Bioconversion of Callus-Produced Precursors to Silymarin Derivatives in Silybum marianum Leaves for the Production of Bioactive Compounds
Abstract
1. Introduction
2. Results
2.1. Bioconversion of Callus-Produced Compounds to Silymarin Derivatives
2.1.1. Leaf Discs for Bioconversion
2.1.2. Leaf Age Greatly Affects Silymarin Bioconversion
2.1.3. Leaf Extract for Bioconversion
2.2. Enzymes Activities
3. Discussion
4. Materials and Methods
4.1. S. marianum Callus Production
4.2. Callus Eextract Preparation
4.3. Metabolite Profiling in S. marianum Leaves
4.4. Leaf Extracts Prepared in Phosphate or Citric Acid Buffer for Bioconversion
4.5. Chromatographic Analysis
4.6. Samples Preparation for Mass Spectrometric Analysis
4.7. Preparation of Enzymes Extract
4.8. Enzymes Assays
4.9. Protein Determination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESI-MS | Electrospray ionization mass spectrometry |
| HPLC | High performance liquid chromatography |
| DCQ | di-caffeoylquinic acid |
| SA | Silybin A |
| SB | Silybin B |
| ISB-A&B | Isosilybin A&B |
| SD | Silydianin |
| SC | Silychristin |
| TXF | Taxifolin |
| CHS | Chalcone synthase |
| PAL | Phenylalanine ammonia lyase |
| HCT | Shikimate o-hydroxycinnamoyl transferase |
| CCR | Cinnamoyl-CoA reductase |
| CAD | Cinnamyl-alcohol dehydrogenase |
| COMT | Caffeoyl-CoA-methyltransferase |
| F3′H | Flavonoid 3′-monooxygenase |
| F3H | Flavanone-3-hydroxylase |
| CGA | Chlorogenic acid |
| CBB | Coomassie Brilliant blue |
References
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- De Marchis, F.; Balducci, C.; Pompa, A.; Stensland, H.M.F.R.; Guaragno, M.; Pagiotti, R.; Menghini, A.R.; Persichetti, E.; Beccari, T.; Bellucci, M. Human α-mannosidase produced in transgenic tobacco plants is processed in human α-mannosidosis cell lines. Plant Biotechnol. J. 2011, 9, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Nokhodchi, A.; Williams, R.O. Process engineering and pharmaceutical manufacturing technologies. Drug Deliv. Transl. Res. 2018, 8, 1593–1594. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Geraniaceae-Boraginaceae. In Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2000; Volume 2, p. 352. [Google Scholar]
