A Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis—And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder
Abstract
1. Introduction
1.1. Oxidant Stress and Intracellular Calcium Signals—Roles in Alzheimer’s Pathogenesis
1.2. Phycocyanobilin—A Phyconutrient NADPH Oxidase Inhibitor
1.3. Phase Two Induction and Support for Glutathione Synthesis
2. A Comprehensive Antioxidant Strategy May Aid Prevention of Alzheimer’s Disease
2.1. Astaxanthin—Antioxidant Protection for Calcium-Overloaded Mitochondria
2.2. Controlling Amyloid β Production via Modulation of BACE1 and ADAM10 Expression
2.3. Antioxidants May Support Astrocyte Glutamate Uptake
2.4. Antioxidants May Sustain Activity of Amyloid β-Degrading Proteases
2.5. Could Antioxidants Aid Expulsion of Amyloid β from the Brain?
2.6. Antioxidants May Support Cerebrovascular Endothelial Nitric Oxide Synthase Activity
3. Potential Enhancers of Amyloid β Neurotoxicity
3.1. Microglial Production of Interleukin-1β Potentiates Amyloid β Neurotoxicity
3.2. Magnesium Deficiency May Up-Regulate Amyloid β Neurotoxicity
4. Toward an Integrated Nutraceutical/Lifestyle Strategy for Amyloid β Neurotoxicity in Alzheimer’s Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| NAC | N-acetylcysteine |
| RYR2 | ryanodine receptors |
| ER | endoplasmic reticulum |
| IRS-1 | insulin receptor substrate-1 |
| GLT-1 | glutamate |
| PCB | Phycocyanobilin |
| MLT | melatonin |
| HO-1 | heme oxygenase-1 |
| H2S | hydrogen sulfide |
| CBS | cystathionine beta-synthase |
| Se | selenium |
| AST | astaxanthin |
| ETC | electron transport chain |
| APP | amyloid precursor protein |
| sGC | soluble guanylate cyclase |
| NO | nitric oxide |
| PPARα | peroxisome proliferator-activated receptor α |
| PKC | protein kinase C |
| IDE | insulin-degrading enzyme |
| P-GP | P-glycoprotein |
| eNOS | endothelial nitric oxide synthase |
| DDAH | dimethylarginine dimethylaminohydrolase |
| ADMA | asymmetric dimethylarginine |
| IL-1β | interleukin-1β |
| Mg | Magnesium |
| DHA | docosahexaenoic acid |
| NMDA | N-methyl-D-aspartate |
| JNK | c-Jun N-terminal kinase |
| ASK1 | apoptosis signal-regulating kinase 1 |
| NADPH | Reduced form of Nicotinamide adenine dinucleotide phosphate |
| LRP1 | lipoprotein receptor-related protein 1 |
| GFAP | glial fibrillary acidic protein |
| EGCG | epigallocatechin-3-gallate |
| SREBP-2 | sterol regulatory element-binding protein 2 |
| RAGE | receptor for advanced glycation end products |
| LTP | Long-term potentiation |
| TLR | Toll-like receptor |
| HMGB1 | high-mobility group box protein 1 |
| FGF21 | fibroblast growth factor 21 |
| LA | Lipoic acid |
References
- Ronicke, R.; Mikhaylova, M.; Ronicke, S.; Meinhardt, J.; Schröder, U.H.; Fändrich, M.; Reiser, G.; Kreutz, M.R.; Reymann, K.G. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol. Aging 2011, 32, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Koeglsperger, T.; Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011, 31, 6627–6638. [Google Scholar] [CrossRef]
- Brennan-Minnella, A.M.; Shen, Y.; El-Benna, J.; Swanson, R.A. Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis. 2013, 4, e580. [Google Scholar] [CrossRef] [PubMed]
- SanMartin, C.D.; Veloso, P.; Adasme, T.; Lobos, P.; Bruna, B.; Galaz, J.; García, A.; Hartel, S.; Hidalgo, C.; Paula-Lima, A.C. RyR2-Mediated Ca(2+) Release and Mitochondrial ROS Generation Partake in the Synaptic Dysfunction Caused by Amyloid β Peptide Oligomers. Front. Mol. Neurosci. 2017, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, H.; Guo, C.; Zhao, S.; Gong, D.; Zhao, Y. Involvement of IRE α signaling in the hippocampus in patients with mesial temporal lobe epilepsy. Brain Res. Bull. 2011, 84, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef] [PubMed]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Aljanabi, N.M.; Mamtani, S.; Al-Ghuraibawi, M.M.H.; Yadav, S.; Nasr, L. Alzheimer’s and Hyperglycemia: Role of the Insulin Signaling Pathway and GSK-3 Inhibition in Paving a Path to Dementia. Cureus 2020, 12, e6885. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, D.; Wang, Y.; Jiang, T.; Hu, S.; Zhang, M.; Yu, X.; Gong, C.-X. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J. Alzheimers Dis. 2013, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.-Y.; Shi, J.-S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3 β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kwon, Y.T.; Li, M.; Peng, J.; Friedlander, R.M.; Tsai, L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 2000, 405, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Stallings, N.R.; O’Neal, M.A.; Hu, J.; Kavalali, E.T.; Bezprozvanny, I.; Malter, J.S. Pin1 mediates Aβ 42-induced dendritic spine loss. Sci. Signal. 2018, 11, eaap8734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Santini, F.; Breese, R.; Ross, D.; Zhang, X.D.; Stone, D.J.; Ferrer, M.; Townsend, M.; Wolfe, A.L.; Seager, M.A.; et al. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J. Biol. Chem. 2010, 285, 7619–7632. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Chen, H.S.; Zhang, D.; Lipton, S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010, 30, 11246–11250. [Google Scholar] [CrossRef]
