Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu—A Novel Competitive Heme Acquisition Strategy
Abstract
1. Introduction
2. Results and Discussion
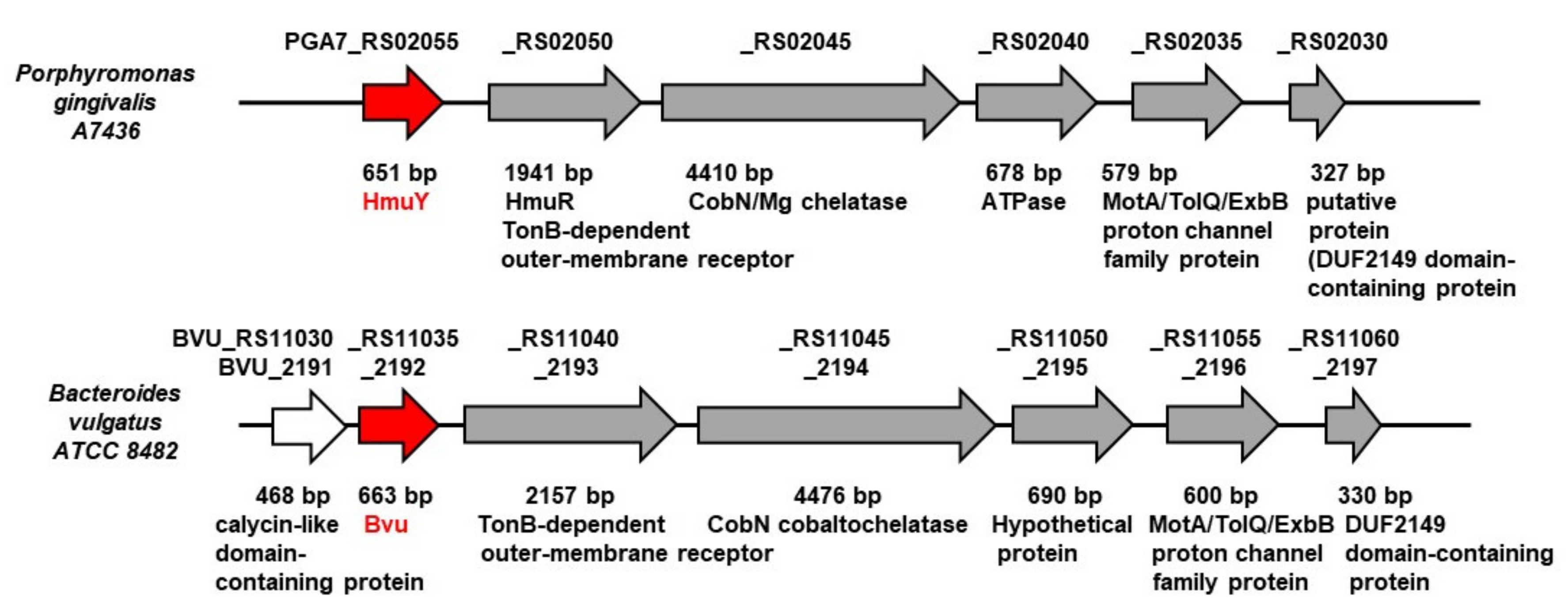
2.1. B. vulgatus Bvu May Belong to the HmuY-Like Family of Hemophore-Like Proteins
2.2. B. vulgatus Bvu Is Expressed at Higher Levels under Low-Iron Conditions
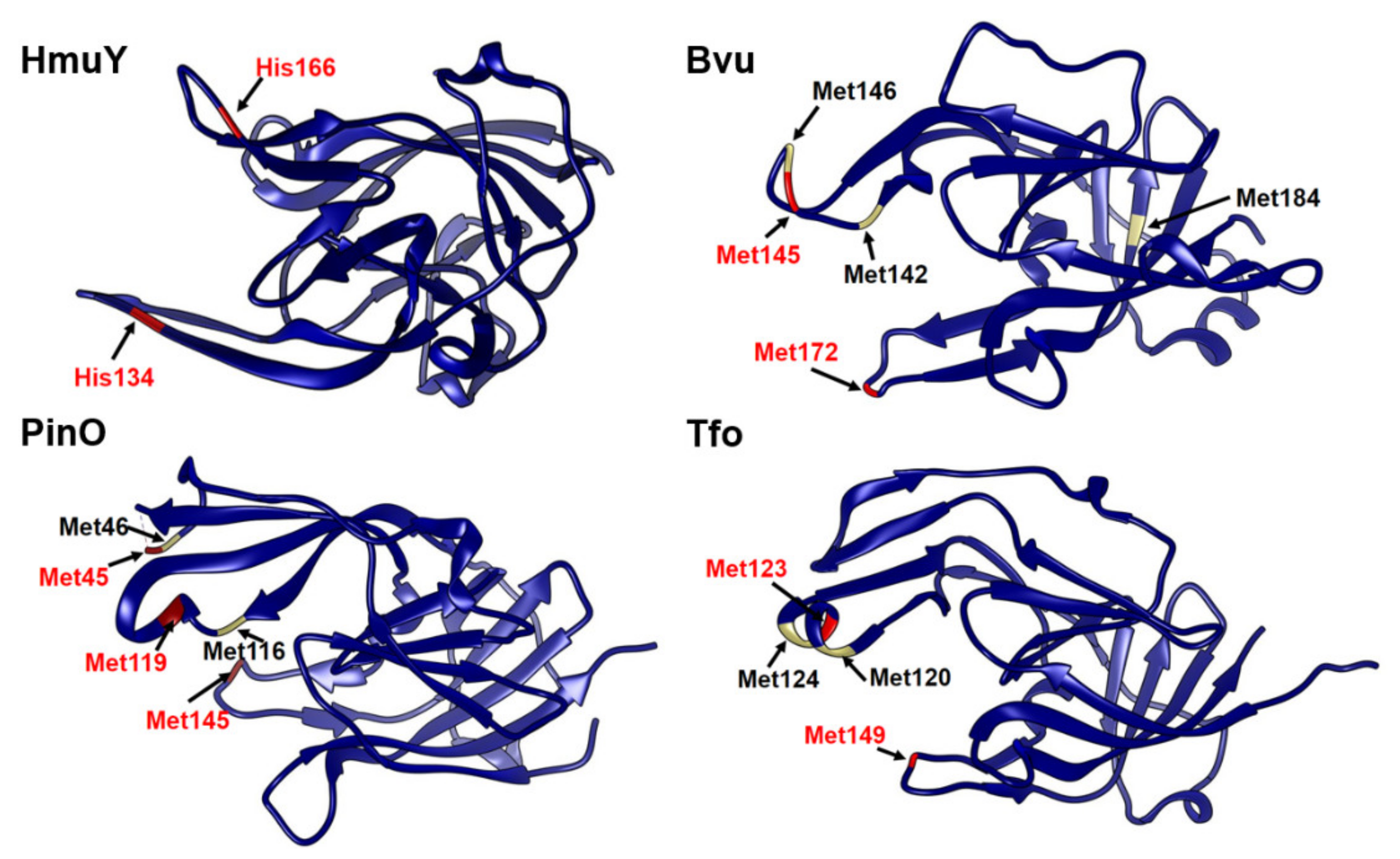
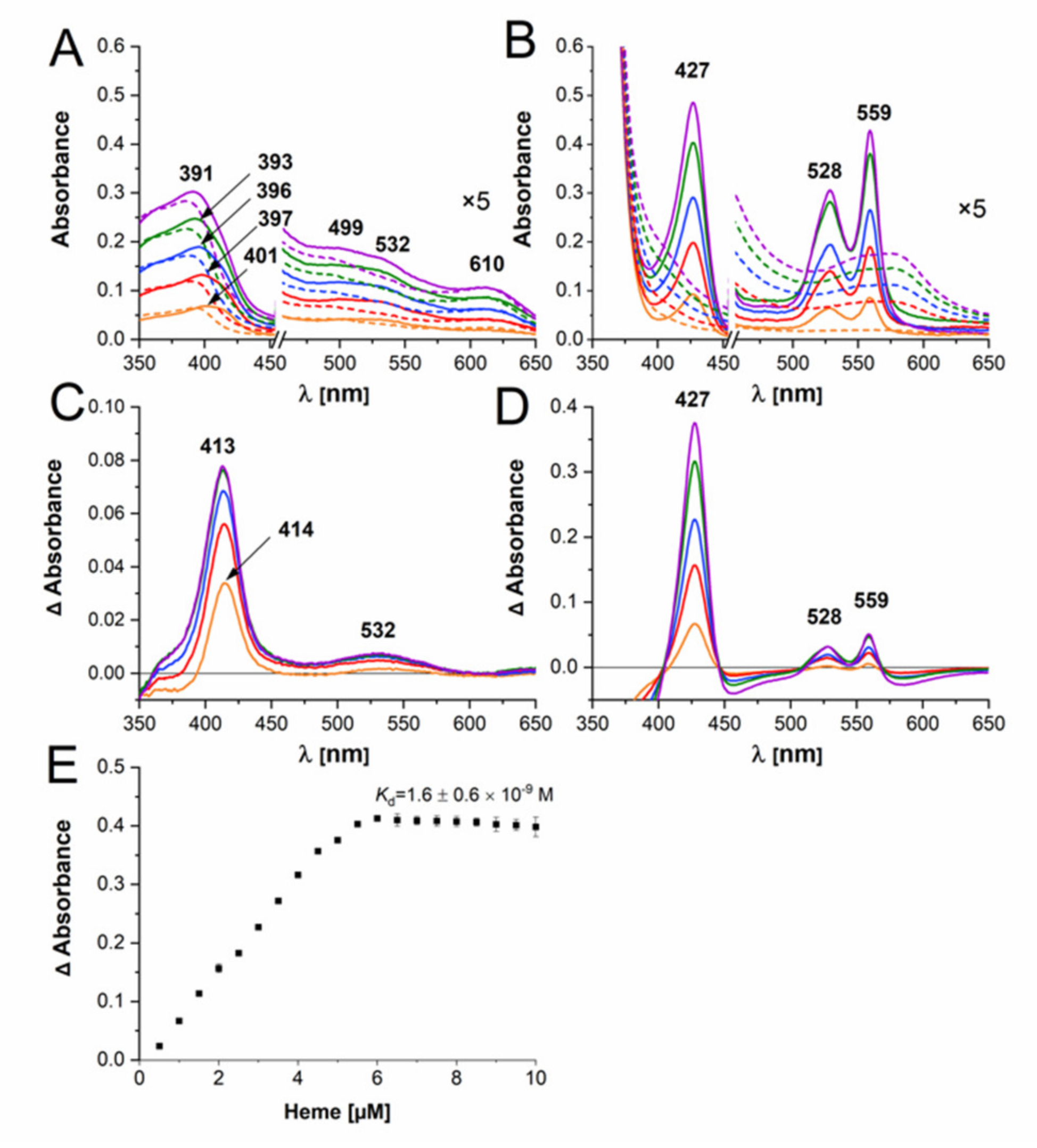
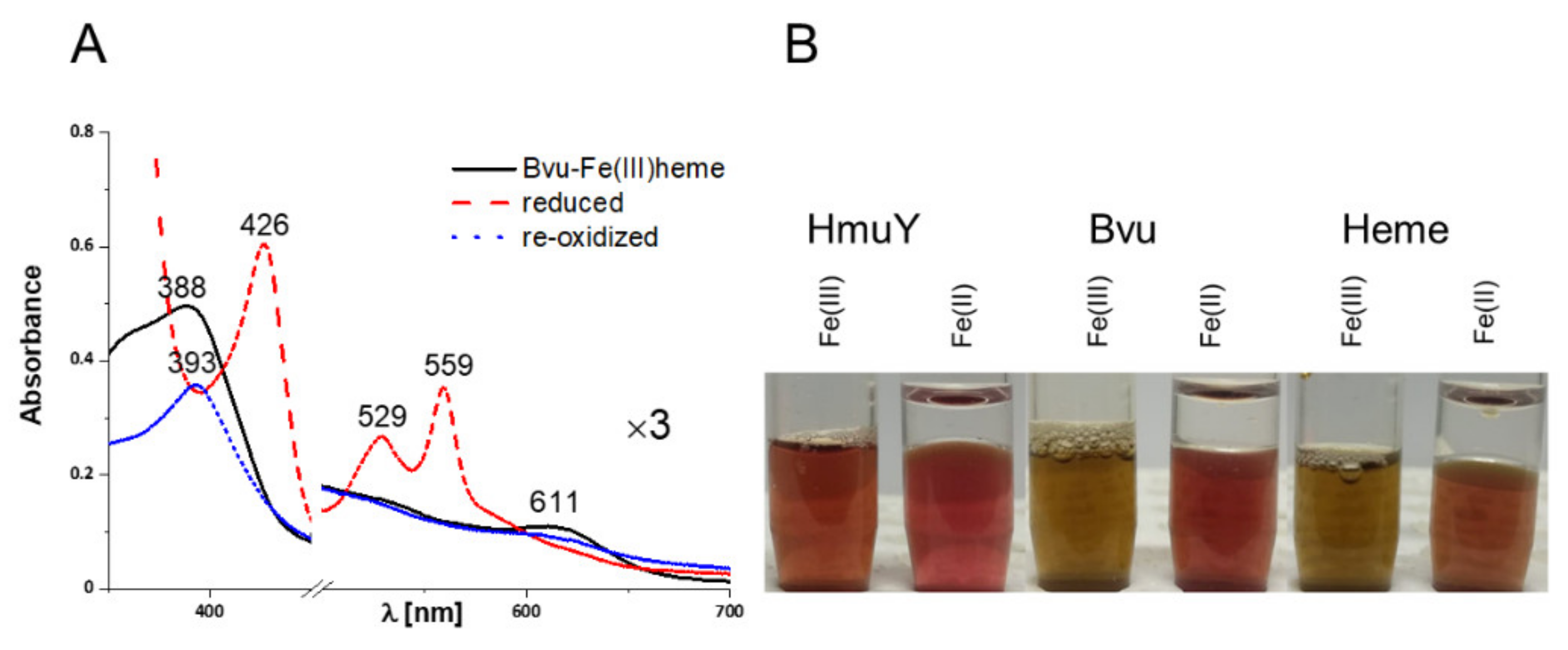
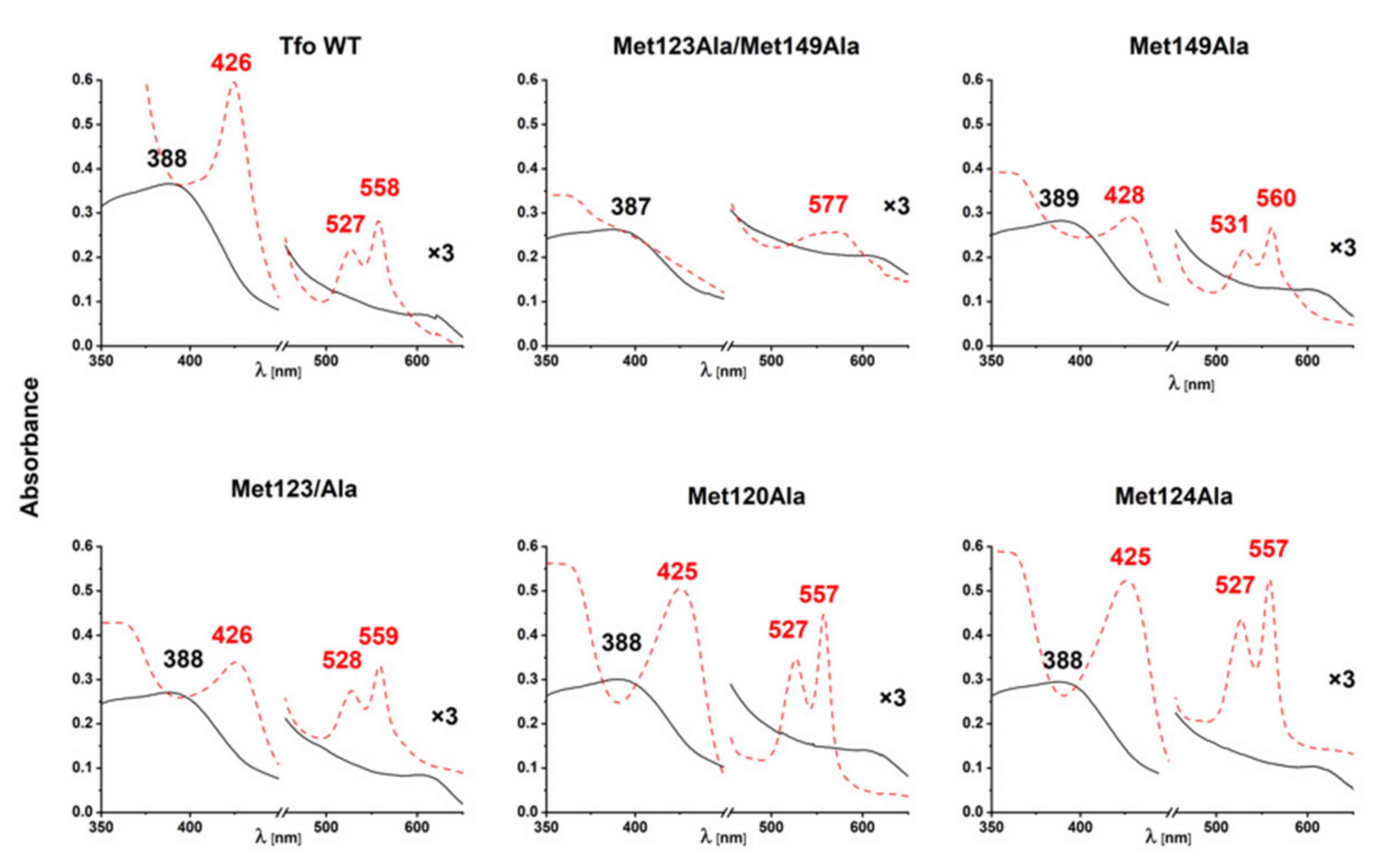
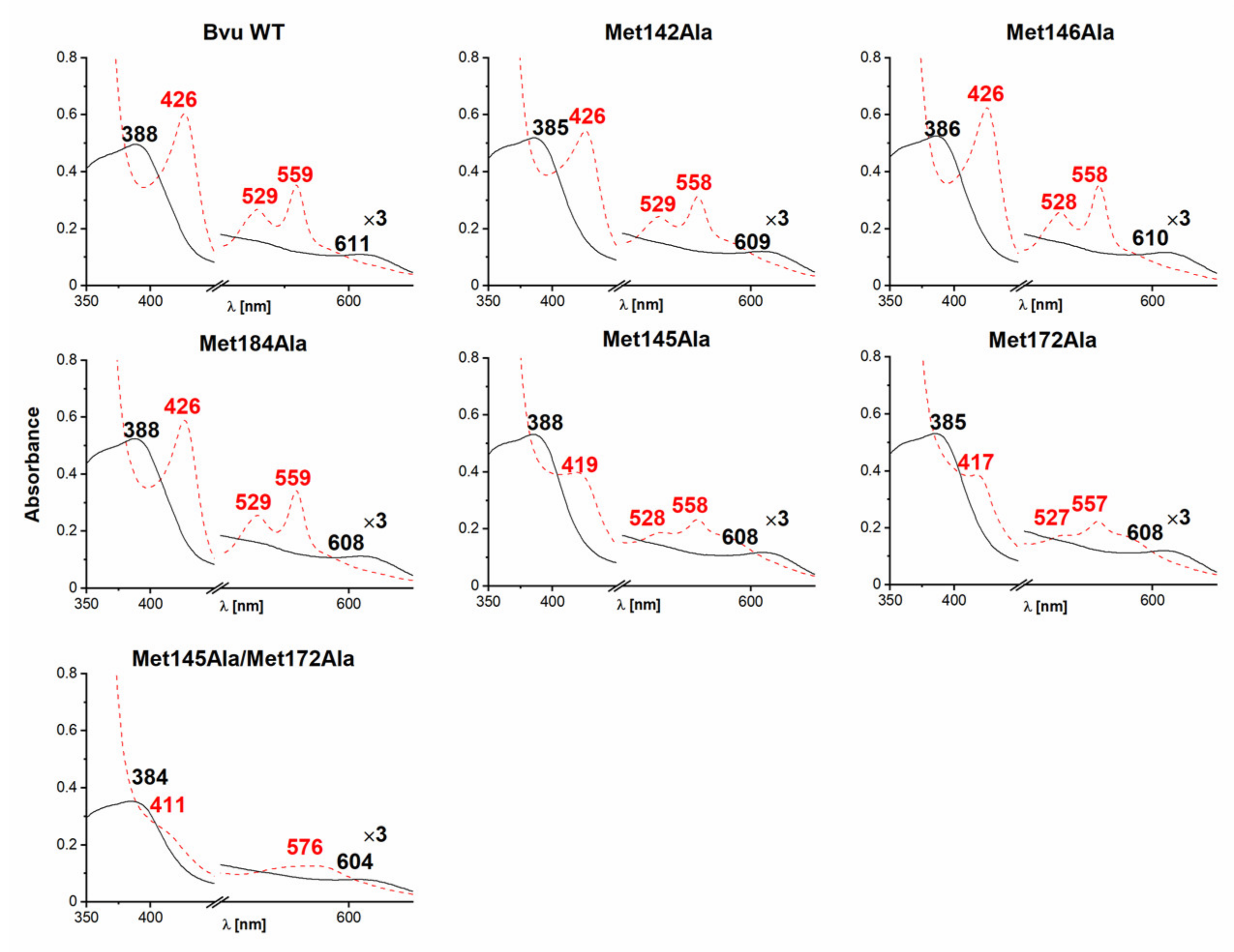
2.3. B. vulgatus Bvu Binds Heme in A Manner Different to P. gingivalis HmuY but Similar to Other HmuY Homologs
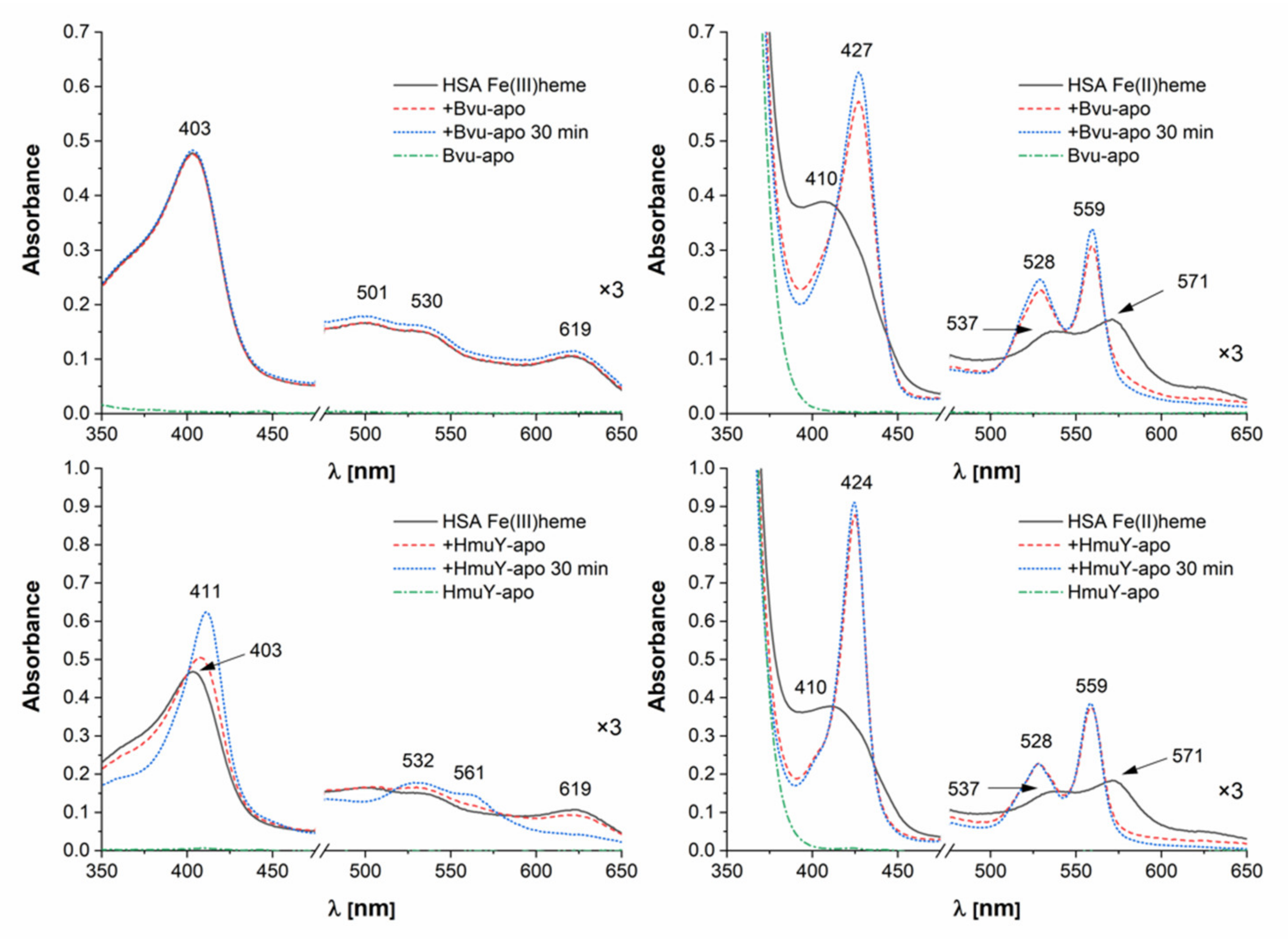
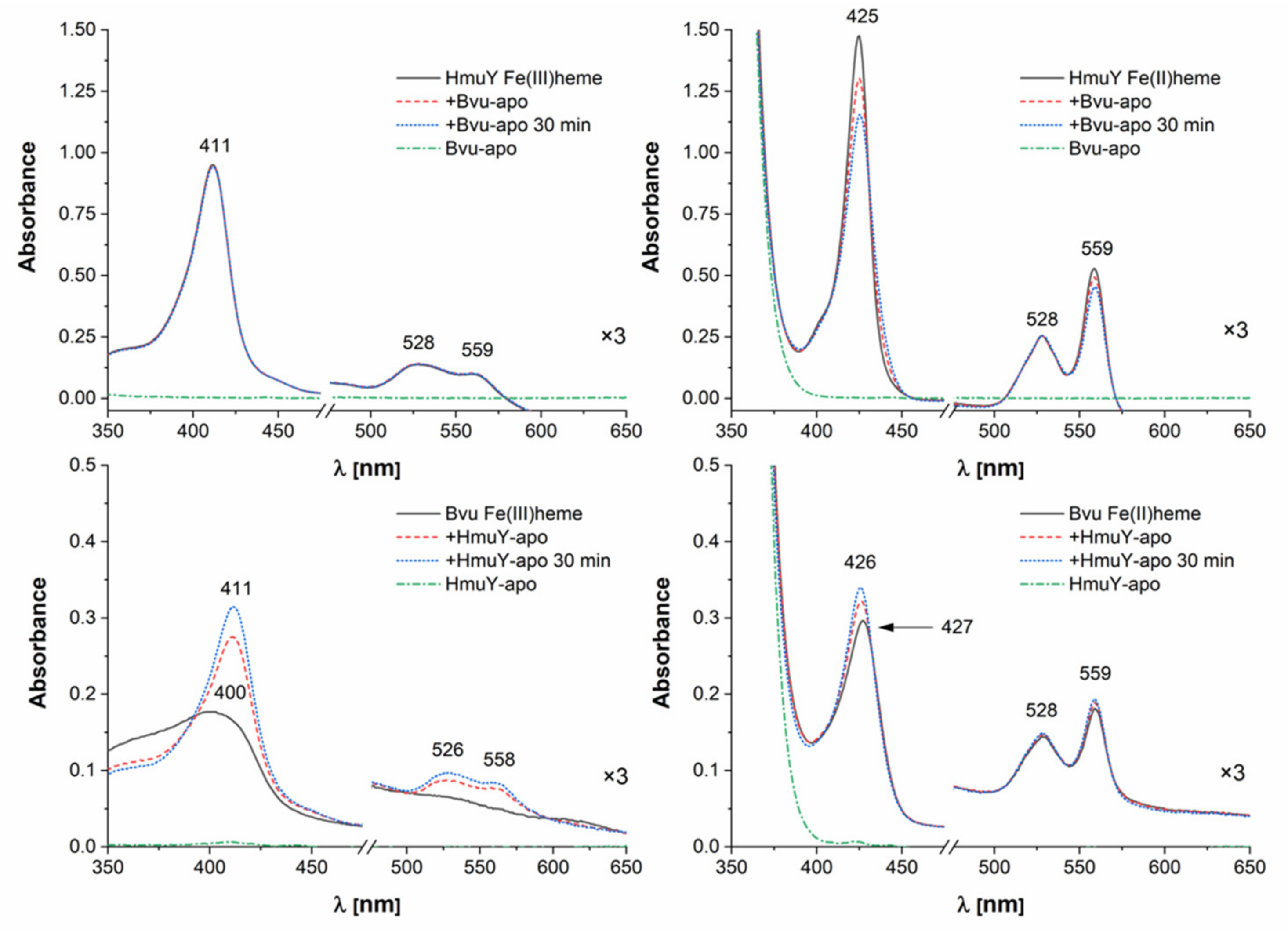
2.4. B. vulgatus Bvu May Sequester Heme from Host Serum Albumin but Only under Reducing Conditions
2.5. B. vulgatus Bvu Is Less Stable as Compared to P. gingivalis HmuY
2.6. Conclusions
3. Materials and Methods
3.1. Bacterial Stains and Growth Conditions
3.2. Plasmid Construction, Site-Directed Mutagenesis, Overexpression, and Purification of Proteins
3.3. Heme-Protein Complex Formation
3.4. Heme Sequestration Experiments
3.5. Determination of Protein Stability
3.6. Proteolytic Digestion
3.7. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HmuY | Heme-binding, hemophore-like protein produced by Porphyromonas gingivalis |
| Tfo | HmuY homolog from Tannerella forsythia |
| PinO | HmuY homolog from Prevotella intermedia |
| PinA | HmuY homolog from Prevotella intermedia |
| Bvu | HmuY homolog from Bacteroides vulgatus |
| HSA | Human serum albumin |
| PPIX | Protoporphyrin IX |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| CBB | Coomassie Brilliant Blue G-250 |