- Radjabian, T.; Rezazadeh, S.H.; Fallah Huseini, H. Analysis of silymarin components in the seed extracts of some milk this-tle ecotypes from Iran by HPLC. Iran. J. Sci. Technol. 2008, 32, 141–146. [Google Scholar] [CrossRef]
- Khalili, M.; Hasanloo, T.; Tabar, S.K.K.; Rahnama, H. Influence of exogenous salicylic acid on flavonolignans and lipoxygenase activity in the hairy root cultures of Silybum marianum. Cell Biol. Int. 2009, 33, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, J.; Li, Z.; Li, X.; Zhou, S.; Liu, C. Preparative chromatographic purification of diastereomers of silybin and their quantification in human plasma by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2008, 862, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Flora, K.; Hahn, M.; Rosen, H.; Benner, K. Milk Thistle (Silybum marianum) for the Therapy of Liver Disease. Am. J. Gastroenterol. 1998, 93, 139–143. [Google Scholar] [CrossRef]
- Alikaridis, F.; Papadakis, D.; Pantelia, K.; Kephalas, T. Flavonolignan production from Silybum marianum transformed and untransformed root cultures. Fitoterapia 2000, 71, 379–384. [Google Scholar] [CrossRef]
- Chambers, C.S.; Valentova, K.; Kren, V. ‘Non-taxifolin’ derived flavonolignans: Phytochemistry and biology. Curr. Pharm. Des. 2015, 21, 5489–5500. [Google Scholar] [CrossRef]
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Svobodová, A.R. Skin Protective Activity of Silymarin and its Flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.W.; Liu, Y.; Graf, T.N.; Oberlies, N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar] [CrossRef]
- Marhol, P.; Bednář, P.; Kolářová, P.; Večeřa, R.; Ulrichová, J.; Tesařová, E.; Vavříková, E.; Kuzma, M.; Křen, V. Pharmacokinetics of pure silybin diastereoisomers and identification of their metabolites in rat plasma. J. Funct. Foods 2015, 14, 570–580. [Google Scholar] [CrossRef]