- Scimemi, A.; Meabon, J.S.; Woltjer, R.L.; Sullivan, J.M.; Diamond, J.S.; Cook, D.G. Amyloid-β1–42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J. Neurosci. 2013, 33, 5312–5318. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, P.; Green, P.S.; Watson, G.S.; Marques, M.A.; Tanaka, K.; Meeker, K.D.; Meabon, J.S.; Li, N.; Zhu, P.; Olson, V.G.; et al. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J. Alzheimers Dis. 2011, 26, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.P.; Nakano, M.; Kubota, K.; Himuro, N.; Mizoguchi, S.; Chikenji, T.; Otani, M.; Mizue, Y.; Nagaishi, K.; Fujimiya, M. Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci. Rep. 2018, 8, 1712. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sun, B.; Institoris, A.; Zhan, X.; Guo, W.; Song, Z.; Liu, Y.; Hiess, F.; Boyce, A.K.J.; Ni, M.; et al. Limiting RyR2 Open Time Prevents Alzheimer’s Disease-Related Neuronal Hyperactivity and Memory Loss but Not β-Amyloid Accumulation. Cell Rep. 2020, 32, 108169. [Google Scholar] [CrossRef] [PubMed]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Praticò, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, M.A.; Stallings, N.R.; Malter, J.S. Alzheimer’s Disease, Dendritic Spines, and Calcineurin Inhibitors: A New Approach? ACS Chem. Neurosci. 2018, 9, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela, G.; Rastellini, C.; Cicalese, L. Reduced Incidence of Dementia in Solid Organ Transplant Patients Treated with Calcineurin Inhibitors. J. Alzheimers Dis. 2015, 47, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taillé, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: Implications for protection against endotoxic shock in rats. FASEB J. 2005, 19, 1890–1892. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ishikawa, K.; Itabe, H.; Maruyama, Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol. Cell. Biochem. 2006, 291, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Roberts, S.J.; Datla, S.; Dusting, G.J. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension 2006, 48, 950–957. [Google Scholar] [CrossRef]
- McCarty, M.F. Clinical potential of Spirulina as a source of phycocyanobilin. J. Med. Food 2007, 10, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef]
- Terry, M.J.; Maines, M.D.; Lagarias, J.C. Inactivation of phytochrome- and phycobiliprotein-chromophore precursors by rat liver biliverdin reductase. J. Biol. Chem. 1993, 268, 26099–26106. [Google Scholar] [CrossRef]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef]
- Bannu, S.M.; Lomada, D.; Gulla, S.; Chandrasekhar, T.; Reddanna, P.; Reddy, M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr. Drug Metab. 2019, 20, 967–976. [Google Scholar] [CrossRef]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini Rev. Med. Chem. 2017, 17, 1173–1193. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical Application of Spirulina platensis Derived C-Phycocyanin. Evid. Based Complement. Alternat. Med. 2016, 2016, 7803846. [Google Scholar] [PubMed]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; Cordeiro de Almeida, A.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M. Neuroprotective Activities of Spirulina platensis in the 6-OHDA Model of Parkinson’s Disease Are Related to Its Anti-Inflammatory Effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef]
- Tobón-Velasco, J.C.; Palafox-Sánchez, V.; Mendieta, L.; García, E.; Santamaría, A.; Chamorro-Cevallos, G.; Daniel Limón, I. Antioxidant effect of Spirulina (Arthrospira) maxima in a neurotoxic model caused by 6-OHDA in the rat striatum. J. Neural Transm. 2013, 120, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, P.C. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef]
- Chamorro, G.; Pérez-Albiter, M.; Serrano-García, N.; Mares-Sámano, J.J.; Rojas, P. Spirulina maxima pretreatment partially protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Nutr. Neurosci. 2006, 9, 207–212. [Google Scholar] [CrossRef]
- Pavón-Fuentes, N.; Marín-Prida, J.; Llópiz-Arzuaga, A.; Falcón-Cama, V.; Campos-Mojena, R.; Cervantes-Llanos, M.; Beatriz Piniella-Matamoros, B.; Pentón-Arias, E.; Pentón-Rol, G. Phycocyanobilin reduces brain injury after endothelin-1- induced focal cerebral ischaemia. Clin. Exp. Pharmacol. Physiol. 2020, 47, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Thaakur, S.; Sravanthi, R. Neuroprotective effect of Spirulina in cerebral ischemia-reperfusion injury in rats. J. Neural Transm. 2010, 117, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Marín-Prida, J.; Pavón-Fuentes, N.; Llópiz-Arzuaga, A.; Fernández-Massó, J.R.; Delgado-Roche, L.; Mendoza-Marí, Y.; Santana, S.P.; Cruz-Ramírez, A.; Valenzuela-Silva, C.; Nazábal-Gálvez, M.; et al. Phycocyanobilin promotes PC12 cell survival and modulates immune and inflammatory genes and oxidative stress markers in acute cerebral hypoperfusion in rats. Toxicol. Appl. Pharmacol. 2013, 272, 49–60. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A.; et al. C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain Res. Bull. 2011, 86, 42–52. [Google Scholar]
