Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth
Abstract
1. Introduction
2. Zinc (Zn) Trafficking in Sperm Transport
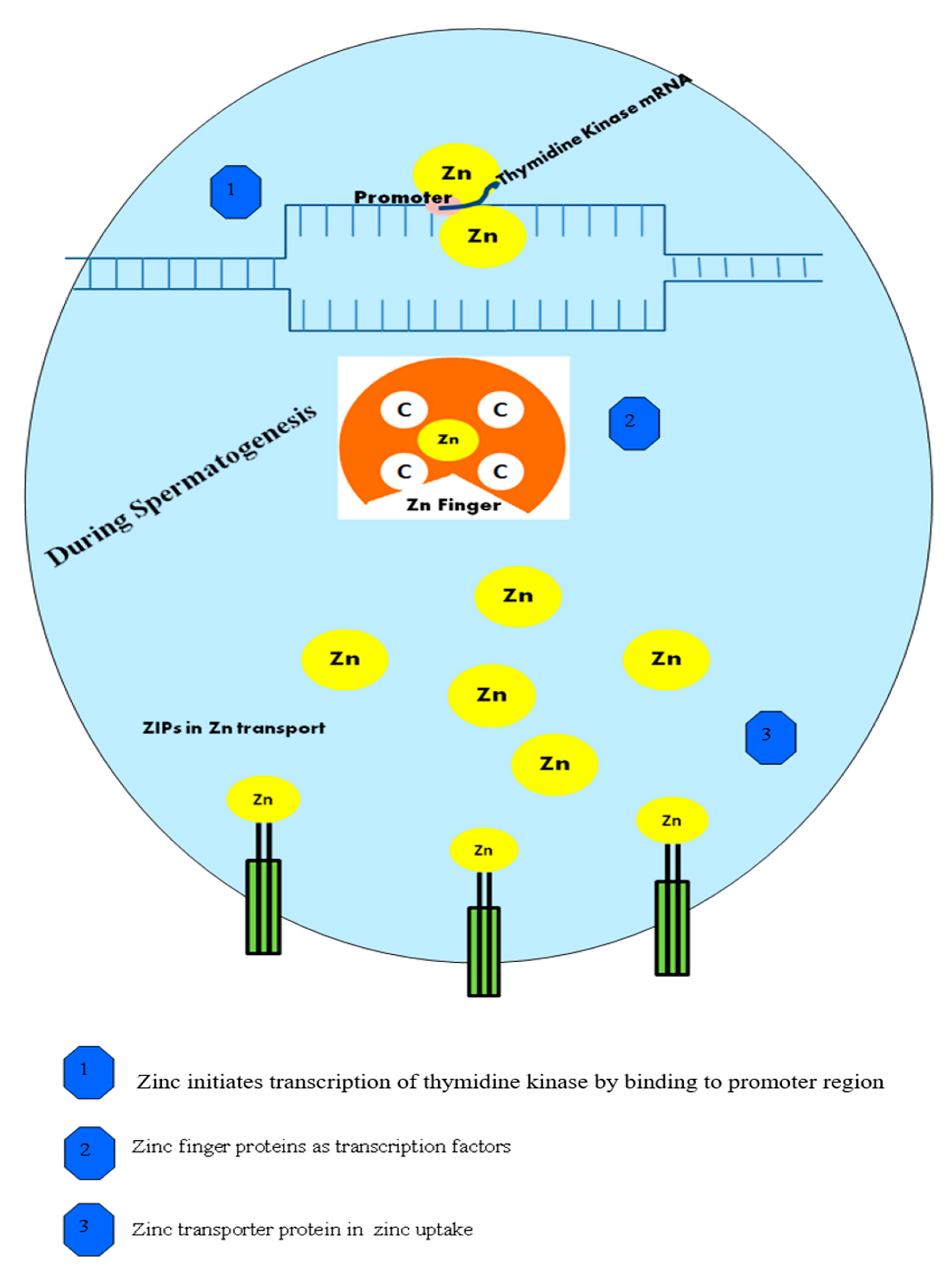
3. Role of Zn in Normal Spermatogenesis
4. Action of Zn in the Testes Phase
5. Significance of Zn in the Prostate
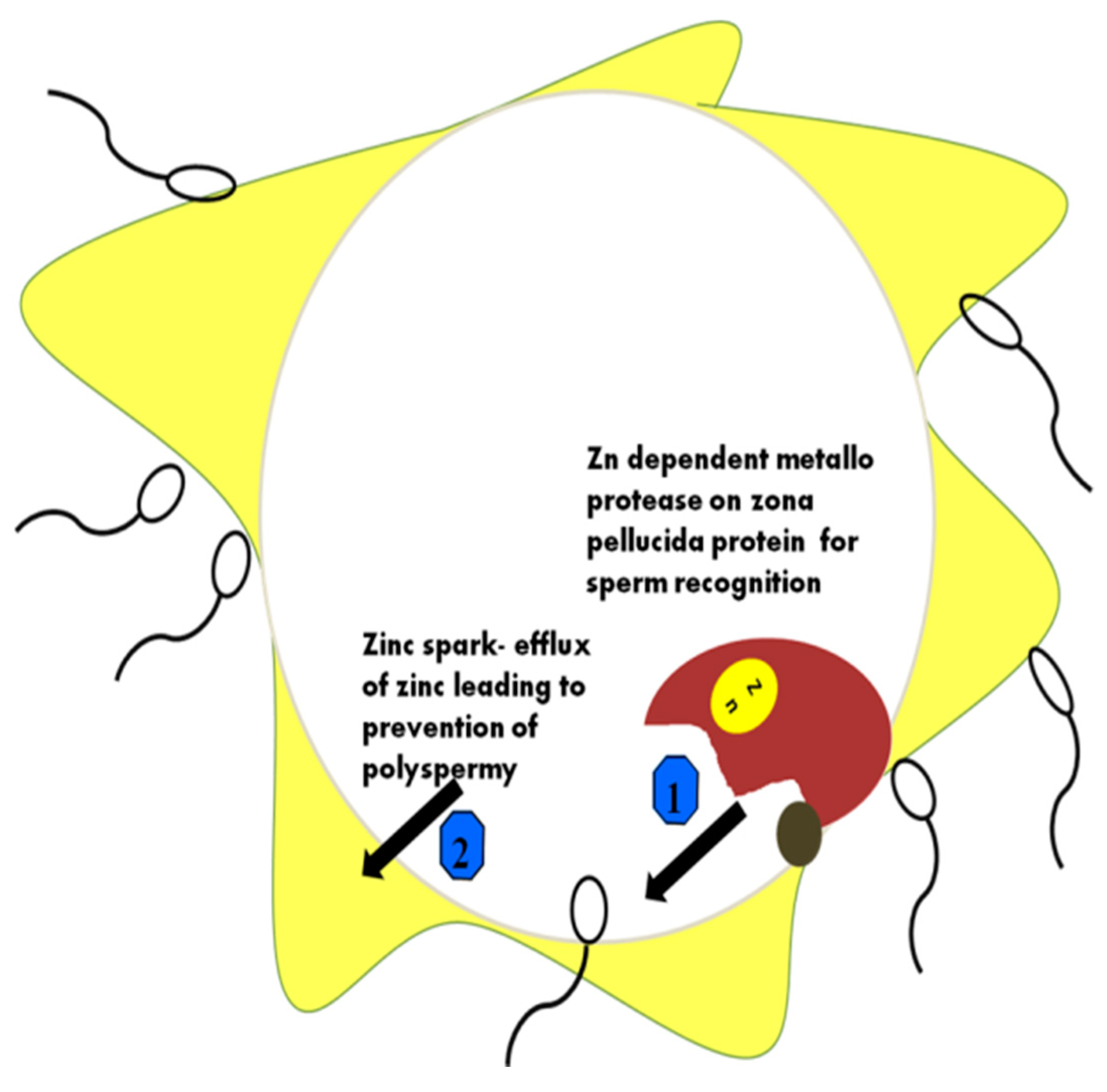
6. The Mechanism of Action of Zn in Capacitation
7. Mechanism of Zn in Human Seminal Vesicles
8. Role of Zn in Major Sex Hormones
9. Role of Zn in Prostasomes and Sperm-Binding Activity
10. Role of Zn in Anti-Cell Death and Anti-Apoptosis
11. Zn and Its Significance in Estrogen
12. Zn as a Regulator in the Female Reproductive Tract
13. Zn Supplementation for Male Fertility
14. Roles of Zn in Maternal, Perinatal, and Postnatal Healthcare
15. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69. [Google Scholar] [PubMed]
- Shukla, A.K.; Tiwari, P.K.; Pakhare, A.; Prakash, C. Zinc and iron in soil, plant, animal and human health. Indian J. Fertil. 2016, 12, 133–149. [Google Scholar]
- Prasad, A.S. Trace metals in growth and sexual maturation. In Metabolism of Trace Metals in Man Volume I (1984): Developmental Aspects; CRC Press: New York, NY, USA, 2017. [Google Scholar]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kovacik, A.; Tirpak, F.; Tomka, M.; Miskeje, M.; Tvrda, E.; Arvay, J.; Fik, M. Trace elements content in semen and their interactions with sperm quality and RedOx status in freshwater fish Cyprinus carpio: A correlation study. J. Trace Elem. Med. Biol. 2018, 50, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Harchegani, A.B. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Nadjarzadeh, A.; Mehrsai, A.; Mostafavi, E.; Gohari, M.R.; Shidfar, F. The association between dietary antioxidant intake and semen quality in infertile men. Med. J. Islamic Repub. Iran 2013, 27, 204. [Google Scholar]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells–immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Gandhi, J.; Hernandez, R.J.; Chen, A.; Smith, N.L.; Sheynkin, Y.R.; Joshi, G.; Khan, S.A. Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote 2017, 25, 103–110. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41. [Google Scholar] [CrossRef]
- Brazdova, A. Study of Immunological Properties of Sperm and Seminal Plasma Antigens: Anti-Seminal and Anti-Sperm Antibodies in Female Immune Infertility: Characterization of Targeted Proteins. Doctoral dissertation, Universite Pierre et Marie Curie-Paris VI, Paris, France, 2014. [Google Scholar]
- Egwurugwu, J.N.; Ifedi, C.U.; Uchefuna, R.C.; Ezeokafor, E.N.; Alagwu, E.A. Effects of zinc on male sex hormones and semen quality in rats. Niger. J. Physiol. Sci. 2013, 28, 17–22. [Google Scholar] [PubMed]
- Roscioli, E.; Hamon, R.; Lester, S.; Murgia, C.; Grant, J.; Zalewski, P. Zinc-rich inhibitor of apoptosis proteins (IAPs) as regulatory factors in the epithelium of normal and inflamed airways. Biometals 2013, 26, 205–227. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Almashhedy, L.A.; Alsalman, A.S. The key role of zinc in enhancement of total antioxidant levels in spermatozoa of patients with asthenozoospermia. Am. J. Respir. Cell Mol. Biol. 2013, 1, 52–61. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Garolla, A.; Cosci, I.; Menegazzo, M.; Ferigo, M.; Gandin, V.; De Toni, L. Role of zinc trafficking in male fertility: From germ to sperm. Hum. Reprod. 2014, 29, 1134–1145. [Google Scholar] [CrossRef]
- Babaei, H.; Abshenas, J. Zinc therapy improves adverse effects of long term administration of copper on epididymal sperm quality of rats. Iran. J. Reprod. Med. 2013, 11, 577–582. [Google Scholar]
- Kambe, T.; Hashimoto, A.; Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014, 71, 3281–3295. [Google Scholar] [CrossRef]