- Pourová, J.; Applová, L.; Macáková, K.; Vopršalová, M.; Migkos, T.; Bentanachs, R.; Biedermann, D.; Petrásková, L.; Tvrdý, V.; Hrubša, M.; et al. The Effect of Silymarin Flavonolignans and Their Sulfated Conjugates on Platelet Aggregation and Blood Vessels Ex Vivo. Nutrients 2019, 11, 2286. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H.-J. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Tighe, S.P.; Akhtar, D.; Iqbal, U.; Ahmed, A. Chronic Liver Disease and Silymarin: A Biochemical and Clinical Review. J. Clin. Transl. Hepatol. 2020, 8, 1–5. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Baradaran, A.; Rafieian, M. Oxidative stress and the paradoxical effects of antioxidants. J. Res. Med. Sci. 2013, 18, 629. [Google Scholar] [PubMed]
- Delmas, D.; Xiao, J.; Vejux, A.; Aires, V. Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules 2020, 25, 2009. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. PLANT RESPONSES TO INSECT HERBIVORY: The Emerging Molecular Analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Makoi, J.H.; Belane, A.K.; Chimphango, S.B.; Dakora, F.D. Seed flavonoids and anthocyanins as markers of enhanced plant defence in nodulated cowpea (Vigna unguiculata L. Walp.). Field Crop. Res. 2010, 118, 21–27. [Google Scholar] [CrossRef]
- Schlüter, P.M.; Schiestl, F.P. Molecular mechanisms of floral mimicry in orchids. Trends Plant Sci. 2008, 13, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, F.; Ragg, H.; Fautz, E.; Kuhn, D.N.; Hahlbrock, K. UV-induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc. Natl. Acad. Sci. USA 1983, 80, 2591–2593. [Google Scholar] [CrossRef]
- Knogge, W.; Schmelzer, E.; Weissenböck, G. The role of chalcone synthase in the regulation of flavonoid biosynthesis in developing oat primary leaves. Arch. Biochem. Biophys. 1986, 250, 364–372. [Google Scholar] [CrossRef]
