Fibrinogen Replacement Therapy for Traumatic Coagulopathy: Does the Fibrinogen Source Matter?
Abstract
1. Introduction
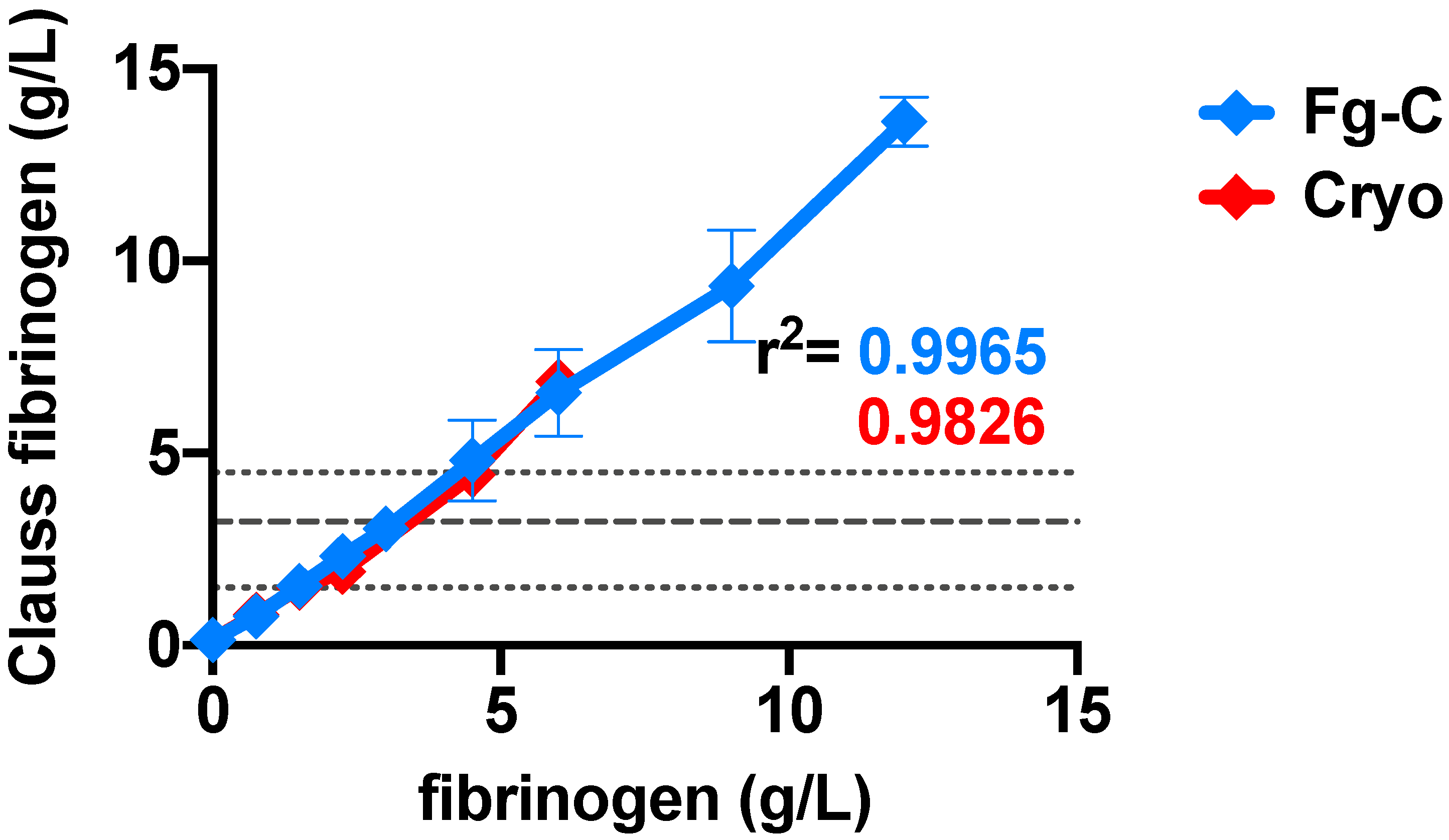
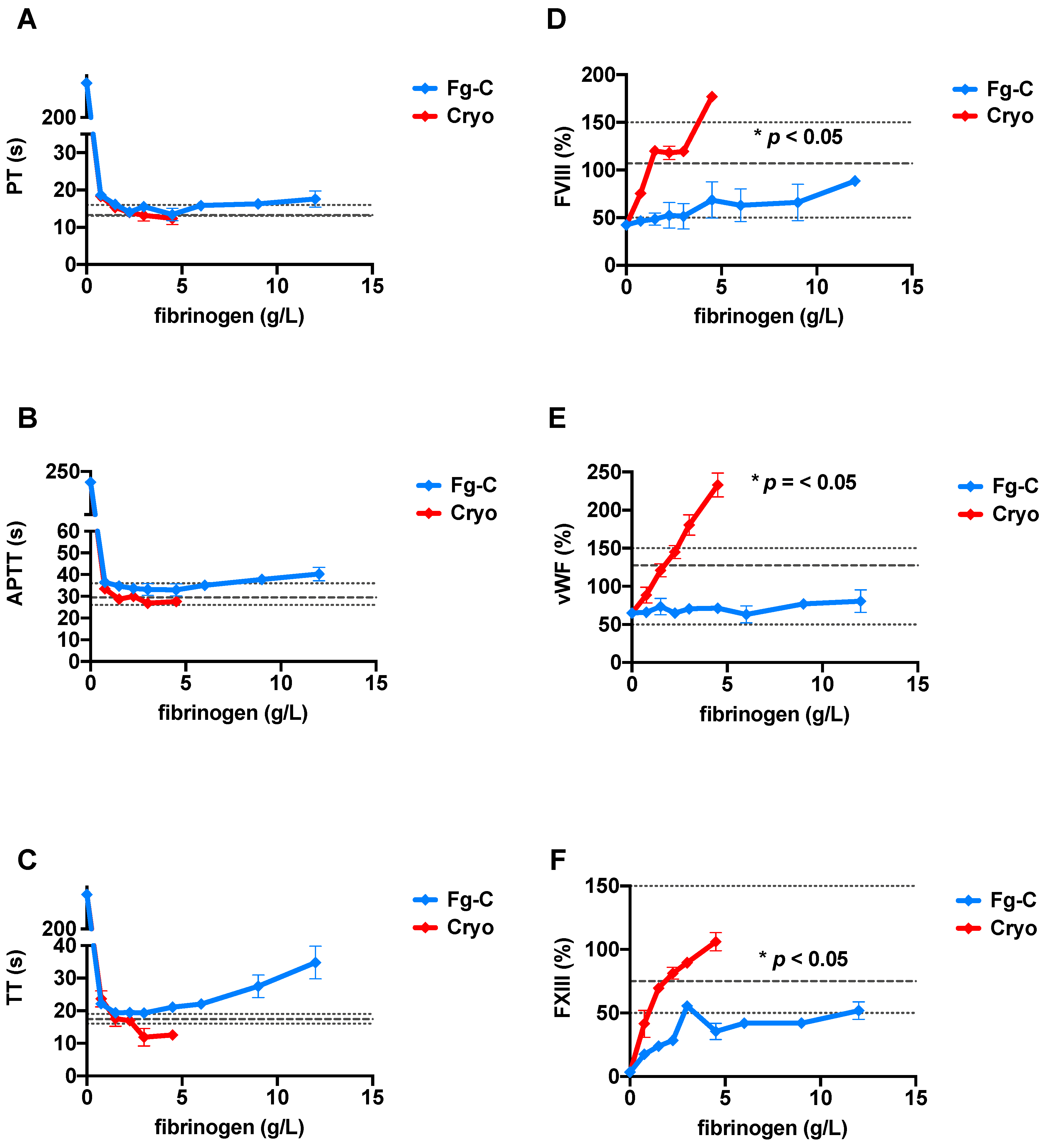
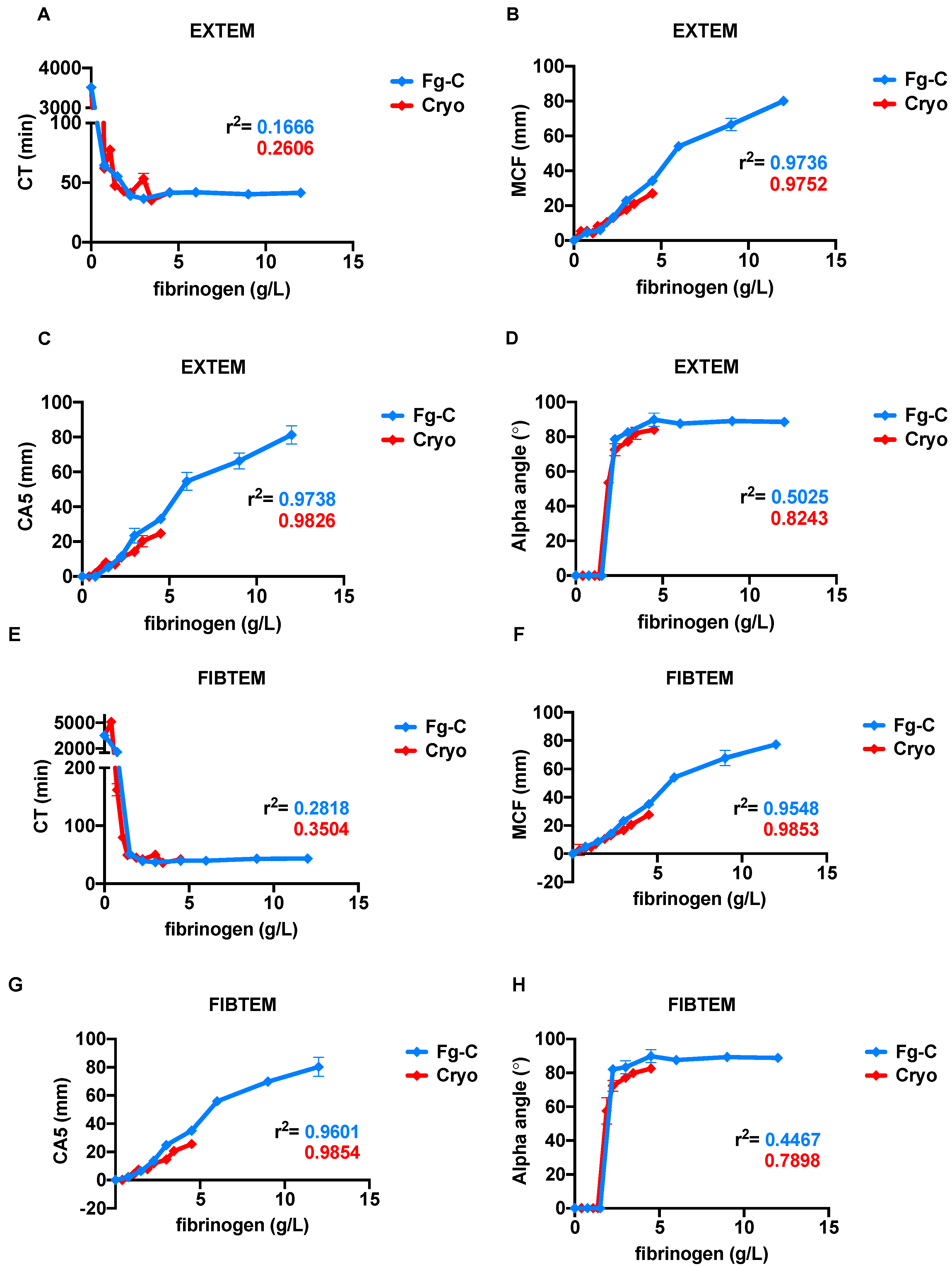
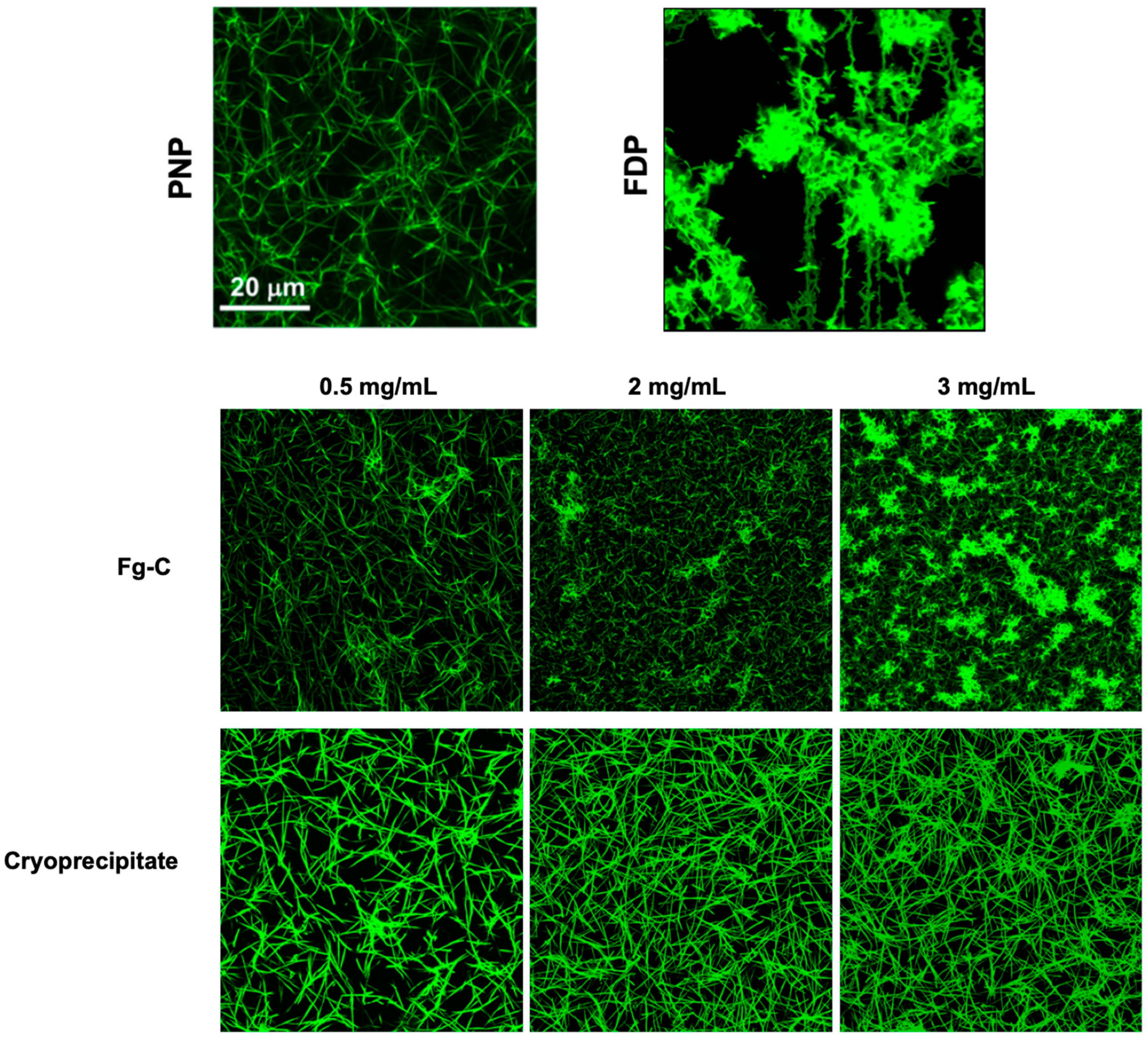
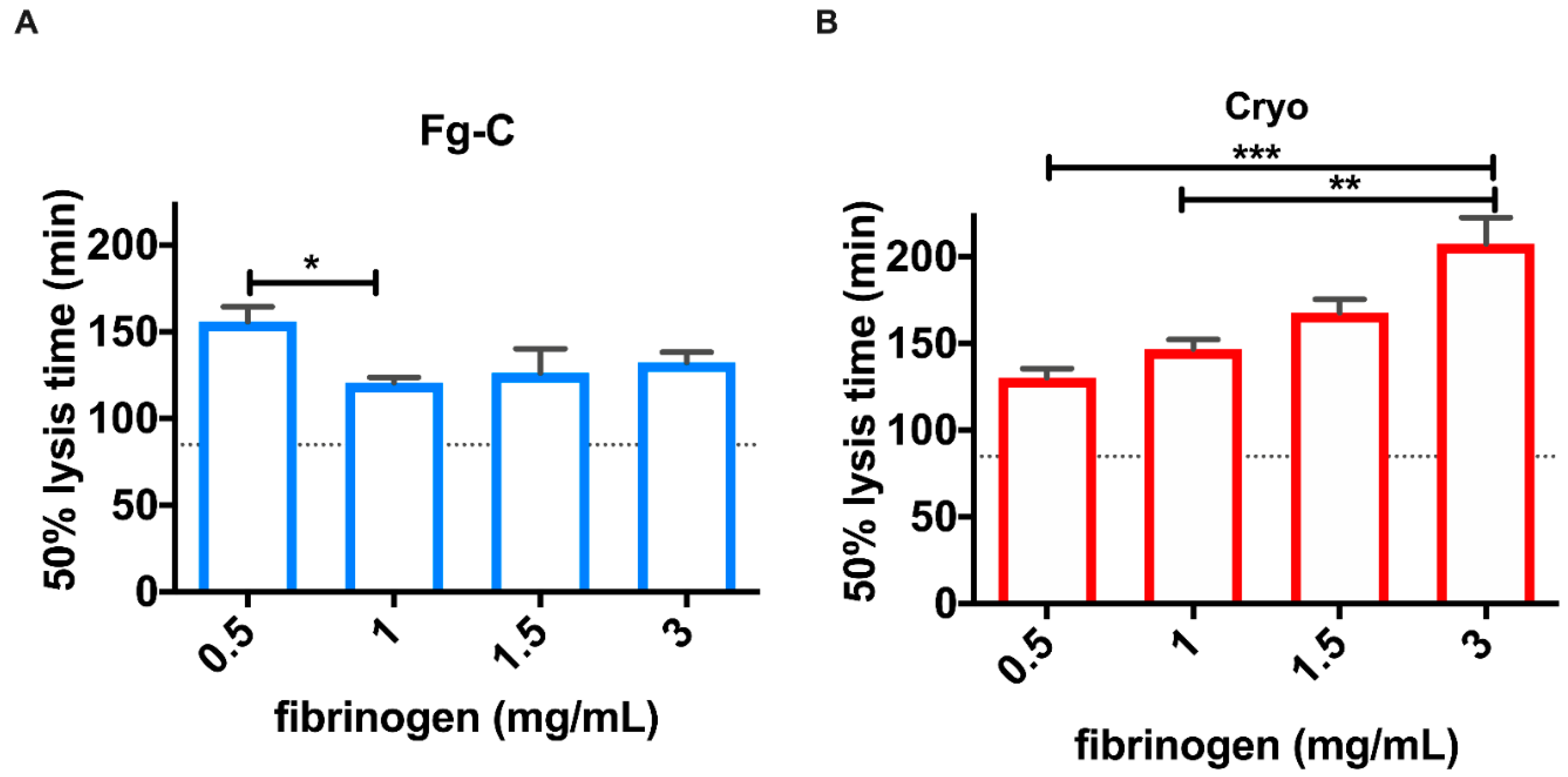
2. Results
3. Discussion
4. Methods
4.1. Fibrinogen Sources
4.2. Standard Laboratory Tests
4.3. ROTEM
4.4. Thrombin Generation
4.5. Confocal Microscopy
4.6. Clot Lysis
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norton, R.; Kobusingye, O. Injuries. N. Engl. J. Med. 2013, 368, 1723–1730. [Google Scholar] [CrossRef]
- Curry, N.; Hopewell, S.; Dorée, C.; Hyde, C.; Brohi, K.; Stanworth, S. The acute management of trauma hemorrhage: A systematic review of randomized controlled trials. Crit. Care 2011, 15, R92. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Del Junco, D.J.; Fox, E.E.; Wade, C.E.; Cohen, M.J.; Schreiber, M.A.; Alarcon, L.H.; Bai, Y.; Brasel, K.J.; Bulger, E.M.; et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study. JAMA Surg. 2013, 148, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.J.; Christie, S.A. New understandings of post injury coagulation and resuscitation. Int. J. Surg. 2016, 33, 242–245. [Google Scholar] [CrossRef]
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute Traumatic Coagulopathy. J. Trauma Inj. Infect. Crit. Care 2003, 54, 1127–1130. [Google Scholar] [CrossRef]
- MacLeod, J.B.A.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early Coagulopathy Predicts Mortality in Trauma. J. Trauma Inj. Infect. Crit. Care 2003, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Maegele, M.; Lefering, R.; Yucel, N.; Tjardes, T.; Rixen, D.; Paffrath, T.; Simanski, C.; Neugebauer, E.; Bouillon, B. Early coagulopathy in multiple injury: An analysis from the German Trauma Registry on 8724 patients. Injury 2007, 38, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, S.T.; Myllyla, G.J.; Vahtera, E.M. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth. Analg. 1995, 81, 360–365. [Google Scholar] [PubMed]
- Floccard, B.; Rugeri, L.; Faure, A.; Denis, M.S.; Boyle, E.M.; Peguet, O.; Levrat, A.; Guillaume, C.; Marcotte, G.; Vulliez, A.; et al. Early coagulopathy in trauma patients: An on-scene and hospital admission study. Injury 2012, 43, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hagemo, J.S.; Stanworth, S.; Juffermans, N.P.; Brohi, K.; Cohen, M.J.; Johansson, P.I.; Røislien, J.; Eken, T.; Næss, P.A.; Gaarder, C. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: A multicentre observational study. Crit. Care 2014, 18, R52. [Google Scholar] [CrossRef]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion Mechanisms in Platelet Function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef]
- Savage, B.; Saldívar, E.; Ruggeri, Z.M. Initiation of Platelet Adhesion by Arrest onto Fibrinogen or Translocation on von Willebrand Factor. Cell 1996, 84, 289–297. [Google Scholar] [CrossRef]
- Law, D.A.; Nannizzi-Alaimo, L.; Phillips, D.R. Outside-in integrin signal transduction. Alpha IIb beta 3-(GP IIb IIIa) tyrosine phosphorylation induced by platelet aggregation. J. Biol. Chem. 1996, 271, 10811–10815. [Google Scholar] [CrossRef]
- Bale, M.D.; Ferry, J.D. Strain enhancement of elastic modulus in fine fibrin clots. Thromb. Res. 1988, 52, 565–572. [Google Scholar] [CrossRef]
- Mockros, L.; Roberts, W.; Lorand, L. Viscoelastic properties of ligation-inhibited fibrin clots. Biophys. Chem. 1974, 2, 164–169. [Google Scholar] [CrossRef]