- Vickram, A.S.; Das, R.; Srinivas, M.S.; Rao, K.A.; Jayaraman, G.; Sridharan, T.B. Prediction of Zn concentration in human seminal plasma of Normospermia samples by Artificial Neural Networks (ANN). J. Assist. Reprod. Genet. 2013, 30, 453–459. [Google Scholar]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2013, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Duncan, F.E.; Que, E.L.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 2014, 20, 1077–1089. [Google Scholar] [CrossRef]
- Ellis, R.E.; Stanfield, G.M. The regulation of spermatogenesis and sperm function in nematodes. Semin. Cell Dev. Biol. 2014, 29, 17–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Zhai, X.; Dai, J.; Jiang, X.; Wang, G.; Li, W.; Cai, L. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol. Mech. Methods 2013, 23, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Hou, J.; Liu, Y.; Liu, Y.; Yang, H.; Li, Z.; He, Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 66–75. [Google Scholar] [CrossRef]
- Bradbury, N.A. All cells have a sex: Studies of sex chromosome function at the cellular level. In Principles of Gender-Specific Medicine; Academic Press: Cambridge, MA, USA, 2017; pp. 269–290. [Google Scholar]
- Olesen, I.A.; Joensen, U.N.; Petersen, J.H.; Almstrup, K.; Rajpert-De Meyts, E.; Carlsen, E.; McLachlan, R.; Juul, A.; Jørgensen, N. Decrease in semen quality and Leydig cell function in infertile men: A longitudinal study. Hum. Reprod. 2018, 33, 1963–1974. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Azemi, M.K.; Al-Maghrebi, M.; Mathew, C.T.; Omu, F.E.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Memon, A. Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-dawley rat model. Indian J. Urol. IJU: J. Urol. Soc. India 2015, 31, 57–64. [Google Scholar]
- Walczak–Jedrzejowska, R.; Wolski, J.K.; Slowikowska–Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60. [Google Scholar] [CrossRef]
- Bolanca, I.; Obhodas, J.; Ljiljak, D.; Matjacic, L.; Kuna, K. Synergetic effects of K, Ca, Cu and Zn in human semen in relation to parameters indicative of spontaneous hyperactivation of spermatozoa. PLoS ONE 2016, 11, e0152445. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Ohtsuka, E.; Inoue, A.; Odaka, M.; Ohshima, H.; Tamura, N.; Yoshida, K.; Sako, N.; Baba, T.; Kashiwabara, S.; et al. Abnormal spermatogenesis and male infertility in testicular zinc finger protein Zfp318-knockout mice. Dev. Growth Differ. 2016, 58, 600–608. [Google Scholar] [CrossRef]
- Ecco, G.; Imbeault, M.; Trono, D. KRAB zinc finger proteins. Development 2017, 144, 2719–2729. [Google Scholar] [CrossRef]
- Lim, K.H.; Park, S.G. Transcriptional regulation of KRAB-ZFPs in cancer. Mol. Cell. Toxicol. 2015, 11, 389–394. [Google Scholar] [CrossRef]
- Harchegani, A.B.; Dahan, H.; Tahmasbpour, E.; Shahriary, A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum. Fertil. 2020, 23, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Converse, A.; Berg, H.A. ZIP9, a novel membrane androgen receptor and zinc transporter protein. Gen. Comp. Endocrinol. 2018, 257, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Karweina, D.; Kreuzer-Redmer, S.; Müller, U.; Franken, T.; Pieper, R.; Baron, U.; Olek, S.; Zentek, J.; Brockmann, G.A. The zinc concentration in the diet and the length of the feeding period affect the methylation status of the ZIP4 zinc transporter gene in piglets. PLoS ONE 2015, 10, e0143098. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.R.; Madhu, P.; Reddy, K.P.; Reddy, P.S. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol. Ind. Health 2017, 33, 265–276. [Google Scholar] [CrossRef]
