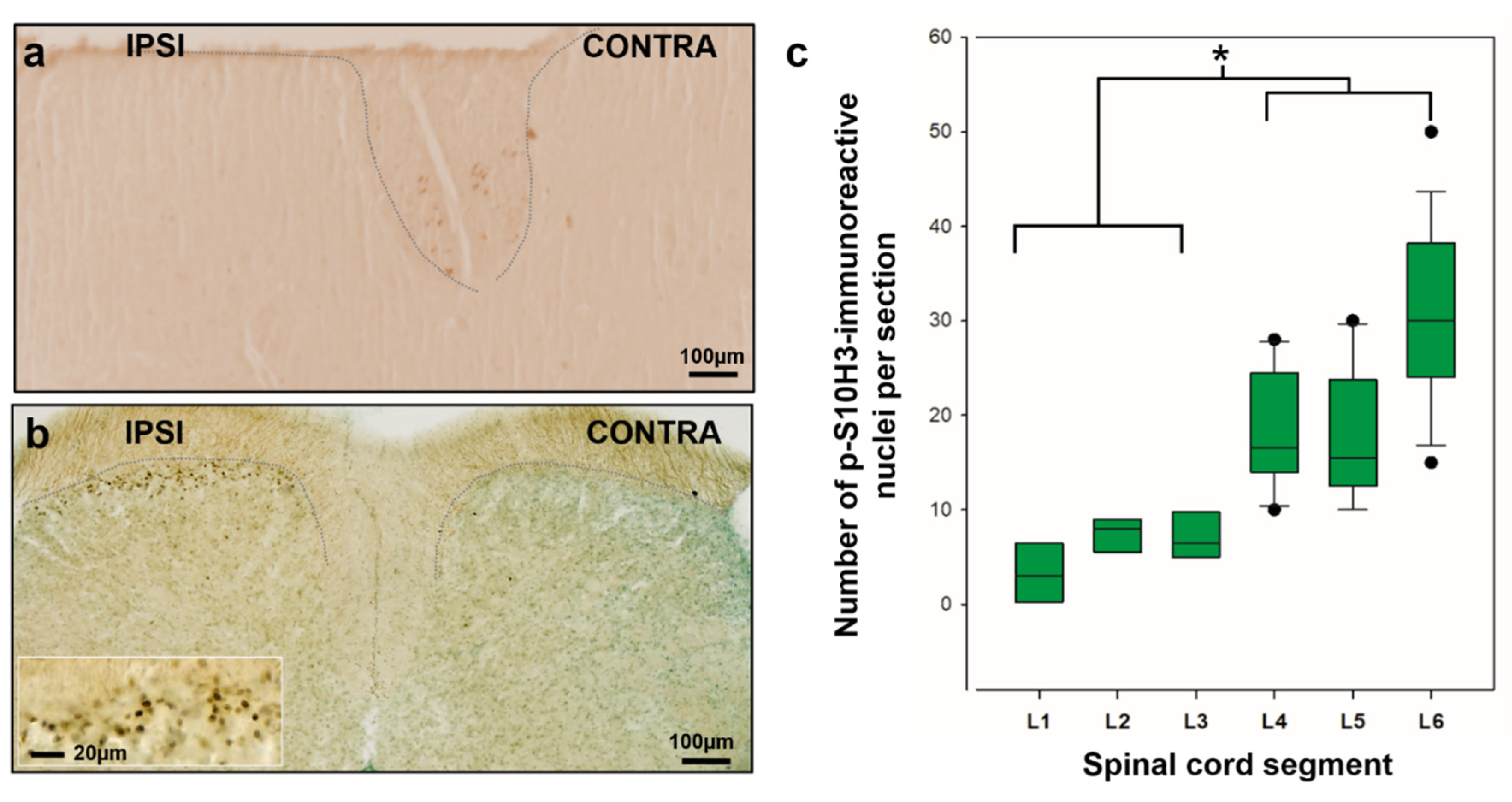

| L6 | L5 | L4 | L3 | L2 | L1 | |
|---|---|---|---|---|---|---|
| Mean number of p-S10H3-positive neurons # | 30.7 ± 3.1 | 17.7± 2.7 | 18.5 ± 2.3 | 7.5 ± 1.5 | 7.5 ± 0.8 | 3.25 ± 2.7 |
| Thickness of a given segment in mm * | 1.45 | 1.18 | 1.31 | 1.45 | 1.58 | 1.31 |
| Number of 80 μm-thick sections per segment £ | 18.1 | 14.7 | 16.3 | 18.1 | 19.2 | 16.3 |
| Total number of p-S10H3+ neurons per segment € | 557 ± 57 | 260.9 ± 40 | 301.5 ± 38 | 135.7 ± 28 | 144 ± 17 | 52.9 ± 44 |
| p-S10H3 # | IR ## | Double ### | Double/p-S10H3 | Double/IR | |
|---|---|---|---|---|---|
| Pax-2 | 150 | 198 | 36 | 24.00% | 18.10% |
| (n = 6) | |||||
| Lmx1b | 297 | 711 | 219 | 73.70% | 30.80% |
| (n = 9) | |||||
| VGAT | 420 | 538 | 67 | 15.90% | 12.40% |
| (n = 16) | |||||
| Vglut2 | 287 | 831 | 128 | 44.50% | 15.40% |
| (n = 16) |
| p-S10H3 # | IR ## | Double ### | Double/p-S10H3 | Double/IR | |
|---|---|---|---|---|---|
| somatostatin | 78 | 146 | 47 | 60.20% | 32.10% |
| (n = 3) | |||||
| Dynorphin * | 277 | 151 | 95 | 34.20% | 62.90% |
| (n = 7) | |||||
| Dynorphin $ | 202 | 186 | 78 | 38.60% | 41.90% |
| (n = 10) | |||||
| calretinin | 385 | 253 | 33 | 8.30% | 12.60% |
| (n = 11) | |||||
| nNOS | 248 | 72 | 8 | 3.20% | 11.10% |
| (n = 7) | |||||
| NPY | 154 | 6 | 1 | 0.60% | 16.60% |
| (n = 5) | |||||
| parvalbumin | 93 | 36 | 2 | 2.10% | 5.50% |
| (n = 3) |
| Name | Species | Dilution | Supplier | Cat. No. |
|---|---|---|---|---|
| p-S10H3 | rabbit | 1:600 | Abcam (Cambridge, UK) | ab5176 |
| p-S10H3 | mouse | 1:1000 | Abcam (Cambridge, UK) | ab14955 |
| GFP | chicken | 1:2000 | Abcam (Cambridge, UK) | ab13970 |
| RFP | rat | 1:1000 | Chromotec (Planegg-Martinsried, Germany) | 5f8-100 |
| Choleratoxin B | goat | 1:2000 | List Biol Labs (Campbell, CA, USA) | 703 |
| NK1R | guinea pig | 1:25000 | Merck, KGaA (Darmstadt, Germany) | AB15810 |
| IBA-1 | guinea pig | 1:500 | SYSY (Göttingen, Germany) | 234 004 |
| NeuroTrace 500/525 Nissl | 1:1000 | Thermo Fisher Sci. Inc. (Waltham, MA, USA) | N21480 | |