| BM | Basal medium |
| Hm | Basal medium supplemented with heme |
| DIP | Basal medium lacking heme, supplemented with iron chelator, dipyridyl |
| PBS | 20 mM sodium phosphate buffer, pH 7.4, containing 140 mM NaCl |
References
- Wexler, H.M.; Daya, S.; Berns, K.I. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human micro-biome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Waidmann, M.; Bechtold, O.; Frick, J.S.; Lehr, H.A.; Schubert, S.; Dobrindt, U.; Loeffler, J.; Bohn EAutenrieth, I.B. Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology 2003, 125, 162–177. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteoides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Bamba, T.; Matsuda, H.; Endo, M.; Fujiyama, Y. The pathogenic role of Bacteroides vulgatus in patients with ulcerative colitis. J. Gastroenterol. 1995, 30, 45–47. [Google Scholar] [PubMed]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Llambes, F.; Arias-Herrera, S.; Caffesse, R. Relationship between diabetes and periodontal infection. World J. Diabetes 2015, 6, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, N.; Kato, T.; Matsuda, Y.; Yokoji, M.; Yamada, M.; Nakajima, T.; Kondo, N.; Endo, N.; Yamamoto, R.; et al. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis though modulation of the gut microbiota and gut immune system. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Du Teil Espina, M.; Gabarrini, G.; Harmsen, H.J.M. Talk to your gut: The oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol. Rev. 2018, 43, 1–18. [Google Scholar] [CrossRef]