- Lo, C.M.; Carroll, K.S. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013, 288, 26480–26488. [Google Scholar]
- Bindoli, A.; Rigobello, M.P. Principles in redox signaling: From chemistry to functional significance. Antioxid. Redox Signal. 2013, 18, 1557–1593. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Forman, H.J. Glutathione in defense and signaling: Lessons from a small thiol. Ann. NY Acad. Sci. 2002, 973, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Shelton, M.D.; Chock, P.B.; Mieyal, J.J. Glutaredoxin: Role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox Signal. 2005, 7, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Parsons, Z.D.; Gates, K.S. Thiol-dependent recovery of catalytic activity from oxidized protein tyrosine phosphatases. Biochemistry 2013, 52, 6412–6423. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dinkova-Kostova, A.T.; Talalay, P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Natl. Acad. Sci. USA 2008, 105, 15926–15931. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Surh, Y.J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005, 224, 171–184. [Google Scholar] [CrossRef]
- Kensler, T.W.; Egner, P.A.; Agyeman, A.S.; Visvanathan, K.; Groopman, J.D.; Chen, J.G.; Chen, T.Y.; Fahey, J.W.; Talalay, P. Keap1-nrf2 signaling: A target for cancer prevention by sulforaphane. Top. Curr. Chem. 2013, 329, 163–177. [Google Scholar] [PubMed]
- Abed, D.A.; Goldstein, M.; Albanyan, H.; Jin, H.; Hu, L. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B 2015, 5, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.-M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Fareid, M.A.; Abdel Hameed, R.S.; Abdel Moneim, A.E. The Protective Effects of Melatonin on Aluminum-Induced Hepatotoxicity and Nephrotoxicity in Rats. Oxid. Med. Cell Longev. 2020, 2020, 7375136. [Google Scholar] [CrossRef]
- GarcÃa, J.A.; Volt, H.; Venegas, C.; Doerrier, C.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. Disruption of the NF- κB/NLRP3 connection by melatonin requires retinoid-related orphan receptor-α and blocks the septic response in mice. FASEB J. 2015, 29, 3863–3875. [Google Scholar] [CrossRef] [PubMed]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J. Pineal Res. 2015, 59, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Hua, N.; Zang, X.; Huang, T.; He, L. Melatonin ameliorates Aβ (1–42) -induced Alzheimer’s cognitive deficits in mouse model. J. Pharm. Pharmacol. 2018, 70, 70–80. [Google Scholar] [CrossRef]
- Song, C.; Li, M.; Xu, L.; Shen, Y.; Yang, H.; Ding, M.; Liu, X.; Xie, Z. Mitochondrial biogenesis mediated by melatonin in an APPswe/PS1dE9 transgenic mice model. Neuroreport 2018, 29, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Jürgenson, M.; Zharkovskaja, T.; Noortoots, A.; Morozova, M.; Beniashvili, A.; Zapolski, M.; Zharkovsky, A. Effects of the drug combination memantine and melatonin on impaired memory and brain neuronal deficits in an amyloid-predominant mouse model of Alzheimer’s disease. J. Pharm. Pharmacol. 2019, 71, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qiu, X.; Wang, Y.; Liu, J.; Li, Q.; Jiang, H.; Li, S.; Song, C. Long-term oral melatonin alleviates memory deficits, reduces amyloid-β deposition associated with downregulation of BACE1 and mitophagy in APP/PS1 transgenic mice. Neurosci. Lett. 2020, 735, 135192. [Google Scholar] [CrossRef] [PubMed]
- Strasky, Z.; Zemankova, L.; Nemeckova, I.; Rathouska, J.; Wong, R.J.; Muchova, L.; Subhanova, I.; Vanikova, J.; Vanova, K.; Vitek, L.; et al. Spirulina platensis and phycocyanobilin activate atheroprotective heme oxygenase-1: A possible implication for atherogenesis. Food Funct. 2013, 4, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Qin, S. Therapeutic effect of phycocyanin on acute liver oxidative damage caused by X-ray. Biomed. Pharmacother. 2020, 130, 110553. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Winter, G.M.; Rogers, W.J.; Lam, J.C.; Denison, M.S. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch. Biochem. Biophys. 1998, 357, 155–163. [Google Scholar] [CrossRef]
- Vítek, L. Bilirubin as a signaling molecule. Med. Res. Rev. 2020, 40, 1335–1351. [Google Scholar] [CrossRef]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef]