- Chemek, M.; Mimouna, S.B.; Boughammoura, S.; Delbès, G.; Messaoudi, I. Protective role of zinc against the toxicity induced by exposure to cadmium during gestation and lactation on testis development. Reprod. Toxicol. 2016, 63, 151–160. [Google Scholar] [CrossRef]
- Torabi, F.; Shafaroudi, M.M.; Rezaei, N. Combined protective effect of zinc oxide nanoparticles and melatonin on cyclophosphamide-induced toxicity in testicular histology and sperm parameters in adult Wistar rats. Int. J. Reprod. Biomed. 2017, 15, 403–412. [Google Scholar] [CrossRef]
- Tirabassi, G.; Biagioli, A.; Balercia, G. Bone benefits of testosterone replacement therapy in male hypogonadism. Panminerva Med. 2014, 56, 151–163. [Google Scholar]
- Sarwar, N.; Ishaq, W.; Farid, G.; Shaheen, M.R.; Imran, M.; Geng, M.; Hussain, S. Zinc–cadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotoxicol. Environ. Saf. 2015, 122, 528–536. [Google Scholar] [CrossRef]
- Bayer, A.R. Zinc Dynamics during Murine Gamete and Embryo Development. Ph.D. Thesis, Northwestern University, Chicago, IL, USA, 2018. [Google Scholar]
- Lee, Y.A.; Kim, Y.H.; Ha, S.J.; Kim, K.J.; Kim, B.J.; Kim, B.G.; Choi, S.-H.; Kim, I.-C.; Schmidt, J.A.; Ryu, B.Y. Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose. J. Anim. Sci. 2014, 92, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.V.C.; Lo, S.T.; Chen, S.; Preihs, C.; Chirayil, S.; Zhang, S.; Kapur, P.; Li, W.-H.; De Leon-Rodriguez, L.M.; Lubag, A.J.M.; et al. Zinc-sensitive MRI contrast agent detects differential release of Zn (II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. USA 2016, 113, E5464–E5471. [Google Scholar] [CrossRef] [PubMed]
- Niwas Jangir, R.; Chand Jain, G. Diabetes mellitus induced impairment of male reproductive functions: A review. Curr. Diabetes Rev. 2014, 10, 147–157. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Thévenod, F.; Lee, W.K. Toxicology of cadmium and its damage to mammalian organs. In Cadmium: From Toxicity to Essentiality; Springer: Dordrecht, The Netherlands, 2013; Volume 11, pp. 415–490. [Google Scholar]
- Verze, P.; Cai, T.; Lorenzetti, S. The role of the prostate in male fertility, health and disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef]
- Prashanth, L.; Kattapagari, K.K.; Chitturi, R.T.; Baddam, V.R.R.; Prasad, L.K. A review on role of essential trace elements in health and disease. J. Dr. NTR Univ. Health Sci. 2015, 4, 75–85. [Google Scholar]
- Agarwal, A.; Durairajanayagam, D.; Halabi, J.; Peng, J.; Vazquez-Levin, M. Proteomics, oxidative stress and male infertility. Reprod. Biomed. Online 2014, 29, 32–58. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Rametse, C.L.; Olivier, A.J.; Masson, L.; Barnabas, S.; McKinnon, L.R.; Ngcapu, S.; Liebenberg, L.J.; Jaumdally, S.Z.; Gray, C.M.; Jaspan, H.B.; et al. Role of semen in altering the balance between inflammation and tolerance in the female genital tract: Does it contribute to HIV risk? Viral Immunol. 2014, 27, 200–206. [Google Scholar] [CrossRef]
- Jeng, H.A.; Huang, Y.L.; Pan, C.H.; Diawara, N. Role of low exposure to metals as male reproductive toxicants. Int. J. Environ. Health Res. 2015, 25, 405–417. [Google Scholar] [CrossRef]
- Franz, M.C.; Anderle, P.; Bürzle, M.; Suzuki, Y.; Freeman, M.R.; Hediger, M.A.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Shusterman, E.; Beharier, O.; Shiri, L.; Zarivach, R.; Etzion, Y.; Campbell, C.R.; Lee, I.-H.; Okabayashi, K.; Dinudom, A.; Cook, D.I.; et al. ZnT-1 extrudes zinc from mammalian cells functioning as a Zn2+ /H+ exchanger. Metallomics 2014, 6, 1656–1663. [Google Scholar] [CrossRef]
- Gangwar, D.K.; Atreja, S.K. Signalling events and associated pathways related to the mammalian sperm capacitation. Reprod. Domest. Anim. 2015, 50, 705–711. [Google Scholar] [CrossRef]