- Seedorf, H.; Griffin, N.W.; Ridaura, V.K.; Reyes, A.; Cheng, J.; Rey, F.E.; Smith, M.I.; Simon, G.M.; Scheffrahn, R.H.; Woebken, D.; et al. Bacteria from Diverse Habitats Colonize and Compete in the Mouse Gut. Cell 2014, 159, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.R.; Bergonia, H.A.; Gerdes, S.; Smith, C.J. Bacteroides fragilis requires the ferrous-iron transporter FeoAB and the CobN-like proteins BtuS1 and BtuS2 for assimilation of iron released from heme. Microbiol. Open 2018, 8, e669. [Google Scholar]
- Smalley, J.W.; Olczak, T. Haem acquisition mechanisms of Porphyromonas gingivalis-strategies used in polymicrobial community in a haem-limited host environment. Mol. Oral Microbiol. 2017, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Sosicka, P.; Olczak, M. HmuY is an important virulence factor for Porphyromonas gingivalis growth in the heme-limited host environment and infection of macrophages. Biochem. Biophys. Res. Commun. 2015, 467, 748–753. [Google Scholar] [CrossRef]
- Young, G.P.; Rose, I.S.; John, D.J.B. Haem in the gut. I. Fate of haemoproteins and the absorption of haem. J. Gastroenterol. Hepatol. 1989, 4, 537–545. [Google Scholar] [CrossRef]
- Young, G.P.; John, D.J.B.S.; Rose, I.S.; Blake, D. Haem in the gut. Part II. Faecal excretion of haem and haem-derived porphyrins and their detection. J. Gastroenterol. Hepatol. 1990, 5, 194–203. [Google Scholar] [CrossRef]
- Bielecki, M.; Antonyuk, S.; Strange, R.W.; Smalley, J.W.; Mackiewicz, P.; Śmiga, M.; Stępień, P.; Olczak, M.; Olczak, T. Tannerella forsythia Tfo belongs to Porphyromonas gingivalis HmuY-like family of proteins but differs in heme-binding properties. Biosci. Rep. 2018, 38, BSR20181325. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, M.; Antonyuk, S.; Strange, R.W.; Siemińska, K.; Smalley, J.W.; Mackiewicz, P.; Śmiga, M.; Cowan, M.; Capper, M.J.; Ślęzak, P.; et al. Prevotella intermedia produces two proteins homologous to Porphyromonas gingivalis HmuY but with different heme coordination mode. Biochem. J. 2020, 477, 381–405. [Google Scholar] [CrossRef]
- Ślęzak, P.; Śmiga, M.; Smalley, J.W.; Siemińska, K.; Olczak, T. Porphyromonas gingivalis HmuY and Streptococcus gordonii GAPDH—Novel Heme Acquisition Strategy in the Oral Microbiome. Int. J. Mol. Sci. 2020, 21, 4150. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Hantke, K. Hemin uptake system of Yersinia enterocolitica: Similarities with TonB-dependent systems in gram-negative bacteria. EMBO J. 1992, 11, 4359–4367. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.R.; Rivera, M. Heme Uptake and Metabolism in Bacteria. In Metallomics and the Cell; Springer: Berlin, Germany, 2012; Volume 12, pp. 279–332. [Google Scholar] [CrossRef]
- Olczak, T.; Sroka, A.; Potempa, J.; Olczak, M. Porphyromonas gingivalis HmuY and HmuR: Further characterization of a novel mechanism of heme utilization. Arch. Microbiol. 2007, 189, 197–210. [Google Scholar] [CrossRef]
- Olczak, T.; Wojtowicz, H.; Ciuraszkiewicz, J.; Olczak, M. Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol. 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Wang, Y.; Jin, Y.; Wang, C. Heme competition triggers and increase in the pathogenic potential of Por-phyromonas gingivalis in Porphyromonas gingivalis-Candida albicans mixed biofilm. Front. Microbiol. 2020, 11, 3063. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.-L.; Sztajer, H.; Jarek, M.; Bhuju, S.; Wagner-Döbler, I. Worlds Apart—Transcriptome Profiles of Key Oral Microbes in the Periodontal Pocket Compared to Single Laboratory Culture Reflect Synergistic Interactions. Front. Microbiol. 2018, 9, 124. [Google Scholar] [CrossRef]