- Dröge, W. Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2355–2372. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- More, J.; Galusso, N.; Veloso, P.; Montecinos, L.; Finkelstein, J.P.; Sanchez, G.; Paula-Lima, A. N-Acetylcysteine Prevents the Spatial Memory Deficits and the Redox-Dependent RyR2 Decrease Displayed by an Alzheimer’s Disease Rat Model. Front. Aging Neurosci. 2018, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; OKeefe, J.H.; McCarty, M.F. Boosting endogenous production of vasoprotective hydrogen sulfide via supplementation with taurine and N-acetylcysteine: A novel way to promote cardiovascular health. Open Heart 2017, 4, e000600. [Google Scholar] [CrossRef] [PubMed]
- Vandini, E.; Ottani, A.; Zaffe, D.; Calevro, A.; Canalini, F.; Cavallini, G.M.; Rossi, R.; Guarini, S.; Giuliani, D. Mechanisms of Hydrogen Sulfide against the Progression of Severe Alzheimer’s Disease in Transgenic Mice at Different Ages. Pharmacology 2019, 103, 50–60. [Google Scholar] [CrossRef]
- Cao, L.; Cao, X.; Zhou, Y.; Nagpure, B.V.; Wu, Z.-Y.; Hu, L.F.; Yang, Y.; Sethi, G.; Moore, P.K.; Bian, J.-S. Hydrogen sulfide inhibits ATP-induced neuroinflammation and Aβ 1–42 synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav. Immun. 2018, 73, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, Y.; Liu, H.; Yin, C.; Li, X.; Gong, Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: A novel mechanism mediated by the activation of Nrf2. Pharmacol. Biochem. Behav. 2016, 150–151, 207–216. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhao, Y.; Yu, B.; Xu, G.-G.; Wang, W.; Zhan, J.-Q.; Tang, Z.-Y.; Wang, T.; Wei, B. GluN2B-containing NMDA receptors contribute to the beneficial effects of hydrogen sulfide on cognitive and synaptic plasticity deficits in APP/PS1 transgenic mice. Neuroscience 2016, 335, 170–183. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Yan, N.; Chen, X.S.; Qi, Y.W.; Yan, Y.; Cai, Z. Hydrogen sulfide down-regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacol. Rep. 2016, 68, 975–982. [Google Scholar] [CrossRef]
- He, X.L.; Yan, N.; Zhang, H.; Qi, Y.-W.; Zhu, L.-J.; Liu, M.-J.; Yan, Y. Hydrogen sulfide improves spatial memory impairment and decreases production of Aβ in APP/PS1 transgenic mice. Neurochem. Int. 2014, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, D.; Ottani, A.; Zaffe, D.; Galantucci, M.; Strinati, F.; Lodi, R.; Guarini, S. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol. Learn. Mem. 2013, 104, 82–91. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. A diet rich in taurine, cysteine, folate, B(12) and betaine may lessen risk for Alzheimer’s disease by boosting brain synthesis of hydrogen sulfide. Med. Hypotheses 2019, 132, 109356. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qu, J.; Li, Q.; Cui, M.; Wang, J.; Zhang, K.; Liu, X.; Feng, H.; Chen, Y. Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino Acids 2018, 50, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, H.V.; Yoon, J.H.; Kang, B.R.; Cho, S.M.; Lee, S.; Kim, J.Y.; Kim, J.W.; Cho, Y.; Woo, J.; et al. Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer’s disease. Sci. Rep. 2014, 4, 7467. [Google Scholar] [CrossRef]
- Jang, H.; Lee, S.; Choi, S.L.; Kim, H.Y.; Baek, S.; Kim, Y. Taurine Directly Binds to Oligomeric Amyloid-β and Recovers Cognitive Deficits in Alzheimer Model Mice. Adv. Exp. Med. Biol. 2017, 975, 233–241. [Google Scholar] [PubMed]
- Stadtman, T.C. Selenium biochemistry. Mammalian selenoenzymes. Ann. NY Acad. Sci. 2000, 899, 399–402. [Google Scholar] [CrossRef]
- Ye, Y.; Qu, J.; Pu, Y.; Rao, S.; Xu, F.; Wu, C. Selenium Biofortification of Crop Food by Beneficial Microorganisms. J. Fungi 2020, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C. Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Alexander, J.; Aaseth, J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS ONE 2016, 11, e0157541. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Myung, S.K.; Jeon, Y.J.; Kim, Y.; Chang, Y.J.; Ju, W.; Seo, H.G.; Huh, B.Y. Effects of selenium supplements on cancer prevention: Meta-analysis of randomized controlled trials. Nutr. Cancer 2011, 63, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Berr, C.; Arnaud, J.; Akbaraly, T.N. Selenium and cognitive impairment: A brief-review based on results from the EVA study. Biofactors 2012, 38, 139–144. [Google Scholar] [CrossRef]
- Yang, Y.W.; Liou, S.H.; Hsueh, Y.M.; Lyu, W.-S.; Liu, C.-S.; Liu, H.-J.; Chung, M.-C.; Hung, P.-H.; Chung, C.-J. Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: A case-control study using propensity score matching. Toxicol. Appl. Pharmacol. 2018, 356, 8–14. [Google Scholar] [CrossRef]
- Sun, H. Associations of Spatial Disparities of Alzheimer’s Disease Mortality Rates with Soil Selenium and Sulfur Concentrations and Four Common Risk Factors in the United States. J. Alzheimers Dis. 2017, 58, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, K.; Sun, X.; Qin, S.; Wu, M.; Qin, L.; Wang, Y.; Li, Z.; Zhong, X.; Wei, X. A cross-sectional study of blood selenium concentration and cognitive function in elderly Americans: National Health and Nutrition Examination Survey 2011–2014. Ann. Hum. Biol. 2020, 47, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Judd, P.A.; Long, A.; Butcher, M.; Caygill, C.P.; Diplock, A.T. Vegetarians and vegans may be most at risk from low selenium intakes. BMJ 1997, 314, 1834. [Google Scholar] [CrossRef][Green Version]