- Shen, L.; Lorand, L. Contribution of fibrin stabilization to clot strength. Supplementation of factor XIII-deficient plasma with the purified zymogen. J. Clin. Investig. 1983, 71, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Aoki, N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J. Clin. Investig. 1980, 65, 290–297. [Google Scholar] [CrossRef]
- Fraser, S.R.; Booth, N.A.; Mutch, N.J. The antifibrinolytic function of factor XIII is exclusively expressed through α2-antiplasmin cross-linking. Blood 2011, 117, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Rourke, C.; Curry, N.; Khan, S.; Taylor, R.; Raza, I.; Davenport, R.; Stanworth, S.; Brohi, K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J. Thromb. Haemost. 2012, 10, 1342–1351. [Google Scholar] [CrossRef]
- Morrison, J.J.; Ross, J.D.; DuBose, J.J.; Jansen, J.O.; Midwinter, M.J.; Rasmussen, T.E. Association of Cryoprecipitate and Tranexamic Acid With Improved Survival Following Wartime Injury. JAMA Surg. 2013, 148, 218–225. [Google Scholar] [CrossRef]
- Innerhofer, P.; Westermann, I.; Tauber, H.; Breitkopf, R.; Fries, D.; Kastenberger, T.; El Attal, R.; Strasak, A.; Mittermayr, M. The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury 2013, 44, 209–216. [Google Scholar] [CrossRef]
- Marsden, M.; Benger, J.; Brohi, K.; Curry, N.; Foley, C.; Green, L.; Lucas, J.; Rossetto, A.; Stanworth, S.; Thomas, H.; et al. Coagulopathy, cryoprecipitate and CRYOSTAT-2: Realising the potential of a nationwide trauma system for a national clinical trial. Br. J. Anaesth. 2019, 122, 164–169. [Google Scholar] [CrossRef]
- Novak, A.; Stanworth, S.J.; Curry, N. Do we still need cryoprecipitate? Cryoprecipitate and fibrinogen concentrate as treatments for major hemorrhage—How do they compare? Expert Rev. Hematol. 2018, 11, 351–360. [Google Scholar] [CrossRef]