- Mendoza, A.D.; Woodruff, T.K.; Wignall, S.M.; O’Halloran, T.V. Zinc availability during germline development impacts embryo viability in Caenorhabditis elegans. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 194–202. [Google Scholar] [CrossRef]
- Seredenina, T.; Demaurex, N.; Krause, K.H. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid. Redox Signal. 2015, 23, 490–513. [Google Scholar] [CrossRef]
- González-Fernández, L.; Macías-García, B.; Loux, S.C.; Varner, D.D.; Hinrichs, K. Focal adhesion kinases and calcium/calmodulin-dependent protein kinases regulate protein tyrosine phosphorylation in stallion sperm. Biol. Reprod. 2013, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, M.V.; Salazar, I.L.; Curcio, M.; Canzoniero, L.M.; Duarte, C.B. Role of the ubiquitin–proteasome system in brain ischemia: Friend or foe? Prog. Neurobiol. 2014, 112, 50–69. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; Lotti, F.; Favilla, V.; Morgia, G.; Calogero, A.E. Seminal vesicles and diabetic neuropathy: Ultrasound evaluation after prolonged treatment with a selective phosphodiesterase-5 inhibitor. Andrology 2013, 1, 245–250. [Google Scholar] [CrossRef]
- Stasinou, T.; Bourdoumis, A.; Owegie, P.; Kachrilas, S.; Buchholz, N.; Masood, J. Calcification of the vas deferens and seminal vesicles: A review. Can. J. Urol. 2015, 22, 7594–7598. [Google Scholar] [PubMed]
- Puppo, V.; Puppo, G. Comprehensive review of the anatomy and physiology of male ejaculation: Premature ejaculation is not a disease. Clin. Anat. 2016, 29, 111–119. [Google Scholar] [CrossRef]
- Roan, N.R.; Liu, H.; Usmani, S.M.; Neidleman, J.; Müller, J.A.; Avila-Herrera, A.; Gawanbacht, A.; Zirafi, O.; Chu, S.; Dong, M.; et al. Liquefaction of semen generates and later degrades a conserved semenogelin peptide that enhances HIV infection. J. Virol. 2014, 88, 7221–7234. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Gokul, S.; Agarwal, A. Semen hyperviscosity: Causes, consequences, and cures. Front. Biosci. 2013, 5, 224–231. [Google Scholar]
- Silverberg, K.M.; Turner, T. Evaluation of sperm. In Textbook of Assisted Reproductive Techniques: Volume 1: Laboratory Perspectives; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hamad, A.W.R.; Al-Daghistani, H.I.; Shquirat, W.D.; Abdel-Dayem, M.; Al-Swaifi, M. Sodium, potassium, calcium and copper levels in seminal plasma are associated with sperm quality in fertile and infertile men. Biochem Pharm. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Boshoff, N.H. The Influence of Genotype on Sperm Motility and Sperm Head Morphometry of Merino (Ovis aries) Sheep. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Barak, S.; Baker, H.W.G. Clinical Management of Male Infertility; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kaltsas, G., et al., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279160/ (accessed on 20 February 2021).
- Marques, P.I.F. An Evolutionary Perspective into the Role Of Kallikreins (KLKs) in Male Reproductive Biology; Universidade do Porto (Portugal), ProQuest Dissertations Publishing: Porto, Portugal, 2016. [Google Scholar]
- Peterson, M.P.; Rosvall, K.A.; Taylor, C.A.; Lopez, J.A.; Choi, J.H.; Ziegenfus, C.; Tang, H.; Colbourne, J.K.; Ketterson, E.D.; Tang, H.; et al. Potential for sexual conflict assessed via testosterone-mediated transcriptional changes in liver and muscle of a songbird. J. Exp. Biol. 2014, 217, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Teerds, K.J.; Huhtaniemi, I.T. Morphological and functional maturation of Leydig cells: From rodent models to primates. Hum. Reprod. Update 2015, 21, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Chi, Z.H.; Zhang, X.; Liang, D.; Wang, X.; Zhao, Y.; Zhang, L.; Zhang, P. A potential role for zinc transporter 7 in testosterone synthesis in mouse Leydig tumor cells. Int. J. Mol. Med. 2016, 37, 1619–1626. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S. Thyroid disorders and semen quality. Biomed. Pharmacol. J. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Maremanda, K.P.; Khan, S.; Jena, G. Zinc protects cyclophosphamide-induced testicular damage in rat: Involvement of metallothionein, tesmin and Nrf2. Biochem. Biophys. Res. Commun. 2014, 445, 591–596. [Google Scholar] [CrossRef]
