Aging Studies on Food Packaging Films Containing β-Cyclodextrin-Grafted TiO2 Nanoparticles
Abstract
1. Introduction
2. Results
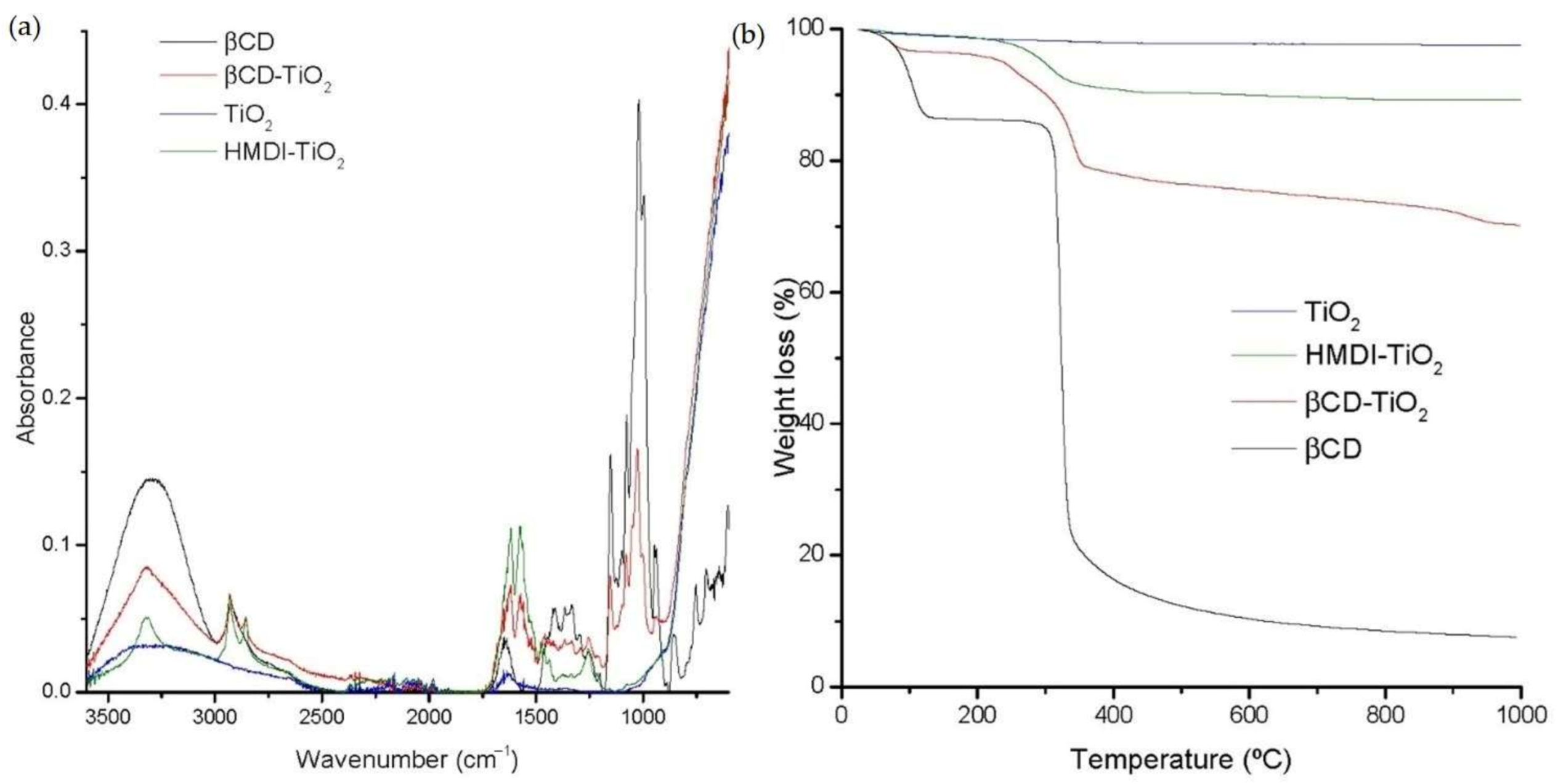
2.1. Characterization of NP Surface Modification
2.2. Accelerated Aging Tests of the Polymeric Films. Evaluation of Degradation
2.2.1. Degradation Studies in Temperature and Humidity Stability Chamber
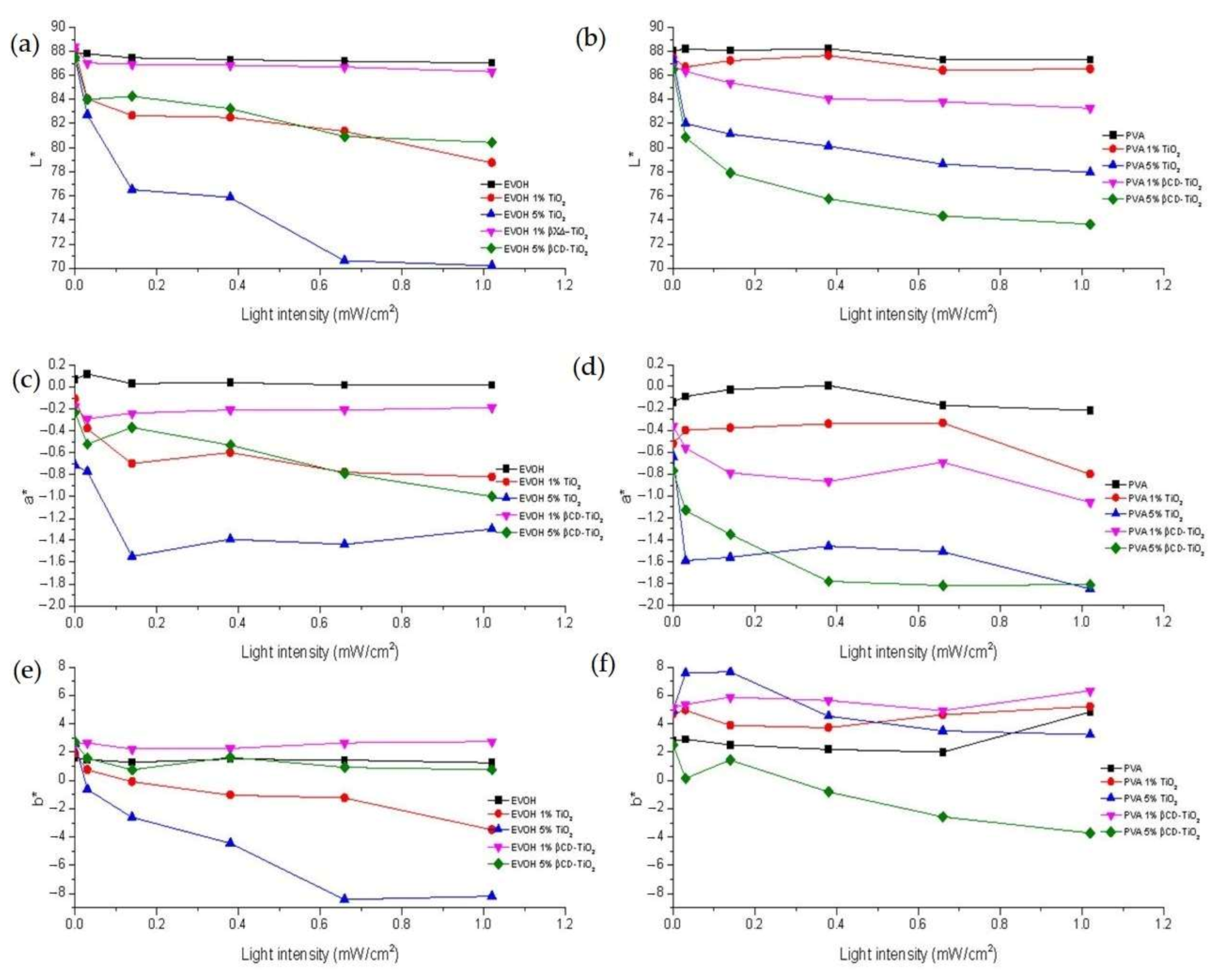
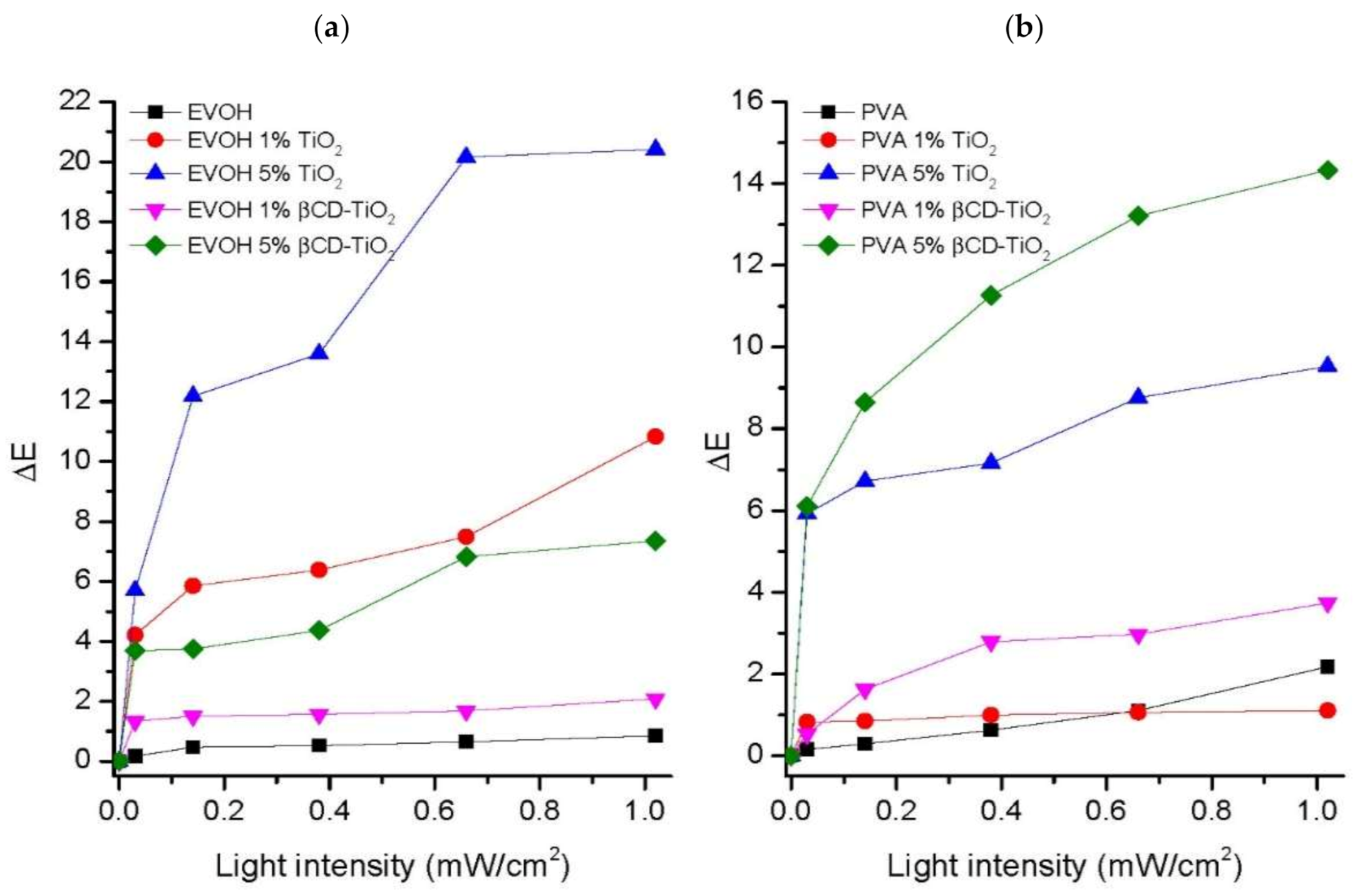
2.2.2. Photodegradation Studies
2.2.3. FTIR Characterization
3. Materials and Methods
3.1. Materials
3.2. Nanoparticle Surface Modification
3.3. Preparation and Characterization of Polymeric Composite Films
3.4. Accelerated Aging Tests
3.5. Evaluation of Composite Films Degradation
3.5.1. Film Color Change
3.5.2. FTIR Film Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues: Scientific status summary. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; Van Beest, M.; De Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Cha, D.S.; Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Damaj, Z.; Joly, C.; Guillon, E. Toward New Polymeric Oxygen Scavenging Systems: Formation of Poly(vinyl alcohol). Packag. Technol. Sci. 2015, 28, 293–302. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Development of flexible antimicrobial films using essential oils as active agents. J. Agric. Food Chem. 2007, 55, 8814–8824. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Lan, W.; Liang, X.; Lan, W.; Ahmed, S.; Liu, Y.; Qin, W. Electrospun polyvinyl alcohol/d-limonene fibers prepared by ultrasonic processing for antibacterial active packaging material. Molecules 2019, 24, 767. [Google Scholar] [CrossRef]