- Curry, N.; Rourke, C.; Davenport, R.; Stanworth, S.; Brohi, K. Fibrinogen replacement in trauma haemorrhage. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, A5. [Google Scholar] [CrossRef]
- Longstaff, C.; Fibrinolysis, T.S.O. Development of Shiny app tools to simplify and standardize the analysis of hemostasis assay data: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2017, 15, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Blombäck, B.; Hogg, D.H.; Gårdlund, B.; Hessel, B.; Kudryk, B. Fibrinogen and fibrin formation. Thromb. Res. 1976, 8, 329–346. [Google Scholar] [CrossRef]
- Rahemeyer, N.; Sorensen, B.S. Fibrinogen concentrate for management of bleeding. J. Thromb. Haemost. 2011, 9, 1–5. [Google Scholar] [CrossRef]
- Martini, W.Z.; Holcomb, J.B. Acidosis and Coagulopathy. Ann. Surg. 2007, 246, 831–835. [Google Scholar] [CrossRef]
- Rossaint, R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernández-Mondéjar, E.; Hunt, B.J.; Komadina, R.; Nardi, G.; Neugebauer, E.; et al. Management of bleeding following major trauma: An updated European guideline. Crit. Care 2010, 14, R52. [Google Scholar] [CrossRef]
- Chowdhury, P.; Saayman, A.G.; Paulus, U.; Findlay, G.P.; Collins, P.W. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br. J. Haematol. 2004, 125, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Davenport, R.; Raza, I.; Glasgow, S.; De’Ath, H.D.; Johansson, P.I.; Curry, N.; Stanworth, S.; Gaarder, C.; Brohi, K. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensiv. Care Med. 2014, 41, 239–247. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef]
- Davenport, R.; Manson, J.; De’ath, H.; Platton, S.; Coates, A.; Allard, S.; Hart, D.; Pearse, R.; Pasi, K.J.; Maccallum, P.; et al. Functional definition and characterization of acute traumatic coagulopathy. Crit. Care Med. 2011, 39, 2652–2658. [Google Scholar] [CrossRef]