- Jimenez-Garcia, S.; Vazquez-Cruz, M.; Guevara-González, R.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Feregrino-Perez, A. Current Approaches for Enhanced Expression of Secondary Metabolites as Bioactive Compounds in Plants for Agronomic and Human Health Purposes. Pol. J. Food Nutr. Sci. 2013, 63, 67–78. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxidative Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanjari, S.; Shobbar, Z.S.; Ebrahimi, M.; Hasanloo, T.; Sadat-Noori, S.-A.; Tirnaz, S. Chalcone synthase genes from milk thistle (Silybum marianum): Isolation and expression analysis. J. Genet. 2015, 94, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Corchete, P. Gene expression and flavonolignan production in fruits and cell cultures of Silybum marianum. J. Plant Physiol. 2016, 192, 111–117. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N. Silymarin for Liver Disease. In Liver Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 621–631. [Google Scholar]
- Merlini, L.; Zanarotti, A.; Pelter, A.; Rochefort, M.P.; Hänsel, R. Biomimetic synthesis of natural silybin. J. Chem. Soc. Chem. Commun. 1979, 16, 695. [Google Scholar] [CrossRef]
- Althagafy, H.S.; Meza-Avina, M.E.; Oberlies, N.H.; Croatt, M.P. Mechanistic Study of the Biomimetic Synthesis of Flavonolignan Diastereoisomers in Milk Thistle. J. Org. Chem. 2013, 78, 7594–7600. [Google Scholar] [CrossRef]
- Pelter, A.; Hänsel, R. The structure of silybin (silybum substance E6), the first flavonolignan. Tetrahedron Lett. 1968, 9, 2911–2916. [Google Scholar] [CrossRef]
- Simanek, V.; Křen, V.; Ulrichová, J.; Vicar, J.; Cvak, L. Silymarin: What is in the name…? An appeal for a change of editorial policy. Hepatology 2000, 32, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Why do lupin cell cultures fail to produce alkaloids in large quantities? Plant Cell Tissue Organ Cult. 1987, 8, 103–111. [Google Scholar] [CrossRef]