- Maes, C.; Luyten, W.; Herremans, G.; Peeters, R.; Carleer, R.; Buntinx, M. Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review. Polym. Rev. 2018, 58, 209–246. [Google Scholar] [CrossRef]
- Mokwena, K.K.; Tang, J. Ethylene Vinyl Alcohol: A Review of Barrier Properties for Packaging Shelf Stable Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lagaron, J.M.; Giménez, E.; Saura, J.J. Degradation of high barrier ethylene-vinyl alcohol copolymer under mild thermal-oxidative conditions studied by thermal analysis and infrared spectroscopy. Polym. Int. 2001, 50, 635–642. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Yang, Y.; Ke, Q. Heterogeneous photocatalytic oxidation of polyvinyl alcohol in water. J. Photochem. Photobiol. A Chem. 2001, 142, 85–89. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). CFR—Code of Federal Regulations Title 21. 2020. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=73.575 (accessed on 14 January 2021).
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Adams, L.; Lyon, D.; Alvarez, P. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Free Radical Biology & Medicine Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [PubMed]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- Mihaly-Cozmuta, A.; Peter, A.; Mihaly-Cozmuta, L.; Nicula, C.; Crisan, L.; Baia, L.; Turila, A. Active packaging system based on Ag/TiO2 nanocomposite used for extending the shelf life of bread. Chemical and microbiological investigations. Packag. Technol. Sci. 2015, 28, 271–284. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; Fernández-Martín, F.; De Andrés, A.; Jimenez-Riobóo, R.J.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Self-sterilized EVOH-TiO2 nanocomposites: Interface effects on biocidal properties. Adv. Funct. Mater. 2008, 18, 1949–1960. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; Salama, H.H.; El-Sayed, H.S.; Dufresne, A. Evaluation of bionanocomposites as packaging material on properties of soft white cheese during storage period. Carbohydr. Polym. 2015, 132, 274–285. [Google Scholar] [CrossRef]