| CGRP | guinea pig | 1:5000 | Peninsula (San Carlos, CA, USA) | T-5027 |
| Isolectin IB4 * | 1:5000 | Thermo Fisher Sci. Inc. (Waltham, MA, USA) | I21414 | |
| NeuN | mouse | 1:1000 | Millipore (Burlington, MA, USA) | MAB377 |
| calretinin | mouse | 1:6000 | Swant (Marly, Switzerland) | 6B3 |
| parvalbumin | mouse | 1:2000 | Swant (Marly, Switzerland) | PV235 |
| neuropeptid Y | guinea pig | 1:600 | Novus Biol (Centennial, CO, USA) | NB100-1624 |
| nNOS | goat | 1:1000 | Novus Biol (Centennial, CO, USA) | NB100-858 |
| somatostatin | rabbit | 1:200 | GeneTex (Irvine, CA, USA) | GTX133119 |
| substance P | rat | 1:100 | Santa Cruz (Dallas, TX, USA) | sc-21715 |
| Pax-2 | rabbit | 1:400 | Thermo Fisher Sci. Inc. (Waltham, MA, USA) | 71-6000 |
| Lmx1b | rabbit | 1:400 | Abcam (Cambridge, UK) | ab66941 |
| prodynorphin | guinea pig | 1:600 | GeneTex (Irvine, CA, USA) | GTX10280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, A.; Mészár, Z.; Sivadó, M.; Bácskai, T.; Végh, B.; Kókai, É.; Nagy, I.; Szücs, P. Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents. Int. J. Mol. Sci. 2021, 22, 2297. https://doi.org/10.3390/ijms22052297
Varga A, Mészár Z, Sivadó M, Bácskai T, Végh B, Kókai É, Nagy I, Szücs P. Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents. International Journal of Molecular Sciences. 2021; 22(5):2297. https://doi.org/10.3390/ijms22052297
Chicago/Turabian StyleVarga, Angelika, Zoltán Mészár, Miklós Sivadó, Tímea Bácskai, Bence Végh, Éva Kókai, István Nagy, and Péter Szücs. 2021. "Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents" International Journal of Molecular Sciences 22, no. 5: 2297. https://doi.org/10.3390/ijms22052297
APA StyleVarga, A., Mészár, Z., Sivadó, M., Bácskai, T., Végh, B., Kókai, É., Nagy, I., & Szücs, P. (2021). Spinal Excitatory Dynorphinergic Interneurons Contribute to Burn Injury-Induced Nociception Mediated by Phosphorylated Histone 3 at Serine 10 in Rodents. International Journal of Molecular Sciences, 22(5), 2297. https://doi.org/10.3390/ijms22052297