- Sobiecki, J.G. Vegetarianism and colorectal cancer risk in a low-selenium environment: Effect modification by selenium status? A possible factor contributing to the null results in British vegetarians. Eur. J. Nutr. 2017, 56, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food. Forum Nutr. 2009, 61, 129–135. [Google Scholar]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A. Calcium and cell death: The mitochondrial connection. Calcium Signal. Dis. 2007, 45, 481–506. [Google Scholar]
- Huang, C.; Wen, C.; Yang, M.; Li, A.; Fan, C.; Gan, D.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Astaxanthin Improved the Cognitive Deficits in APP/PS1 Transgenic Mice Via Selective Activation of mTOR. J. Neuroimmune Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Yan, R. A Close Look at BACE1 Inhibitors for Alzheimer’s Disease Treatment. CNS Drugs 2019, 33, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Zagórska, A.; Dulak, J. HIF-1: The knowns and unknowns of hypoxia sensing. Acta Biochim. Pol. 2004, 51, 563–585. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Xi, M.M.; Guo, Y.; Hai, C.X.; Yang, W.L.; Qin, X.J. NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylases activity. Toxicol. Lett. 2014, 224, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef]
- Fedele, A.O.; Whitelaw, M.L.; Peet, D.J. Regulation of gene expression by the hypoxia-inducible factors. Mol. Interv. 2002, 2, 229–243. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Wang, R.; Cui, J.; Lipton, S.A.; Liao, F.F.; Zhang, Y.W. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J. Biol. Chem. 2007, 282, 10873–10880. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: Impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2017, 140, 536–549. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Monteleone, D.; Giliberto, L.; Fornaro, M.; Borghi, R.; Tamagno, E.; Tabaton, M. Amyloid-beta42 activates the expression of BACE1 through the JNK pathway. J. Alzheimers Dis. 2011, 27, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Saadipour, K.; Tiberi, A.; Lombardo, S.; Grajales, E.; Montroull, L.; Mañucat-Tan, N.B.; LaFrancois, J.; Cammer, M.; Mathews, P.M.; Scharfman, H.E.; et al. Regulation of BACE1 expression after injury is linked to the p75 neurotrophin receptor. Mol. Cell. Neurosci. 2019, 99, 103395. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.D.; Wang, R.; Li, J.J.; Zhang, Y.W.; Xu, H.; Liao, F.F. Differential regulation of BACE1 expression by oxidative and nitrosative signals. Mol. Neurodegener. 2011, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.A.; d’Uscio, L.V.; Katusic, Z.S. Supplementation of nitric oxide attenuates AbPP and BACE1 protein in cerebral microcirculation of eNOS-deficient mice. J. Alzheimers Dis. 2013, 33, 29–33. [Google Scholar] [CrossRef]
- Borniquel, S.; Valle, I.; Cadenas, S.; Lamas, S.; Monsalve, M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006, 20, 1889–1891. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Itoh, H.; Tsujimoto, H.; Tamura, N.; Fukunaga, Y.; Sone, M.; Yamahara, K.; Taura, D.; Inuzuka, M.; Sonoyama, T.; et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 2009, 58, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, L.; Lim, Y.M.; Blondrath, K.; Eleftheriadou, I.; Lombardero, L.; Birch, A.M.; Mirzaei, N.; Irvine, E.E.; Mazarakis, N.D.; Sastre, M. PPARgamma-coactivator-1alpha gene transfer reduces neuronal loss and amyloid-beta generation by reducing beta-secretase in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. USA 2016, 113, 12292–12297. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, L.; Parr, C.; Bogdanovic, N.; Willem, M.; Sastre, M. PPARγ co-activator-1α (PGC-1α) reduces amyloid-β generation through a PPARγ -dependent mechanism. J. Alzheimers Dis. 2011, 25, 151–162. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schmidt, P.M.; Nedvetsky, P.I.; Nedvetskaya, T.Y.; Kumar, A.H.S.; Meurer, S.; Deile, M.; Taye, A.; Knorr, A.; Lapp, H.; et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Invest. 2006, 116, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Martin, E.; Sharina, I.; Esposito, I.; Szabo, C.; Bucci, M.; Cirino, G.; Papapetropoulos, A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharmacol. Res. 2016, 111, 556–562. [Google Scholar] [CrossRef]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef]
- Vesely, D.L. Biotin enhances guanylate cyclase activity. Science 1982, 216, 1329–1330. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kamiyama, M.; Kamiyama, S.; Horiuchi, K.; Ohinata, K.; Shirakawa, H.; Furukawa, Y.; Komai, M. Antihypertensive effect of biotin in stroke-prone spontaneously hypertensive rats. Br. J. Nutr. 2008, 99, 756–763. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. In type 1 diabetics, high-dose biotin may compensate for low hepatic insulin exposure, promoting a more normal expression of glycolytic and gluconeogenic enyzymes and thereby aiding glycemic control. Med. Hypotheses 2016, 95, 45–48. [Google Scholar] [CrossRef]