- Acharyya, S. Inflammation and Ageing: Probable role in Male infertility. Chem. Biol. Lett. 2020, 7, 99–112. [Google Scholar]
- Carruthers, M. Testosterone deficiency syndrome: Cellular and molecular mechanism of action. Curr. Aging Sci. 2013, 6, 115–124. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Aalberts, M.; Stout, T.A.; Stoorvogel, W. Prostasomes: Extracellular vesicles from the prostate. Reproduction 2014, 147, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, M.; Sostaric, E.; Wubbolts, R.; Wauben, M.W.; Nolte, E.N.; Gadella, B.M.; Stout, T.A.; Stoorvogel, W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.S.; Samad, H.A.; Latheef, S.K.; Chakraborty, S.; Dhama, K.; Sridharan, T.B.; Sundaram, T.; Gulothungan, G. Human prostasomes an extracellular vesicle–Biomarkers for male infertility and prostrate cancer: The journey from identification to current knowledge. Int. J. Biol. Macromol. 2020, 146, 946–958. [Google Scholar] [CrossRef]
- Liu, Y.; Batchuluun, B.; Ho, L.; Zhu, D.; Prentice, K.J.; Bhattacharjee, A.; Pourasgari, F.; Hardy, A.B.; Taylor, K.M.; Gaisano, H. Characterization of Zinc Influx Transporters (ZIPs) in Pancreatic β Cells Roles in Regulating Cytosolic Zinc Homeostasis and Insulin Secretion. J. Biol. Chem. 2015, 290, 18757–18769. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Van Saen, D.; Tournaye, H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Hum. Reprod. 2013, 28, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Quinn, B.A.; Das, S.K.; Dash, R.; Emdad, L.; Dasgupta, S.; Wang, X.-Y.; Dent, P.; Reed, J.C.; Pellecchia, M.; et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, D.; Guo, B.; Deng, W.; Chi, Z.H.; Cai, Y.; Wang, L.; Ma, J. Zinc transporter 5 and zinc transporter 7 induced by high glucose protects peritoneal mesothelial cells from undergoing apoptosis. Cell. Signal. 2013, 25, 999–1010. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Evgeni, E.; Charalabopoulos, K.; Asimakopoulos, B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J. Reprod. Infertil. 2014, 15, 2. [Google Scholar]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 1–14. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- McCord, M.C.; Aizenman, E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhou, X.; Cao, Y.; Li, C. Preventive effects of supplemental dietary zinc on heat-induced damage in the epididymis of boars. J. Therm. Biol. 2017, 64, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Queiroz-Hazarbassanov, N.; Bernardi, M.M.; Felicio, L.F. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015, 130, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Barsony, J.; Manigrasso, M.B.; Xu, Q.; Tam, H.; Verbalis, J.G. Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats. Age 2013, 35, 271–288. [Google Scholar] [CrossRef]
- Garcia Barros, R.; Franciosi, F.; Dall Acqua, P.; Dieci, C.; Lodde, V.; Luciano, A. Zinc supplementation during in vitro culture of bovine growing oocytes. In Proceedings of the Technologies and controversies in reproduction. International conference proceedings; 2019. Available online: https://fertilityconference.org/wp-content/uploads/2018/12/Fertility_2019_abstracts_book-updated (accessed on 20 February 2021).