- Haas, T.; Fries, D.; Velik-Salchner, C.; Reif, C.; Klingler, A.; Innerhofer, P. The In Vitro Effects of Fibrinogen Concentrate, Factor XIII and Fresh Frozen Plasma on Impaired Clot Formation After 60% Dilution. Anesth. Analg. 2008, 106, 1360–1365. [Google Scholar] [CrossRef]
- Asmis, L.M.; Seifert, B.; Spahn, D.R.; Theusinger, O.M.; Baulig, W. In Vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM®). Thromb. Haemost. 2010, 104, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.H. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J. Biomater. Appl. 1993, 7, 309–352. [Google Scholar] [CrossRef]
- Stief, T.W. Inhibition of Thrombin in Plasma by Heparin or Arginine. Clin. Appl. Thromb. 2007, 13, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A. Thrombin Generation Assay and Its Application in the Clinical Laboratory. Clin. Chem. 2016, 62, 699–707. [Google Scholar] [CrossRef]
- Lisman, T.; Caldwell, S.H.; Burroughs, A.K.; Northup, P.G.; Senzolo, M.; Stravitz, R.T.; Tripodi, A.; Trotter, J.F.; Valla, D.-C.; Porte, R.J. Hemostasis and thrombosis in patients with liver disease: The ups and downs. J. Hepatol. 2010, 53, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.B.; Beavis, J.; Harper, S.; Baker, P.; Desbourough, M.J.R.; Curry, N.; Stanworth, S.J.; Laffan, M.A. Coagulation status of critically ill patients with and without liver disease assessed using a novel thrombin generation analyser. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Butenas, S.; van’t Veer, C.; Mann, K.G. “Normal” thrombin generation. Blood 1999, 94, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Mintz, P.D.; Blatt, P.M.; Kuhns, W.J.; Roberts, H.R. Antithrombin III in fresh frozen plasma, cryoprecipitate, and cryoprecipitate-depleted plasma. Transfusion 1979, 19, 597–598. [Google Scholar] [CrossRef]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef]