- McCarty, M.F. cGMP may have trophic effects on beta cell function comparable to those of cAMP, implying a role for high-dose biotin in prevention/treatment of diabetes. Med. Hypotheses 2006, 66, 323–328. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; DiNicolantonio, J.J. Neuroprotective potential of high-dose biotin. Med. Hypotheses 2017, 109, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ferguson, A.; Cervinski, M.A.; Lynch, K.L.; Kyle, P.B. AACC Guidance Document on Biotin Interference in Laboratory Tests. J. Appl. Lab. Med. 2020, 5, 575–587. [Google Scholar] [CrossRef]
- Corbett, G.T.; Gonzalez, F.J.; Pahan, K. Activation of peroxisome proliferator-activated receptor α stimulates ADAM10-mediated proteolysis of APP. Proc. Natl. Acad. Sci. USA 2015, 112, 8445–8450. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.J. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kim, J.Y.; Jun, H.J.; Kim, S.-J.; Lee, J.-H.; Hoang, M.H.; Hwang, K.-Y.; Um, S.-J.; Chang, H.I.; Lee, S.-J. The natural carotenoid astaxanthin, a PPAR-α agonist and PPAR-γ antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Fanaee-Danesh, E.; Gali, C.C.; Tadic, J.; Zandl-Lang, M.; Kober, A.C.; Agujetas, V.R.; de Dios, C.; Tam-Amersdorfer, C.; Stracke, A.; Albrecher, N.M.; et al. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer’s disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2224–2245. [Google Scholar] [CrossRef] [PubMed]
- Storck, S.E.; Meister, S.; Nahrath, J.; Meißner, J.N.; Schubert, N.; Di Spiezio, A.; Baches, S.; Vandenbroucke, R.E.; Bouter, Y.; Prikulis, I.; et al. Endothelial LRP1 transports amyloid-beta(1–42) across the blood-brain barrier. J. Clin. Invest. 2016, 126, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zhang, X.; Meng, X.; Xu, P.; Zou, X.; Qu, S. Amyloid-beta peptide decreases expression and function of glutamate transporters in nervous system cells. Int. J. Biochem. Cell Biol. 2017, 85, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wyssenbach, A.; Quintela, T.; Llavero, F.; Zugaza, J.L.; Matute, C.; Alberdi, E. Amyloid β-induced astrogliosis is mediated by β1-integrin via NADPH oxidase 2 in Alzheimer’s disease. Aging Cell 2016, 15, 1140–1152. [Google Scholar] [CrossRef]
- Kalandadze, A.; Wu, Y.; Robinson, M.B. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J. Biol. Chem. 2002, 277, 45741–45750. [Google Scholar] [CrossRef]
- Sheldon, A.L.; Gonzãlez, M.I.; Krizman-Genda, E.N.; Susarla, B.T.; Robinson, M.B. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochem. Int. 2008, 53, 296–308. [Google Scholar] [CrossRef]
- Martínez-Villarreal, J.; García-Tardón, N.; Ibáñez, I.; Giménez, C.; Zafra, F. Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia 2012, 60, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- García-Tardón, N.; González-González, I.M.; Martínez-Villarreal, J.; Fernández-Sánchez, E.; Giménez, C.; Zafra, F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4–2-dependent ubiquitination but not phosphorylation. J. Biol. Chem. 2012, 287, 19177–19187. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Takase, H.; Nunome, M.; Enomoto, H.; Ito, I.-I.; Gong, J.-S.; Michikawa, M. Amyloid-β Reduces Exosome Release from Astrocytes by Enhancing JNK Phosphorylation. J. Alzheimers Dis. 2016, 53, 1433–1441. [Google Scholar] [CrossRef]
- Gao, K.; Wang, C.R.; Jiang, F.; Wong, A.Y.K.; Su, N.; Jiang, J.H.; Chai, R.C.; Vatcher, G.; Teng, J.; Chen, J.; et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia 2013, 61, 2063–2077. [Google Scholar] [CrossRef]
- Gwoździńska, P.; Buchbinder, B.A.; Mayer, K.; Herold, S.; Morty, R.E.; Seeger, W.; Vadász, I. Hypercapnia Impairs ENaC Cell Surface Stability by Promoting Phosphorylation, Polyubiquitination and Endocytosis of β-ENaC in a Human Alveolar Epithelial Cell Line. Front. Immunol. 2017, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Hallows, K.R.; Bhalla, V.; Oyster, N.M.; Wijngaarden, M.A.; Lee, J.K.; Li, H.; Chandran, S.; Xia, X.; Huang, Z.; Chalkley, R.J.; et al. Phosphopeptide screen uncovers novel phosphorylation sites of Nedd4–2 that potentiate its inhibition of the epithelial Na+ channel. J. Biol. Chem. 2010, 285, 21671–21678. [Google Scholar] [CrossRef]
- Han, X.; Yang, L.; Du, H.; Sun, Q.; Wang, X.; Cong, L.; Liu, X.; Yin, L.; Li, S.; Du, Y. Insulin Attenuates Beta-Amyloid-Associated Insulin/Akt/EAAT Signaling Perturbations in Human Astrocytes. Cell Mol. Neurobiol. 2016, 36, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Cheung, B.S.; Hyman, B.T.; Irizarry, M.C. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002, 59, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Lindholm, K.; Yan, R.; Citron, M.; Xia, W.; Yang, X.L.; Beach, T.; Sue, L.; Wong, P.; Price, D.; et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003, 9, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, T.; Alexopoulos, P.; Tsolakidou, A.; Guo, L.H.; Henriksen, G.; Yousefi, B.H.; Förstl, H.; Sorg, C.; Kurz, A.; Drzezga, A.; et al. Cerebrospinal fluid BACE1 activity and brain amyloid load in Alzheimer’s disease. Sci. World J. 2012, 2012, 712048. [Google Scholar] [CrossRef] [PubMed]