- Gad, D.; Elhaak, M.; Pompa, A.; Mattar, M.; Zayed, M.; Fraternale, D.; Dietz, K.-J. A New Strategy to Increase Production of Genoprotective Bioactive Molecules from Cotyledon-Derived Silybum marianum L. Callus. Genes 2020, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Juvany, M.; Müller, M.; Munné-Bosch, S. Photo-oxidative stress in emerging and senescing leaves: A mirror image? J. Exp. Bot. 2013, 64, 3087–3098. [Google Scholar] [CrossRef] [PubMed]
- Schmundt, D.; Stitt, M.; Jähne, B.; Schurr, U. Quantitative analysis of the local rates of growth of dicot leaves at a high temporal and spatial resolution, using image sequence analysis. Plant J. 1998, 16, 505–514. [Google Scholar] [CrossRef]
- Martin, R.J.; Lauren, D.R.; Smith, W.A.; Jensen, D.J.; Deo, B.; Douglas, J.A. Factors influencing silymarin content and compo-sition in variegated thistle (Silybum marianum). N. Z. J. Crop Hortic. Sci. 2006, 34, 239–245. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Infusions of artichoke and milk thistle represent a good source of phenolic acids and flavonoids. Food Funct. 2014, 6, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Forkmann, G. Flavonoids as Flower Pigments: The Formation of the Natural Spectrum and its Extension by Genetic Engineering. Plant Breed. 1991, 106, 1–26. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.-H. Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef]
- Sánchez-Sampedro, M.A.; Fernández-Tárrago, J.; Corchete, P. Silymarin synthesis and degradation by peroxidases of cell suspension cultures of Silybum marianum. J. Plant Physiol. 2007, 164, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Solekha, R.; Susanto, F.A.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae pv. oryzae. J. Plant Pathol. 2020, 102, 359–365. [Google Scholar] [CrossRef]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef]
- Knogge, W. Tissue-distribution of secondary phenolic biosynthesis in developing primary leaves of Avena sativa L. Planta 1986, 167, 196–205. [Google Scholar] [CrossRef] [PubMed]