- Kubacka, A.; Serrano, C.; Ferrer, M.; Lunsdorf, H.; Bielecki, P.; Cerrada, M.L.; Fernández-García, M.; Fernández-García, M. High-performance dual-action polymer-TiO2 nanocomposite films via melting processing. Nano Lett. 2007, 7, 2529–2534. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Kubacka, A.; Ferrer, M.; Cerrada, M.L.; Fernández-García, M.; Fernández-García, M. Role of TiO2 morphological characteristics in EVOH-TiO2 nanocomposite films: Self-degradation and self-cleaning properties. RSC Adv. 2013, 3, 8541–8550. [Google Scholar] [CrossRef]
- Enescu, D.; Dehelean, A.; Gonçalves, C.; Cerqueira, M.A.; Magdas, D.A.; Fucinos, P.; Pastrana, L.M. Evaluation of the specific migration according to EU standards of titanium from Chitosan/Metal complexes films containing TiO2 particles into different food simulants. A comparative study of the nano-sized vs micro-sized particles. Food Packag. Shelf Life 2020, 26, 100579. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation and characterization of poly(l-lactic acid)/TiO2 nanoparticle nanocomposite films with high transparency and efficient photodegradability. Polym. Degrad. Stab. 2007, 92, 1255–1264. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, M.; Miao, X.; Zhang, I.; Wang, X. Improvement of the Mechanical Properties of Nano-TiO2/Poly(Vinyl Alcohol) Composites by Enhanced Interaction Between Nanofiller and Matrix. Polym. Compos. 2010, 31, 1184–1193. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O.; Gomaa, M.M. Physical and electrochemical properties of PVA/TiO2 nanocomposite membrane. Adv. Polym. Technol. 2018, 37, 3842–3853. [Google Scholar] [CrossRef]