- Weisel, J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004, 112, 267–276. [Google Scholar] [CrossRef]
- Carr, M.E.; Alving, B.M. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagul. Fibrinolysis 1995, 6, 567–573. [Google Scholar] [CrossRef]
- Longstaff, C.; Thelwell, C.; Williams, S.C.; Silva, M.M.C.G.; Szabó, L.; Kolev, K. The interplay between tissue plasminogen activator domains and fibrin structures in the regulation of fibrinolysis: Kinetic and microscopic studies. Blood 2011, 117, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Muga, K.; Boothroyd, E. The effect of fibrin structure on fibrinolysis. J. Biol. Chem. 1992, 267, 24259–24263. [Google Scholar] [CrossRef]
- Undas, A.; Ariëns, R.A. Fibrin Clot Structure and Function. Arter. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef]
- La Corte, A.L.C.; Philippou, H.; Ariëns, R.A. Role of Fibrin Structure in Thrombosis and Vascular Disease. Adv. Protein Chem. Struct. Biol. 2011, 83, 75–127. [Google Scholar] [CrossRef]
- Ariëns, R.A.S. Fibrin(ogen) and thrombotic disease. J. Thromb. Haemost. 2013, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Simurda, T.; Vilar, R.; Zolkova, J.; Ceznerova, E.; Kolkova, Z.; Loderer, D.; Neerman-Arbez, M.; Casini, A.; Brunclikova, M.; Skornova, I.; et al. A Novel Nonsense Mutation in FGB (c.1421G>A; p.Trp474Ter) in the Beta Chain of Fibrinogen Causing Hypofibrinogenemia with Bleeding Phenotype. Biomedicines 2020, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Fatah, K.; Hamsten, A.; Blombäck, B.; Blombäck, M. Fibrin Gel Network Characteristics and Coronary Heart Disease: Relations to Plasma Fibrinogen Concentration, Acute Phase Protein, Serum Lipoproteins and Coronary Atherosclerosis. Thromb. Haemost. 1992, 68, 130–135. [Google Scholar] [CrossRef]