- Szafranski, S.P.; Deng, Z.L.; Tomasch JJarek, M.; Bhuju, S.; Meisinger, C.; Kuhnisch, J.; Sztajer, H.; Wagner-Dobler, I. Functional biomarkers for chronic periodontitis and insights into the roles of Prevotella nigrescens and Fusobacterium nucleatum; a metatranscriptome analysis. NPJ Biofilms Microbiomes 2015, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, H.; Guevara, T.; Tallant, C.; Olczak, M.; Sroka, A.; Potempa, J.; Sola, M.; Olczak, T.; Gomis-Rüth, F.X. Unique Structure and Stability of HmuY, a Novel Heme-Binding Protein of Porphyromonas gingivalis. PLOS Pathog. 2009, 5, e1000419. [Google Scholar] [CrossRef]
- Wojtowicz, H.; Wojaczynski, J.; Olczak, M.; Kroliczewski, J.; Latos-Grazynski, L.; Olczak, T. Heme environment of Porphyromonas gingivalis HmuY heme-binding protein. Biochem. Biophys. Res. Commun. 2009, 382, 178–182. [Google Scholar] [CrossRef]
- Smalley, J.W.; Byrne, D.P.; Birss, A.J.; Wojtowicz, H.; Sroka, A.; Potempa, J.; Olczak, T. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PLoS ONE 2011, 6, e17182. [Google Scholar] [CrossRef]
- Byrne, D.P.; Potempa, J.; Olczak, T.; Smalley, J.W. Evidence of mutualism between two periodontal pathogens: Co-operative haem acquisition by the HmuY haemophore of Porphyromonas gingivalis and the cysteine protease interpain A (InpA) of Prevotella intermedia. Mol. Oral Microbiol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Benedyk, M.; Byrne, D.P.; Glowczyk, I.; Potempa, J.; Olczak, M.; Olczak, T.; Smalley, J.W. Pyocyanin: A contributory factor in haem acquisition and virulence enhancement of Porphyromonas gingivalis in the lung. PLoS ONE 2015, 10, e0118319. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.; Roy, U.; Moshe, S.; Weissman, Z.; Kornitzer, D. Human Serum Albumin Facilitates Heme-Iron Utilization by Fungi. mBio 2020, 11. [Google Scholar] [CrossRef]
- Cao, Y.; Nicoletti, F.P.; De Sanctis, G.; Bocedi, A.; Ciaccio, C.; Gullotta, F.; Fanali, G.; Tundo, G.R.; di Masi, A.; Fasano, M.; et al. Evidence for pH-dependent multiple conformes in iron(II) heme-human serum albumin: Spectroscopic and kinetic investigation of carbon monoxide binding. J. Biol. Inorg. Chem. 2012, 17, 133–147. [Google Scholar] [CrossRef]
- Bocedi, A.; De Sanctis, G.; Ciaccio, C.; Tundo, G.R.; Di Masi, A.; Fanali, G.; Nicoletti, F.P.; Fasano, M.; Smulevich, G.; Ascenzi, P.; et al. Reciprocal allosteric modulation of carbon monooxide and warfarin binding to ferrous human serum heme-albumin. PLoS ONE 2013, 8, e58842. [Google Scholar] [CrossRef] [PubMed]
- Schejter, A.; Plotkin, B.; Vig, I. The reactivity of cytochrome c with soft ligands. FEBS Lett. 1991, 280, 199–201. [Google Scholar] [CrossRef]
- Ayers, P.W.; Parr, R.G.; Pearson, R.G. Elucidating the hard/soft acid/base principle: A perspective based on half-reactions. J. Chem. Phys. 2006, 124, 194107. [Google Scholar] [CrossRef]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free. Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota in humans and mice. Gastroenterology 2014, 147, 1055–1063. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Genet. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Campbell, E.L.; Kominsky, D.J. Hypoxia and Mucosal Inflammation. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 77–100. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Birss, A.J.; Withnall, R.; Silver, J. Interactions of Porphyromonas gingivalis with oxyhaemoglobin and deoxy-haemoglobin. Biochem. J. 2002, 362, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Yates, E.; Bielecki, M.; Olczak, T.; Smalley, J.W. Potential role for Streptococcus gordonii-derived hydrogen peroxide in heme acquisition by Porphyromonas gingivalis. Mol. Oral Microbiol. 2018, 33, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Śmiga, M.; Bielecki, M.; Olczak, M.; Olczak, T. Porphyromonas gingivalis PgFur Is a Member of a Novel Fur Subfamily With Non-canonical Function. Front. Cell. Infect. Microbiol. 2019, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A vis-ualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Wojaczynski, J.; Wojtowicz, H.; Bielecki, M.; Olczak, M.; Smalley, J.W.; Latos-Grazynski, L.; Olczak, T. Iron(III) mesoporphyrin IX and iron(III) deuteroporphyrin IX bind to the Porphyromonas gingivalis HmuY hemophore. Biochem. Biophys. Res. Commun. 2011, 411, 299–304. [Google Scholar] [CrossRef] [PubMed]


| Protein | Unfolding (°C) | Refolding (°C) | ||
|---|---|---|---|---|
| Apo-Protein | Protein-Heme | Apo-Protein | Protein-Heme | |
| P. gingivalis HmuY | 70.8 ± 0.1 | 70.6 ± 0.2 | ND | ND |
| T. forsythia Tfo | 61.4 ± 0.2 | 61.2 ± 0.3 | ND | ND |
| P. intermedia PinO | 69.3 ± 0.2 | 68.8 ± 0.3 | 63.9 ±0.2 | 63.8 ± 0.3 |
| P. intermedia PinA | 62.3 ± 0.3 | 62.0 ± 0.2 | ND | ND |
| B. vulgatus Bvu | 60.7 ± 0.1 | 60.4 ± 0.1 | ND | ND |
| Name | DNA Sequence (5′→3′) | Description |