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef]
- Prasad, A.S. Biochemistry of Zinc; Springer Science & Business Media: New York, NY, USA, 2013; Volume 11. [Google Scholar]
- Dietzel, E.; Wessling, J.; Floehr, J.; Schäfer, C.; Ensslen, S.; Denecke, B.; Rösing, B.; Neulen, J.; Veitinger, T.; Spehr, M.; et al. Fetuin-B, a liver-derived plasma protein is essential for fertilization. Dev. Cell 2013, 25, 106–112. [Google Scholar] [CrossRef]
- Karmilin, K.; Schmitz, C.; Kuske, M.; Körschgen, H.; Olf, M.; Meyer, K.; Hildebrand, A.; Felten, M.; Fridrich, S.; Yiallouros, I.; et al. Mammalian plasma fetuin-B is a selective inhibitor of ovastacin and meprin metalloproteinases. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Roy, B.; Baghel, R.P.S.; Mohanty, T.K.; Mondal, G. Zinc and male reproduction in domestic animals: A Review. Indian J. Anim. Nutr. 2013, 30, 339–350. [Google Scholar]
- Liang, J.; Shang, Y. Estrogen and cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef]
- Holt, W.V.; Fazeli, A. Do sperm possess a molecular passport? Mechanistic insights into sperm selection in the female reproductive tract. MHR: Basic Sci. Reprod. Med. 2015, 21, 491–501. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Jones, S.L.; Flanagan-Cato, L.M.; Blaustein, J.D. Female sexual behavior. In Physiology of Reproduction; Elsevier: New York, NY, USA, 2015; pp. 2287–2370. [Google Scholar]
- Yeste, M.; Jones, C.; Amdani, S.N.; Patel, S.; Coward, K. Oocyte activation deficiency: A role for an oocyte contribution? Hum. Reprod. Update 2016, 22, 23–47. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, C. Biological basis for human capacitation—revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Bleher, R.; Duncan, F.E.; Kong, B.Y.; Gleber, S.C.; Vogt, S.; Chen, S.; Garwin, S.A.; Bayer, A.R.; Dravid, V.P.; et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 2019, 7, 130–139. [Google Scholar] [CrossRef]
- Yi, Y.J.; Sutovsky, M.; Song, W.H.; Sutovsky, P. Protein deubiquitination during oocyte maturation influences sperm function during fertilisation, antipolyspermy defense and embryo development. Reprod. Fertil. Dev. 2015, 27, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Wright, G.J. Izumo meets Juno: Preventing polyspermy in fertilization. Cell Cycle 2014, 13, 2019–2020. [Google Scholar] [CrossRef] [PubMed]
- Powrie, E.A.; Ciocanel, V.; Kreiling, J.A.; Gagnon, J.A.; Sandstede, B.; Mowry, K.L. Using in vivo imaging to measure RNA mobility in Xenopus laevis oocytes. Methods 2016, 98, 60–65. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.J.; Zhu, M.; Fan, T.T.; Zhou, D.M.; Phillips, B.L.; Sparks, D.L. Inhibition mechanisms of Zn precipitation on aluminum oxide by glyphosate: A 31P NMR and Zn EXAFS study. Environ. Sci. Technol. 2013, 47, 4211–4219. [Google Scholar] [CrossRef]
- Hamad, A.M. Molecular and Physical Interactions of Human Sperm with Female Tract Secretions. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2017. [Google Scholar]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm cohort-specific zinc signature acquisition and capacitation-induced zinc flux regulate sperm-oviduct and sperm-zona pellucida interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef]
- Da Ros, V.G.; Muñoz, M.W.; Battistone, M.A.; Brukman, N.G.; Carvajal, G.; Curci, L.; Gómez-Elías, M.D.; Cohen, D.J.; Cuasnicu, P.S. From the epididymis to the egg: Participation of CRISP proteins in mammalian fertilization. Asian J. Androl. 2015, 17, 711. [Google Scholar]
- Robertson, S.A.; Sharkey, D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016, 106, 511–519. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, H.J.; Wang, H.L.; Wang, J.W.; Gou, P.H.; Ye, Z.H.; Wang, Y.L. Influence of hypothyroidism on oxidative stress, c-Fos expression, cell cycle, and apoptosis in rats testes. Toxicol. Environ. Chem. 2015, 97, 1394–1407. [Google Scholar] [CrossRef]
- Palmer, B.F. Sexual Dysfunction in Men and Women with Chronic Kidney Disease and end-stage kidney disease. Adv. Ren. Replace. 2003, 10, 48–60. [Google Scholar] [CrossRef]
- Aarabi, M.; San Gabriel, M.C.; Chan, D.; Behan, N.A.; Caron, M.; Pastinen, T.; Bourque, G.; MacFarlane, A.J.; Zini, A.; Trasler, J. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum. Mol. Genet. 2015, 24, 6301–6313. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lv, H.; Chen, Z.; Wang, L.; Wu, X.; Chen, Z.; Zhang, W.; Liang, R.; Jiang, Z. Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejunal gene expression in weaned piglets. Biol. Trace Elem. Res. 2017, 175, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wendlova, J. Progression of the erectile dysfunction in the population and the possibilities of its regression with bioregeneration. Neuroendocrinol. Lett. 2013, 34, 482–497. [Google Scholar]