- Collet, J.; Allali, Y.; Lesty, C.; Tanguy, M.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered Fibrin Architecture Is Associated with Hypofibrinolysis and Premature Coronary Atherothrombosis. Arter. Thromb. Vasc. Biol. 2006, 26, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Fatah, K.; Silveira, A.; Tornvall, P.; Karpe, F.; Blombäck, M.; Hamsten, A. Proneness to Formation of Tight and Rigid Fibrin Gel Structures in Men with Myocardial Infarction at a Young Age. Thromb. Haemost. 1996, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, J.; Pan-Petesch, B.; Arnaud, B.; Blouch, M.-T.; Abgrall, J.-F. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. Thromb. Haemost. 2008, 99, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Chantarangkul, V.; Martinelli, I.; Bucciarelli, P.; Mannucci, P.M. A shortened activated partial thromboplastin time is associated with the risk of venous thromboembolism. Blood 2004, 104, 3631–3634. [Google Scholar] [CrossRef]
- Winearls, J.; Wullschleger, M.; Wake, E.; Hurn, C.; Furyk, J.; Ryan, G.; Trout, M.; Walsham, J.; Holley, A.; Cohen, J.; et al. Fibrinogen Early In Severe Trauma studY (FEISTY): Study protocol for a randomised controlled trial. Trials 2017, 18, 241. [Google Scholar] [CrossRef]
- Salooja, N.; Perry, D.J. Thrombelastography. Blood Coagul. Fibrinolysis 2001, 12, 327–337. [Google Scholar] [CrossRef]
- Hemker, H.; Giesen, P.; Aldieri, R.; Regnault, V.; De Smed, E.; Wagenvoord, R.; Lecompte, T.; Béguin, S. The Calibrated Automated Thrombogram (CAT): A universal routine test for hyper- and hypocoagulability. Pathophysiol. Haemost. Thromb. 2002, 32, 249–253. [Google Scholar] [CrossRef]