|---|---|---|
| 16S_F | ACACGTATCCAACCTGCCGT | Amplify fragment of B. vulgatus 16S rRNA gene used in qRT-PCR |
| 16S_R | ATGGAACGCATCCCCATCGT | |
| Bvu_F | AGACCCTTCTACCCATTCGGG | Amplify fragment of B. vulgatus bvu gene used in qRT-PCR |
| Bvu_R | CCCATTCCTCCGGTTCTTTCTG | |
| Bvu_FP | CAACCTCGGGATCGAGGGAAGGATGGATGGTATTTTGGAAGGAATA | Amplify bvu gene for overexpression and purification of recombinant N-terminally tagged Bvu protein with 6His tag and maltose-binding protein (MBP), lacking predicted signal peptide sequence |
| Bvu_RP | TATTTAATTACCTGCAGGGAATTCGGATCCTTACAATTCAAACGGATAAATATAATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemińska, K.; Cierpisz, P.; Śmiga, M.; Olczak, T. Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu—A Novel Competitive Heme Acquisition Strategy. Int. J. Mol. Sci. 2021, 22, 2237. https://doi.org/10.3390/ijms22052237
Siemińska K, Cierpisz P, Śmiga M, Olczak T. Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu—A Novel Competitive Heme Acquisition Strategy. International Journal of Molecular Sciences. 2021; 22(5):2237. https://doi.org/10.3390/ijms22052237
Chicago/Turabian StyleSiemińska, Klaudia, Patryk Cierpisz, Michał Śmiga, and Teresa Olczak. 2021. "Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu—A Novel Competitive Heme Acquisition Strategy" International Journal of Molecular Sciences 22, no. 5: 2237. https://doi.org/10.3390/ijms22052237
APA StyleSiemińska, K., Cierpisz, P., Śmiga, M., & Olczak, T. (2021). Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu—A Novel Competitive Heme Acquisition Strategy. International Journal of Molecular Sciences, 22(5), 2237. https://doi.org/10.3390/ijms22052237