- Ewers, M.; Cheng, X.; Zhong, Z.; Nural, H.F.; Walsh, C.; Meindl, T.; Teipel, S.J.; Buerger, K.; He, P.; Shen, Y.; et al. Increased CSF-BACE1 activity associated with decreased hippocampus volume in Alzheimer’s disease. J. Alzheimers Dis. 2011, 25, 373–381. [Google Scholar] [CrossRef]
- Tyler, S.J.; Dawbarn, D.; Wilcock, G.K.; Allen, S.J. alpha- and beta-secretase: Profound changes in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 299, 373–376. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [PubMed]
- Saido, T.; Leissring, M.A. Proteolytic degradation of amyloid β -protein. Cold Spring Harb. Perspect. Med. 2012, 2, a006379. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Sastre, M. Mechanisms of Aβ Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain--implications for Alzheimer diseaser. Nat. Rev. Neurol. 2016, 12, 248. [Google Scholar] [CrossRef]
- Deane, R.; Zlokovic, B.V. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Storck, S.E.; Hartz, A.M.S.; Bernard, J.; Wolf, A.; Kachlmeier, A.; Mahringer, A.; Weggen, S.; Pahnke, J.; Pietrzik, C.U. The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM. Brain Behav. Immun. 2018, 73, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, H.; Tomiyama, T.; Mori, H. Human neprilysin is capable of degrading amyloid beta peptide not only in the monomeric form but also the pathological oligomeric form. Neurosci. Lett. 2003, 350, 113–116. [Google Scholar] [CrossRef]
- Yasojima, K.; McGeer, E.G.; McGeer, P.L. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001, 919, 115–121. [Google Scholar] [CrossRef]
- Yasojima, K.; Akiyama, H.; McGeer, E.G.; McGeer, P.L. Reduced neprilysin in high plaque areas of Alzheimer brain: A possible relationship to deficient degradation of beta-amyloid peptide. Neurosci. Lett. 2001, 297, 97–100. [Google Scholar] [CrossRef]
- Wang, D.S.; Lipton, R.B.; Katz, M.J.; Davies, P.; Buschke, H.; Kuslansky, G.; Verghese, J.; Younkin, S.G.; Eckman, C.; Dickson, D.W. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J. Neuropathol. Exp. Neurol. 2005, 64, 378–385. [Google Scholar] [CrossRef]
- El-Amouri, S.S.; Zhu, H.; Yu, J.; Marr, R.; Verma, I.M.; Kindy, M.S. Neprilysin: An enzyme candidate to slow the progression of Alzheimer’s disease. Am. J. Pathol. 2008, 172, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.; Marr, R.A.; Rockenstein, E.; Crews, L.; Adame, A.; Potkar, R.; Patrick, C.; Gage, F.H.; Verma, I.M.; Masliah, E. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 2008, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Hüttenrauch, M.; Baches, S.; Gerth, J.; Bayer, T.A.; Weggen, S.; Wirths, O. Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015, 44, 1291–1302. [Google Scholar]
- Wang, R.; Wang, S.; Malter, J.S.; Wang, D.S. Effects of HNE-modification induced by Abeta on neprilysin expression and activity in SH-SY5Y cells. J. Neurochem. 2009, 108, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wei, C.; Huang, W.; Bennett, D.A.; Dickson, D.W.; Wang, R.; Wang, D. Distinct subcellular patterns of neprilysin protein and activity in the brains of Alzheimer’s disease patients, transgenic mice and cultured human neuronal cells. Am. J. Transl. Res. 2013, 5, 608–621. [Google Scholar] [PubMed]
- Wang, D.S.; Iwata, N.; Hama, E.; Saido, T.C.; Dickson, D.W. Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2003, 310, 236–241. [Google Scholar] [CrossRef]
- Wang, R.; Malter, J.S.; Wang, D.S. N-acetylcysteine prevents 4-hydroxynonenal- and amyloid-beta-induced modification and inactivation of neprilysin in SH-SY5Y cells. J. Alzheimers Dis 2010, 19, 179–189. [Google Scholar] [CrossRef]
- Zhou, L.; Qian, J.; Liu, J.; Zhao, R.; Li, B.; Wang, R. Identification of the sites of 4-hydroxy-2-nonenal and neprilysin adduction using a linear trap quadrapole Velos Pro-Orbitrap Elite mass spectrometer. Eur. J. Mass Spectrom. 2016, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Han, J.S. The antioxidant xanthorrhizol prevents amyloid-β-induced oxidative modification and inactivation of neprilysin. Biosci. Rep. 2018, 38, BSR20171611. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Sakuma, K.; Kuchide, M.; Tokuda, H.; Maoka, T.; Toyokuni, S.; Oka, S.; Yasuhara, M.; Yoshikawa, T. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxid. Redox Signal. 2003, 5, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Inokuchi, Y.; Shimazawa, M.; Otsubo, K.; Ishibashi, T.; Hara, H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J. Pharm. Pharmacol. 