- Monreal-Pérez, P.; Isasi, J.R.; González-Benito, J.; Olmos, D.; González-Gaitano, G. Cyclodextrin-grafted TiO2 nanoparticles: Synthesis, complexation capacity, and dispersion in polymeric matrices. Nanomaterials 2018, 8, 642. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Fenyvesi, É. Cyclodextrin-enabled polymer composites for packaging. Molecules 2018, 23, 1556. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Simionato, I.; Domingues, F.C.; Nerín, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- Goñi-Ciaurriz, L.; González-Gaitano, G.; Vélaz, I. Cyclodextrin-grafted nanoparticles as food preservative carriers. Int. J. Pharm. 2020, 588, 119664. [Google Scholar] [CrossRef]
- González, E.A.S.; Olmos, D.; Lorente, M.A.; Vélaz, I.; González-Benito, J. Preparation and characterization of polymer composite materials based on PLA/TiO2 for antibacterial packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Gonnet, J.F. Colour effects of co-pigmentation of anthocyanins revisited-1. A colorimetric definition using the CIELAB scale. Food Chem. 1998, 63, 409–415. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Matykiewicz, D. Correlation between processing parameters and degradation of different polylactide grades during twin-screw extrusion. Polymers 2020, 12, 1333. [Google Scholar] [CrossRef]
- Cai, L.H.; Qi, Z.G.; Xu, J.; Guo, B.H.; Huang, Z.Y. Thermo-oxidative degradation of Nylon 1010 films: Colorimetric evaluation and its correlation with material properties. Chin. Chem. Lett. 2017, 28, 949–954. [Google Scholar] [CrossRef]