- El-Garhy, H.A.; Khattab, S.; Moustafa, M.M.; Ali, R.A.; Azeiz, A.Z.A.; Elhalwagi, A.; El Sherif, F. Silybin content and overexpression of chalcone synthase genes in Silybum marianum L. plants under abiotic elicitation. Plant Physiol. Biochem. 2016, 108, 191–202. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Liu, X.; Shang, J.; Zhang, A.; Zhu, Z.; Zha, D. Chalcone synthase (CHS) family members analysis from eggplant (Solanum melongena L.) in the flavonoid biosynthetic pathway and expression patterns in response to heat stress. PLoS ONE 2020, 15, e0226537. [Google Scholar] [CrossRef]
- Zhou, T.-S.; Zhou, R.; Yu, Y.-B.; Xiao, Y.; Li, D.-H.; Xiao, B.; Yu, O.; Yang, Y.-J. Cloning and Characterization of a Flavonoid 3′-Hydroxylase Gene from Tea Plant (Camellia sinensis). Int. J. Mol. Sci. 2016, 17, 261. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Kandasamy, D.; Ullah, C.; Schmidt, A.; Wright, L.P.; Gershenzon, J. Flavanone-3-Hydroxylase Plays an Important Role in the Biosynthesis of Spruce Phenolic Defenses against Bark Beetles and Their Fungal Associates. Front. Plant Sci. 2019, 10, 208. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.-M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Blom, T.J.M.; Sierra, M.; Van Vliet, T.B.; Dijk, M.E.I.F.-V.; De Koning, P.; Van Iren, F.; Verpoorte, R.; Libbenga, K.R. Uptake and accumulation of ajmalicine into isolated vacuoles of cultured cells of Catharanthus roseus (L.) G. Don. and its conversion into serpentine. Planta 1991, 183, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Peroxidase-Catalyzed Formation of Quercetin Quinone Methide–Glutathione Adducts. Arch. Biochem. Biophys. 2000, 378, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; De Forchetti, S.M.; Tigier, H.; Valpuesta, V. A Tomato Peroxidase Involved in the Synthesis of Lignin and Suberin. Plant Physiol. 2000, 122, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, L.; Cheng, S.; Cao, F.; Wang, Y.; Yuan, H. Molecular cloning and function assay of a chalcone isomerase gene (GbCHI) from Ginkgo biloba. Plant Cell Rep. 2010, 30, 49–62. [Google Scholar] [CrossRef]