| PNP | FDP | Cryoprecipitate | Fg-C | |
|---|---|---|---|---|
| Clauss Fg (g/L) | 3.3 ± 0.3 | <0.15 | 6.8 ± 0.1 | 21.6 ± 0.1 |
| FII (%) | 98 ± 4 | 98 ± 7 | 101 ± 12 | <1 |
| FV (%) | 82 | 36 ± 0.7 | 68 ± 9 | <1 |
| FVII (%) | 84 ± 1 | 75 ± 4 | 81 ± 7 | <1 |
| FVIII (%) | 107 ± 14 | 43 ± 5 | 190 ± 0.6 | <1 |
| FIX (%) | 127 ± 25 | 106 ± 3 | 105 ± 8 | 2 |
| FX (%) | 92 ± 7 | 95 ± 3 | 98 ± 14 | <1 |
| FXI (%) | 100 ± 16 | 107 ± 4 | 92 ± 3 | <1 |
| FXIII (%) | 80 ± 7 | <5 | 105 ± 3 | <1 |
| vWF:Ag (%) | 127 ± 33 | 65 ± 1 | 288 ± 66 | 66 |
| α2AP (µg/mL) | 72 ± 20 | 38 ± 3 | 98 ± 8 | 1 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrow, G.B.; Carlier, M.S.A.; Dasgupta, S.; Craigen, F.B.; Mutch, N.J.; Curry, N. Fibrinogen Replacement Therapy for Traumatic Coagulopathy: Does the Fibrinogen Source Matter? Int. J. Mol. Sci. 2021, 22, 2185. https://doi.org/10.3390/ijms22042185
Morrow GB, Carlier MSA, Dasgupta S, Craigen FB, Mutch NJ, Curry N. Fibrinogen Replacement Therapy for Traumatic Coagulopathy: Does the Fibrinogen Source Matter? International Journal of Molecular Sciences. 2021; 22(4):2185. https://doi.org/10.3390/ijms22042185
Chicago/Turabian StyleMorrow, Gael B., Molly S. A. Carlier, Sruti Dasgupta, Fiona B. Craigen, Nicola J. Mutch, and Nicola Curry. 2021. "Fibrinogen Replacement Therapy for Traumatic Coagulopathy: Does the Fibrinogen Source Matter?" International Journal of Molecular Sciences 22, no. 4: 2185. https://doi.org/10.3390/ijms22042185
APA StyleMorrow, G. B., Carlier, M. S. A., Dasgupta, S., Craigen, F. B., Mutch, N. J., & Curry, N. (2021). Fibrinogen Replacement Therapy for Traumatic Coagulopathy: Does the Fibrinogen Source Matter? International Journal of Molecular Sciences, 22(4), 2185. https://doi.org/10.3390/ijms22042185