- Favaro, M.; Mendichi, R.; Ossola, F.; Russo, U.; Simon, S.; Tomasin, P.; Vigato, P.A. Evaluation of polymers for conservation treatments of outdoor exposed stone monuments. Part I: Photo-oxidative weathering. Polym. Degrad. Stab. 2006, 91, 3083–3096. [Google Scholar] [CrossRef]
- Bociaga, E.; Trzaskalska, M. Influence of polymer processing parameters and coloring agents on gloss and color of acrylonitrile-butadiene-styrene terpolymer moldings. Polimery/Polymers 2016, 61, 544–550. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Zhang, Y.; Niu, D.; Du, J. Effects of aqueous chlorine dioxide treatment on enzymatic browning and shelf-life of fresh-cut asparagus lettuce (Lactuca sativa L.). Postharvest Biol. Technol. 2010, 58, 232–238. [Google Scholar] [CrossRef]
- Boun, H.R.; Huxsoll, C.C. Control of Minimally Processed Carrot (Daucus carota) Surface Discoloration Caused by Abrasion Peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar] [CrossRef]

| Additive | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 60 °C | 80 °C | |||||
| L*/a*/b* | ∆E | L*/a*/b* | ∆E | L*/a*/b* | ∆E | L*/a*/b* | ∆E | |
| None | 87.4/0.0/2.2 | 0.7 | 87.4/0.0/2.2 | 0.7 | 87.3/0.0/2.6 | 1.1 | 87.2/0.0/2.6 | 1.2 |
| 1% TiO2 | 87.3/−0.1/2.3 | 0.9 | 87.6/−0.1/3.0 | 1.1 | 85.8/−0.2/3.8 | 3.0 | 85.7/−0.3/6.7 | 5.3 |
| 5% TiO2 | 87.7/−0.5/2.8 | 0.3 | 88.5/−0.5/2.4 | 1.0 | 86.5/−0.9/12 | 9.7 | 85.3/−0.8/15 | 12 |
| 1% βCD-TiO2 | 88.6/−0.2/2.5 | 0.2 | 87.5/−0.2/3.8 | 1.5 | 86.9/−0.2/3.4 | 1.7 | 87.1/−0.3/5.8 | 3.5 |
| 5% βCD-TiO2 | 86.8/−0.2/2.1 | 0.9 | 86.8/−0.3/3.4 | 1.0 | 86.0/−0.6/8.0 | 5.5 | 84.1/−0.6/14 | 12 |
| Additive | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 40 °C | 60 °C | 80 °C | |||||
| L*/a*/b* | ∆E | L*/a*/b* | ∆E | L*/a*/b* | ∆E | L*/a*/b* | ∆E | |