- Boland, M.J.; Wong, E. Purification and Kinetic Properties of Chalcone-Flavanone Isomerase from Soya Bean. JBIC J. Biol. Inorg. Chem. 1975, 50, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.N.; Robbinst, M.P.; Dixon, R.A.; Veltkamp, E. Spontaneous and enzymic rearrangement of naringenin chalcone to flavanone. Phytochemistry 1985, 24, 2267–2269. [Google Scholar] [CrossRef]
- El-Shora, H.M. Properties of phenylalanine ammonia-lyase from marrow cotyledons. Plant Sci. 2002, 162, 1–7. [Google Scholar] [CrossRef]
- Obinata, N.; Yamakawa, T.; Takamiya, M.; Tanaka, N.; Ishimaru, K.; Kodama, T. Effects of Salicylic Acid on the Production of Procyanidin and Anthocyanin in Cultured Grape Cells. Plant Biotechnol. 2003, 20, 105–111. [Google Scholar] [CrossRef]
- Larson, R.L.; Bussard, J.B. Microsomal Flavonoid 3′-Monooxygenase from Maize Seedlings. Plant Physiol. 1986, 80, 483–486. [Google Scholar] [CrossRef]
- Ulbrich, B.; Zenk, M.H. Partial purification and properties of p-hydroxycinnamoyl-CoA: Shikimate-p-hydroxycinnamoyl transferase from higher plants. Phytochemistry 1980, 19, 1625–1629. [Google Scholar] [CrossRef]
- Lüderitz, T.; Grisebach, H. Enzymic Synthesis of Lignin Precursors Comparison of Cinnamoyl-CoA Reductase and Cinnamyl Alcohol: NADP+ Dehydrogenase from Spruce (Picea abies L.) and Soybean (Glycine max L.). JBIC J. Biol. Inorg. Chem. 1981, 119, 115–124. [Google Scholar] [CrossRef]
- Wyrambik, D.; Grisebach, H. Purification and Properties of Isoenzymes of Cinnamyl-Alcohol Dehydrogenase from Soybean-Cell-Suspension Cultures. JBIC J. Biol. Inorg. Chem. 1975, 59, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pakusch, A.-E.; Matern, U. Kinetic Characterization of Caffeoyl-Coenzyme A-Specific 3-O-Methyltransferase from Elicited Parsley Cell Suspensions. Plant Physiol. 1991, 96, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