- Grieger, J.A.; Clifton, V.L. A review of the impact of dietary intakes in human pregnancy on infant birthweight. Nutrients 2015, 7, 153–178. [Google Scholar] [CrossRef]
- Sengupta, P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem. Toxicol. 2013, 36, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Feki-Tounsi, M.; Hamza-Chaffai, A. Cadmium as a possible cause of bladder cancer: A review of accumulated evidence. Environ. Sci. Pollut. Res. 2014, 21, 10561–10573. [Google Scholar] [CrossRef]
- Lehtonen, L.; Gimeno, A.; Parra-Llorca, A.; Vento, M. Early neonatal death: A challenge worldwide. Semin. Fetal Neonatal Med. 2017, 22, 153–160. [Google Scholar] [CrossRef]
- Mridha, M.K.; Matias, S.L.; Chaparro, C.M.; Paul, R.R.; Hussain, S.; Vosti, S.A.; Harding, K.L.; Cummins, J.R.; Day, L.T.; Saha, S.L.; et al. Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am. J. Clin. Nutr. 2016, 103, 236–249. [Google Scholar] [CrossRef]
- Deshpande, J.D.; Joshi, M.M.; Giri, P.A. Zinc: The trace element of major importance in human nutrition and health. Int. J. Med. Sci. Public Health 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Young, S.D.; Briley, A.L.; Broughton Pipkin, F.; Baker, P.N.; Poston, L. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Matern. Child Nutr. 2014, 10, 327–334. [Google Scholar] [CrossRef]
- Fabunmi, T.M.; Onabanjo, O.O.; Oguntona, E.B.; Keshinro, O.O.; Onabanjo, J.A.; Obanla, O.O.; Oyawoye, O.O. Nutrient intakes and nutritional status of mothers and their under-five children in a rural community of Oyo state, Nigeria. Int. J. Child Health Nutr. 2013, 2, 39–49. [Google Scholar]
- Hooper, L.; Bunn, D.; Jimoh, F.O.; Fairweather-Tait, S.J. Water-loss dehydration and aging. Mech. Ageing Dev. 2014, 136, 50–58. [Google Scholar] [CrossRef]
- Jyotsna, S.; Amit, A.; Kumar, A. Study of serum zinc in low birth weight neonates and its relation with maternal zinc. J. Clin. Diagn. Res. 2015, 9, SC01–SC03. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I.; Mkparu, U.C. Micronutrients in pregnancy in low-and middle-income countries. Nutrients 2015, 7, 1744–1768. [Google Scholar] [CrossRef] [PubMed]
- Tadi, K.K.; Alshanski, I.; Mervinetsky, E.; Marx, G.; Petrou, P.; Dimitrios, K.; Yitzchaik, S. Oxytocin-monolayer-based impedimetric biosensor for zinc and copper ions. ACS Omega 2017, 2, 8770–8778. [Google Scholar] [CrossRef] [PubMed]
- Parkash, A.; Haider, N.; Khoso, Z.A.; Shaikh, A.S. Frequency, causes and outcome of neonates with respiratory distress admitted to Neonatal Intensive Care Unit, National Institute of Child Health, Karachi. J. Pak. Med. Assoc. 2015, 65, 771–775. [Google Scholar] [PubMed]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of trace elements for oxidative status and quality of human sperm. Balk. Med. J. 2017, 34, 343–348. [Google Scholar] [CrossRef]
- Qu, F.; Ying, X.; Guo, W.; Guo, Q.; Chen, G.; Liu, Y.; Ding, Z. The role of Zn-α2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction 2007, 134, 569–576. [Google Scholar] [CrossRef]
- Saleh, B.O.M. Status of zinc and copper concentrations in seminal plasma of male infertility and their correlation with various sperm parameters. Iraqi Acad. Sci. J. 2008, 7, 76–80. [Google Scholar]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Dissanayake, D.M.A.B.; Wijesinghe, P.S.; Ratnasooriya, W.D.; Wimalasena, S. Relationship between seminal plasma zinc and semen quality in a subfertile population. J. Hum. Reprod. Sci. 2010, 3, 124–128. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaman, S.; Sajjad, M.; Shoaib, M.; Gilani, G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int. J. Appl. Basic Med. Res. 2011, 1, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Hadwan, M.H.; Almashhedy, L.A.; Alsalman, A.R.S. Oral zinc supplementation restore high molecular weight seminal zinc binding protein to normal value in Iraqi infertile men. BMC Urol. 2012, 12, 32. [Google Scholar] [CrossRef]
- Sundaram, V.; Srinivas, M.; Gurunathan, J.; Rao, K.; Maniyan, R.P.; Balasundaram, S. Influence of trace elements and their correlation with semen quality in fertile and infertile subjects. Turk. J. Med. Sci. 2013, 43, 1000–1007. [Google Scholar] [CrossRef]
- Altaher, Y.M.; Abdrabo, A.A. Levels of Zinc and Copper in seminal plasma of Sudanese infertile males. J. Adv. Med. Med. Res. 2015, 5, 533–538. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Zn Role in Human Reproduction and Infertility | Study Conclusion |
|---|---|---|
| Qu et al., 2007 [138] | Zn-α2-glycoprotein, termed ZAG, plays a major action in sperm motility. | ZAG could be present in human semen, and it could help with proper motility as well as with the signaling pathway known as PKA (Protein Kinase A). |