| None | 88.4/0.0/2.1 | 0.8 | 87.5/−0.2/4.0 | 1.3 | 85.6/−0.1/3.2 | 2.6 | 86.2/−0.2/6.9 | 4.5 |
| 1% TiO2 | 87.1/−0.4/5.2 | 0.6 | 88.7/−0.2/2.4 | 2.7 | 86.8/0.1/7.9 | 3.4 | 86.8/−0.2/8.1 | 3.4 |
| 5% TiO2 | 89.9/−0.6/2.3 | 3.7 | 89.3/−0.7/9.7 | 5.2 | 78.2/2.1/24 | 22 | 83.6/0.5/26 | 22 |
| 1% βCD-TiO2 | 87.4/−0.2/4.8 | 0.8 | 88.8/−0.2/2.9 | 3.0 | 87.2/−0.6/10 | 5.0 | 83.5/−0.9/10 | 6.0 |
| 5% βCD-TiO2 | 87.9/−0.6/2.8 | 1.5 | 87.5/−0.7/6.2 | 3.8 | 85.7/0.1/20 | 17 | 73.8/4.5/31 | 31 |
| Assay | Temperature (°C) | Relative Humidity (%) | Time of Exposure (Weeks) |
|---|---|---|---|
| 1 | 25 | 28 | 1 |
| 2 | 25 | 40 | 2 |
| 3 | 25 | 60 | 2 |
| 4 | 25 | 80 | 2 |
| 5 | 25 | 80 | 1 |
| 6 | 40 | 80 | 2 |
| 7 | 60 | 80 | 2 |
| 8 | 80 | 80 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goñi-Ciaurriz, L.; Senosiain-Nicolay, M.; Vélaz, I. Aging Studies on Food Packaging Films Containing β-Cyclodextrin-Grafted TiO2 Nanoparticles. Int. J. Mol. Sci. 2021, 22, 2257. https://doi.org/10.3390/ijms22052257
Goñi-Ciaurriz L, Senosiain-Nicolay M, Vélaz I. Aging Studies on Food Packaging Films Containing β-Cyclodextrin-Grafted TiO2 Nanoparticles. International Journal of Molecular Sciences. 2021; 22(5):2257. https://doi.org/10.3390/ijms22052257
Chicago/Turabian StyleGoñi-Ciaurriz, Leire, Marta Senosiain-Nicolay, and Itziar Vélaz. 2021. "Aging Studies on Food Packaging Films Containing β-Cyclodextrin-Grafted TiO2 Nanoparticles" International Journal of Molecular Sciences 22, no. 5: 2257. https://doi.org/10.3390/ijms22052257
APA StyleGoñi-Ciaurriz, L., Senosiain-Nicolay, M., & Vélaz, I. (2021). Aging Studies on Food Packaging Films Containing β-Cyclodextrin-Grafted TiO2 Nanoparticles. International Journal of Molecular Sciences, 22(5), 2257. https://doi.org/10.3390/ijms22052257