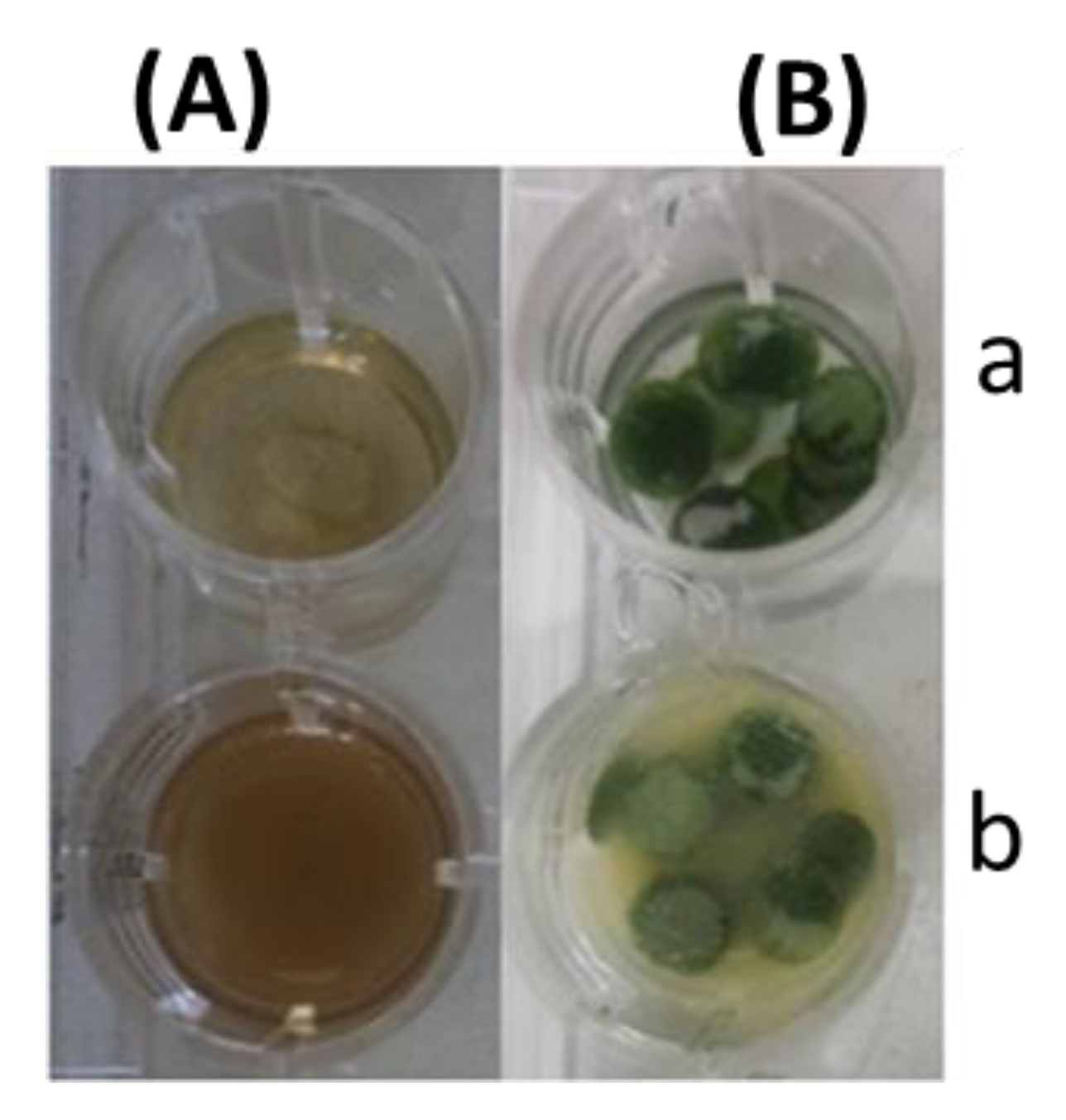
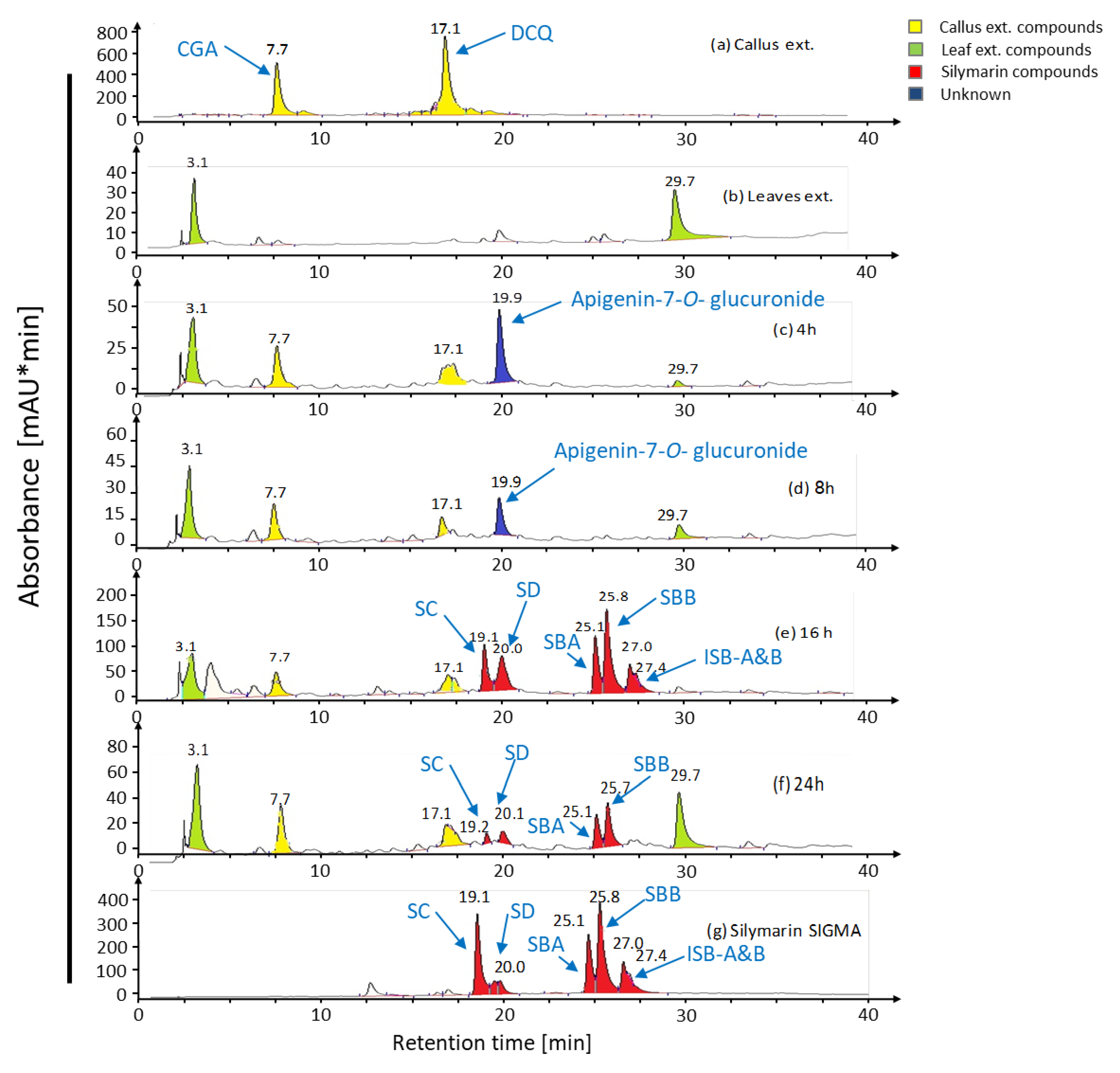

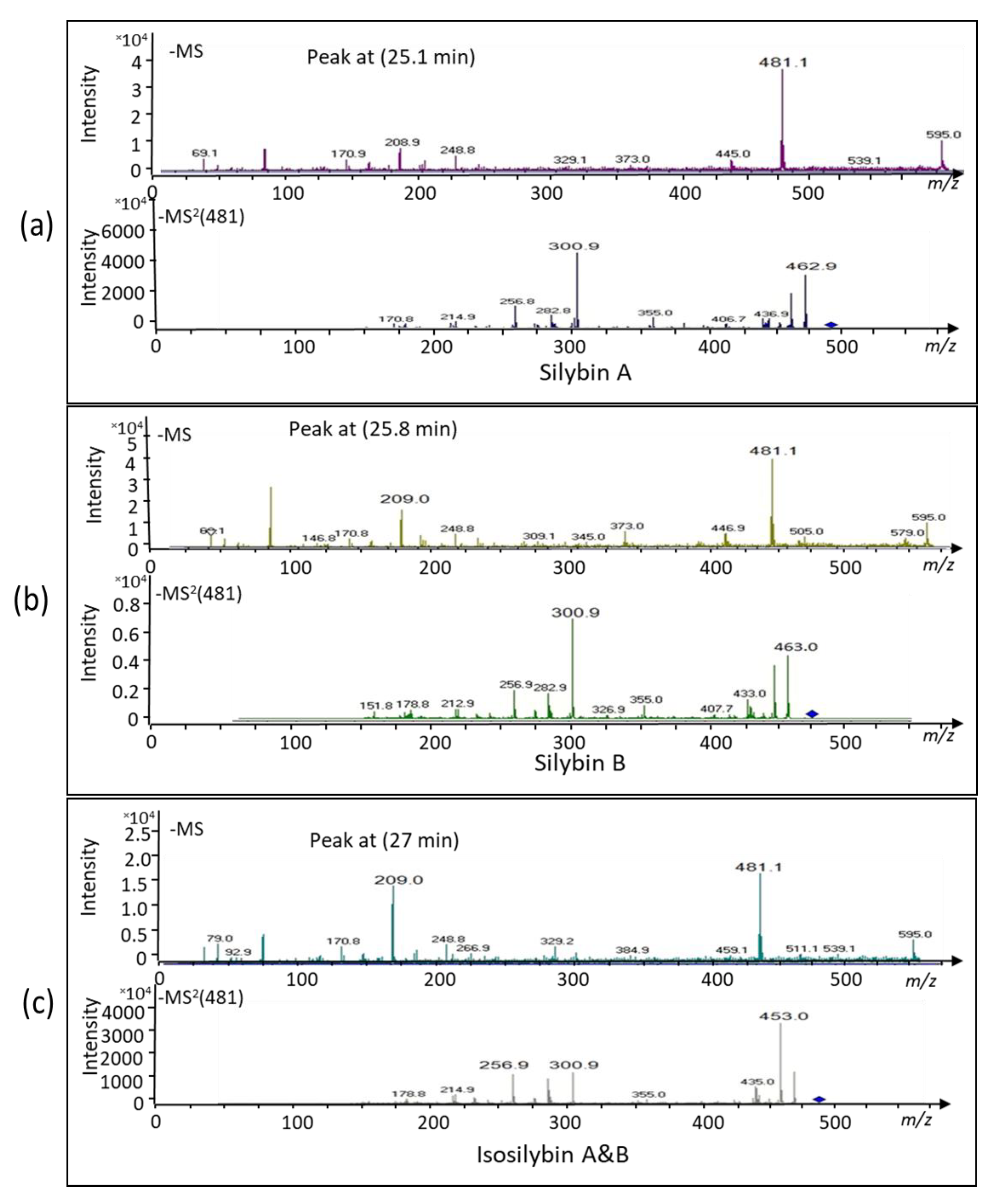

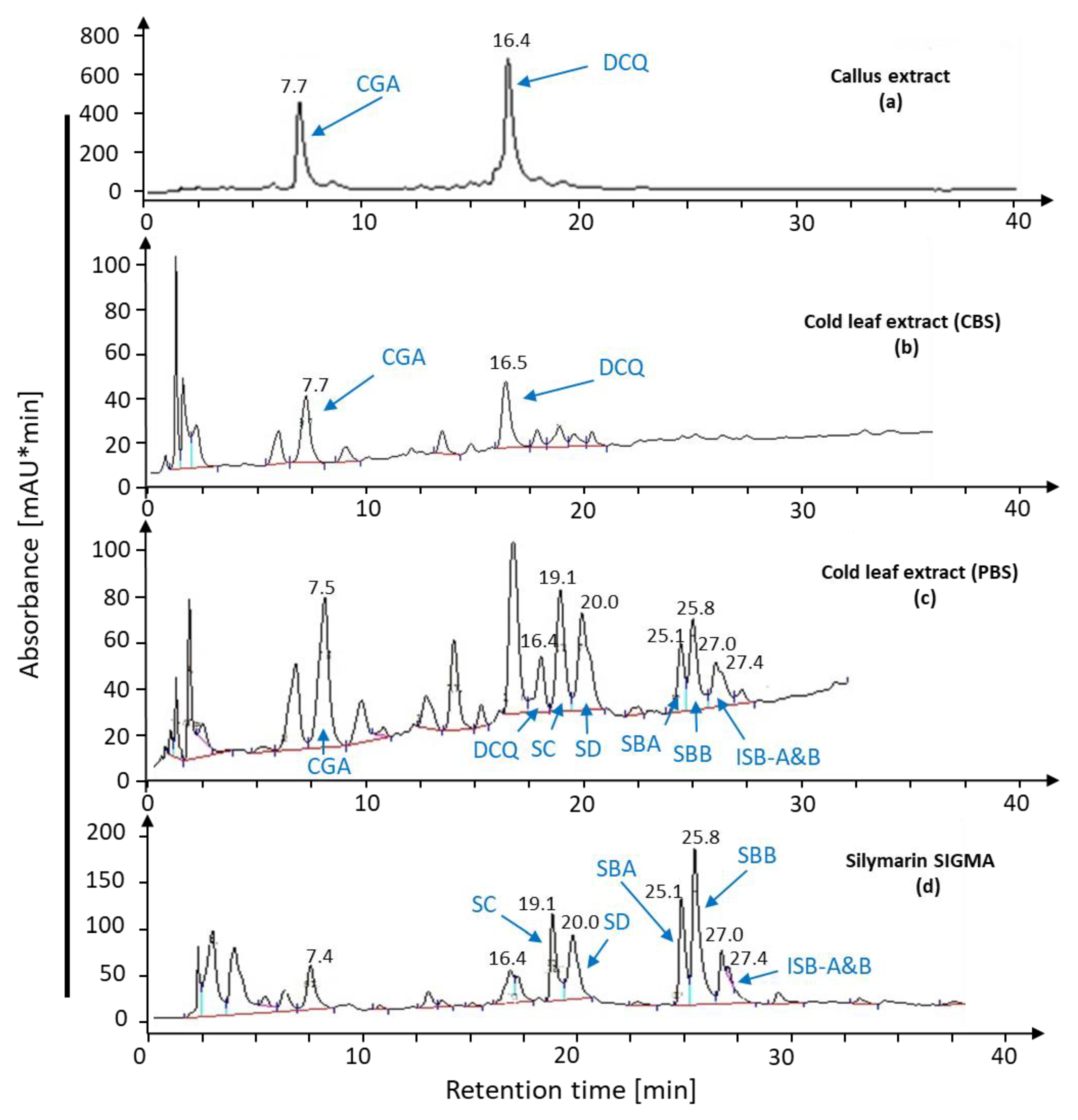
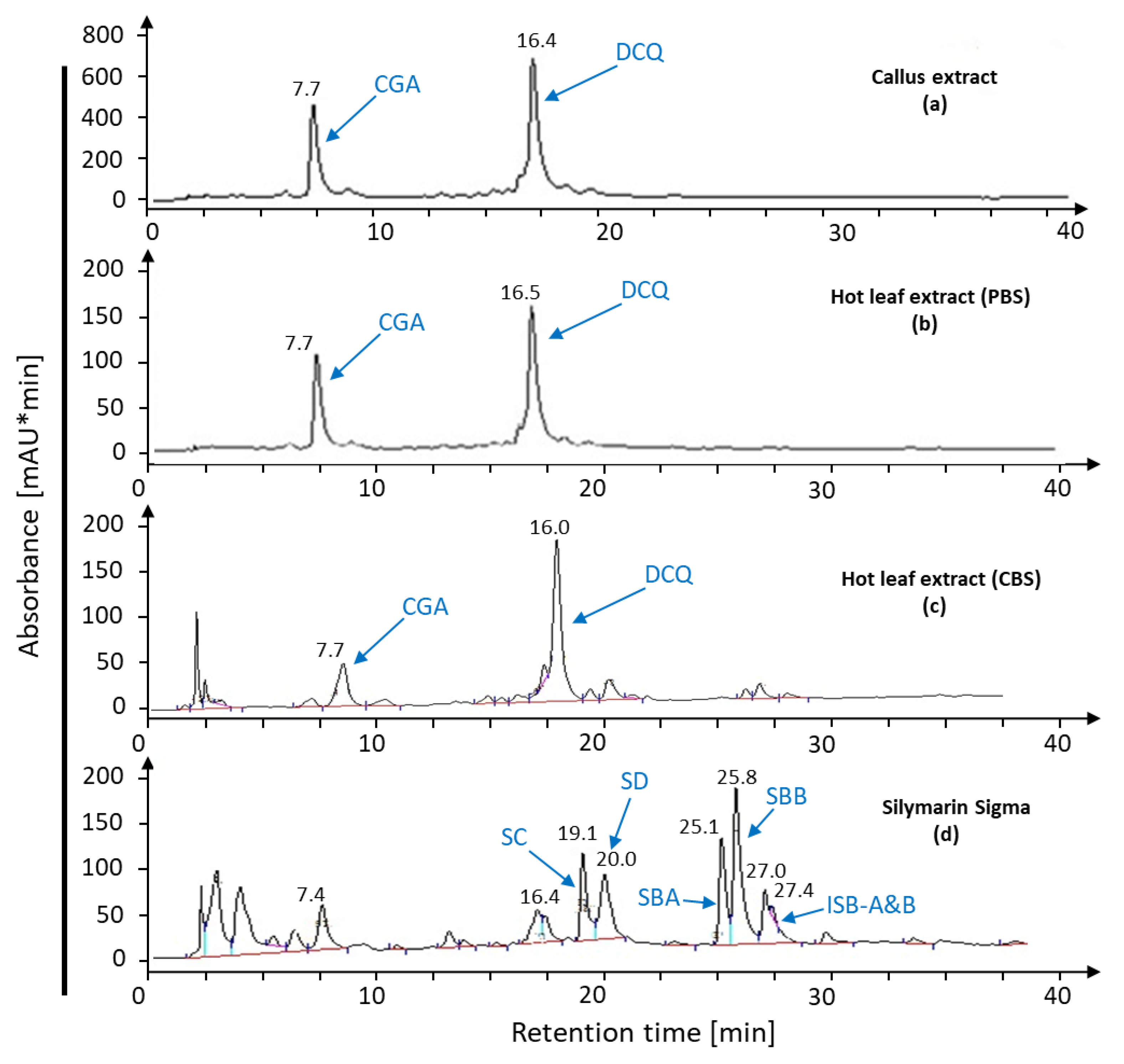

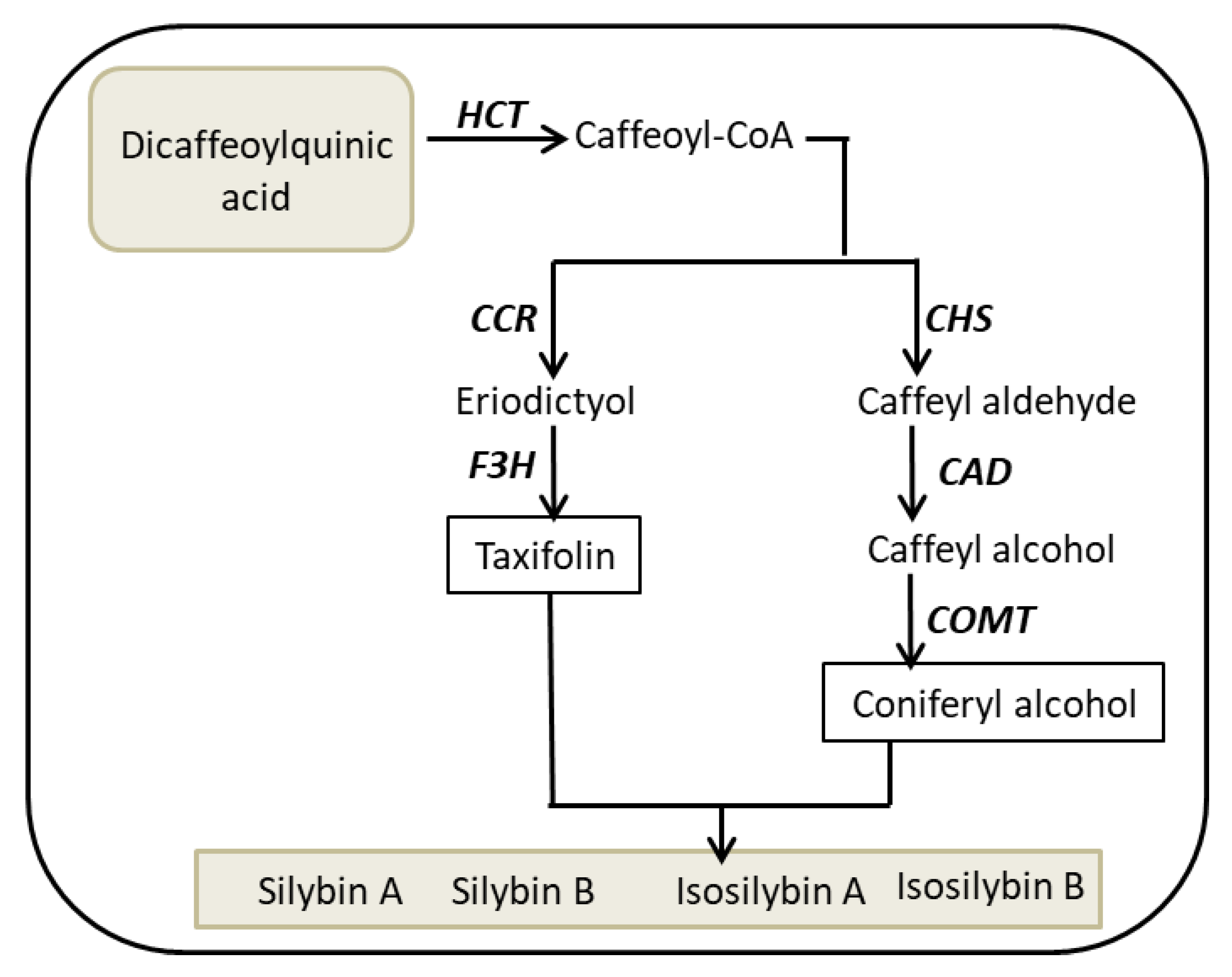
| Content (mg·g DW−1) | SC | SD | SBA | SBB | ISB-AB | Total | |
|---|---|---|---|---|---|---|---|
| Leaf discs | 1.3 ± 0.5 | 2.2 ± 0.8 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.03 | 8.2 | |
| Leaf extract | 1.09 ± 0.5 | 1.13 ± 0.5 | 0.82 ± 0.09 | 0.72 ± 0.05 | 0.86 ± 0.4 | 4.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad, D.; El-Shora, H.; Fraternale, D.; Maricchiolo, E.; Pompa, A.; Dietz, K.-J. Bioconversion of Callus-Produced Precursors to Silymarin Derivatives in Silybum marianum Leaves for the Production of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 2149. https://doi.org/10.3390/ijms22042149
Gad D, El-Shora H, Fraternale D, Maricchiolo E, Pompa A, Dietz K-J. Bioconversion of Callus-Produced Precursors to Silymarin Derivatives in Silybum marianum Leaves for the Production of Bioactive Compounds. International Journal of Molecular Sciences. 2021; 22(4):2149. https://doi.org/10.3390/ijms22042149
Chicago/Turabian StyleGad, Dina, Hamed El-Shora, Daniele Fraternale, Elisa Maricchiolo, Andrea Pompa, and Karl-Josef Dietz. 2021. "Bioconversion of Callus-Produced Precursors to Silymarin Derivatives in Silybum marianum Leaves for the Production of Bioactive Compounds" International Journal of Molecular Sciences 22, no. 4: 2149. https://doi.org/10.3390/ijms22042149
APA StyleGad, D., El-Shora, H., Fraternale, D., Maricchiolo, E., Pompa, A., & Dietz, K.-J. (2021). Bioconversion of Callus-Produced Precursors to Silymarin Derivatives in Silybum marianum Leaves for the Production of Bioactive Compounds. International Journal of Molecular Sciences, 22(4), 2149. https://doi.org/10.3390/ijms22042149