| Saleh, 2008 [139] | Semen contains higher concentrations of Zn and copper than any other body fluid. This helps to maintain sperm quality. | For proper diagnosis of male infertility, Zn and Cu estimation is important. |
| Colagar et al., 2009 [140] | The absence or moderate deficiency of Zn in the seminal plasma leads to increased reactive oxygen species (ROS) and increased oxidative damage, which could result in low sperm quality. | The seminal Zn concentration was found to be significantly positively correlated with sperm count and the normal morphology of sperm. A low or absent Zn intake results in low semen quality and leads to idiopathic male infertility. |
| Dissanayake et al., 2010 [141] | Zn plays major parameter in determining sperm count, normal sperm morphology, and other parameters. | Both the Zn concentration and total amount of Zn per volume of ejaculate were calculated in this study. The total Zn content was termed Zn (T), and it was positively correlated with the sperm count and normal morphology. |
| Khan et al., 2011 [142] | Zn deficiency plays a key act in human male infertility. Zn deficiency is associated with hypogonadism and deficient development of secondary sex characteristics. | Having adequate Zn in the seminal plasma aids in proper sperm functioning. An increased amount of Zn results in decreased sperm motility, but a decreased amount of Zn in the seminal plasma was associated with an increased sperm count. It is very crucial to monitor the Zn content in seminal plasma. |
| Hadwan et al., 2012 [143] | Human seminal Zn is classified into three types of ligands: high, intermediate, and low molecular weight ligands. An increase in the oral supplementation of Zn results in increased sperm motility for asthenospermic patients. | This study concludes that the overall increase in motility in asthenospermic patients following Zn supplementation increases the overall high and low molecular Zn ligand levels. |
| Sundaram et al., 2013 [144] | Zn acts as a cofactor for DNA binding proteins and Zn fingers. | Zn could be the best biochemical marker for major semen anomalies, as well as for the proper diagnosis of human male infertility. |
| Foresta, 2014 [20] | Zn is involved in a number of sperm functions after the post-epididymal phase reaches a maximum. | During the entire lifetime of sperm, Zn trafficking occurs. |
| Altaher and Abdrabo, 2015 [145] | Zn and Cu play major actions in oligospermic and asthenospermic patients. | Zn concentration was significantly lower in cases of azoospermia and oligospermia. |
| Zhao et al., 2016 [146] | Systematic data analysis suggests that Zn concentration is significantly lower than in other fertile groups, which proves the significance of Zn in semen parameters. | Zn supplementation increases the major semen parameters like semen volume, forwarded motility, and normal morphology. |
| Nenkova et al., 2017 [137] | Zn plays a major action in protecting spermatozoa against oxidative stress. | There is evidence that trace elements play an antioxidative role at the time of ejaculation. |
| Fallah et al., 2018 [1] | Zn acts as an antibacterial agent in the female genital tract and even helps in protection from immunological shock. | Zn could be considered as a nutrient marker for male reproductive potential. |
| Mirnamniha et al., 2019 [6] | Zn plays a major function in the incitation of capacitation. | The measurement of Zn in the seminal plasma of idiopathic male infertility is essential. |
| Vickram et al., 2020 [84] | Zn plays a major function in mediating the binding of prostasomes on spermatozoa to transfer essential compounds, which paves the way for fertilization. | Prostasomes are biomarkers for both male infertility and prostate cancer diagnosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021, 22, 2188. https://doi.org/10.3390/ijms22042188
Vickram S, Rohini K, Srinivasan S, Nancy Veenakumari D, Archana K, Anbarasu K, Jeyanthi P, Thanigaivel S, Gulothungan G, Rajendiran N, et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. International Journal of Molecular Sciences. 2021; 22(4):2188. https://doi.org/10.3390/ijms22042188
Chicago/Turabian StyleVickram, Sundaram, Karunakaran Rohini, Subramanian Srinivasan, David Nancy Veenakumari, Kumar Archana, Krishnan Anbarasu, Palanivelu Jeyanthi, Sundaram Thanigaivel, Govindarajan Gulothungan, Nanmaran Rajendiran, and et al. 2021. "Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth" International Journal of Molecular Sciences 22, no. 4: 2188. https://doi.org/10.3390/ijms22042188
APA StyleVickram, S., Rohini, K., Srinivasan, S., Nancy Veenakumari, D., Archana, K., Anbarasu, K., Jeyanthi, P., Thanigaivel, S., Gulothungan, G., Rajendiran, N., & Srikumar, P. S. (2021). Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. International Journal of Molecular Sciences, 22(4), 2188. https://doi.org/10.3390/ijms22042188