2008, 60, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.M.; Girens, R.E.; Larson, S.K.; Jones, M.R.; Restivo, J.L.; Holtzman, D.M.; Cirrito, J.R.; Yuede, C.M.; Zimmerman, S.D.; Timson, B.F. A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 85, 218–224. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, D.; Zhang, X.; Li, T.; Li, J.; Tang, Y.; Le, W. Hypoxia-induced down-regulation of neprilysin by histone modification in mouse primary cortical and hippocampal neurons. PLoS ONE 2011, 6, e19229. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hou, T.; Wang, W.; Luo, Y.; Yan, F.; Jia, J. The Effect of Chronic Cerebral Hypoperfusion on Amyloid-β Metabolism in a Transgenic Mouse Model of Alzheimer’s Disease (PS1V97L). J. Alzheimers Dis. 2018, 62, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Rong, C.; Chen, Y.; Yang, C.; Hu, Q.; Mo, Y.; Zhang, C.; Gu, X.; Zhang, L.; He, W.; et al. (-)-Epigallocatechin-3-gallate attenuates cognitive deterioration in Alzheimer’s disease model mice by upregulating neprilysin expression. Exp. Cell Res. 2015, 334, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, J.; Jiang, C.; Wang, S.; Que, R.; An, L. Up-regulation of neprilysin mediates the protection of fructo-oligosaccharides against Alzheimer’s disease. Food Funct. 2020, 11, 6565–6572. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Lehmann, J.; Mett, J.; Zimmer, V.C.; Grösgen, S.; Stahlmann, C.P.; Hundsdörfer, B.; Haupenthal, V.J.; Rothhaar, T.L.; Herr, C.; et al. Impact of Vitamin D on amyloid precursor protein processing and amyloid- beta peptide degradation in Alzheimer’s disease. Neurodegener. Dis. 2014, 13, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J.; et al. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 2000, 106, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Miller, M.C.; Monahan, R.; Osgood, D.P.; Stopa, E.G.; Silverberg, G.D. P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer’s disease: Preliminary observations. Neurobiol. Aging 2015, 36, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Storck, S.E.; Pietrzik, C.U. Endothelial LRP1—A Potential Target for the Treatment of Alzheimer’s Disease: Theme: Drug Discovery, Development and Delivery in Alzheimer’s Disease Guest Editor: Davide Brambilla. Pharm. Res. 2017, 34, 2637–2651. [Google Scholar] [CrossRef]
- Gali, C.C.; Fanaee-Danesh, E.; Zandl-Lang, M.; Albrecher, N.M.; Tam-Amersdorfer, C.; Stracke, A.; Sachdev, V.; Reichmann, F.; Sun, Y.; Avdili, A.; et al. Amyloid-beta impairs insulin signaling by accelerating autophagy-lysosomal degradation of LRP-1 and IR- β in blood-brain barrier endothelial cells in vitro and in 3XTg-AD mice. Mol. Cell. Neurosci. 2019, 99, 103390. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Kook, S.Y.; Park, J.C.; Mook-Jung, I. Aβ 1–42 reduces P-glycoprotein in the blood-brain barrier through RAGE-NF-κB signaling. Cell Death Dis. 2014, 5, e1299. [Google Scholar] [CrossRef]
- Hartz, A.M.; Zhong, Y.; Wolf, A.; LeVine, H., III; Miller, D.S.; Bauer, B. Aβ 40 Reduces P-Glycoprotein at the Blood-Brain Barrier through the Ubiquitin-Proteasome Pathway. J. Neurosci. 2016, 36, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Choi, S.M.; Whitcomb, D.J.; Kim, B.C. Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis. 2017, 8, e3102. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.A., 3rd; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E.; et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef]
- Brenn, A.; Grube, M.; Peters, M.; Fischer, A.; Jedlitschky, G.; Kroemer, H.K.; Warzok, R.W.; Vogelgesang, S. Beta-Amyloid Downregulates MDR1-P-Glycoprotein (Abcb1) Expression at the Blood-Brain Barrier in Mice. Int. J. Alzheimers Dis. 2011, 2011, 690121. [Google Scholar] [CrossRef]
- Park, L.; Wang, G.; Zhou, P.; Zhou, J.; Pitstick, R.; Previti, M.L.; Younkin, L.; Younkin, S.G.; Van Nostrand, W.E.; Cho, S.; et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc. Natl. Acad. Sci. USA 2011, 108, 5063–5068. [Google Scholar] [CrossRef] [PubMed]
- Carrano, A.; Hoozemans, J.J.; van der Vies, S.M.; Rozemuller, A.J.; van Horssen, J.; de Vries, H.E. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal. 2011, 15, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Anrather, J.; Zhou, P.; Frys, K.; Pitstick, R.; Younkin, S.; Carlson, G.A.; Iadecola, C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J. Neurosci. 2005, 25, 1769–1777. [Google Scholar] [CrossRef]
- Han, B.H.; Zhou, M.L.; Johnson, A.W.; Singh, I.; Liao, F.; Vellimana, A.K.; Nelson, J.W.; Milner, E.; Cirrito, J.R.; Basak, J.; et al. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc. Natl. Acad. Sci. USA 2015, 112, E881–E890. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, L.; Martin, M.; Hsu, C.Y.; Fang, L.; Lin, F.M.; Lin, T.Y.; Geary, M.J.; Geary, G.G.; Zhao, Y.; et al. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation 2015, 131, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Shin, S.M. Induction of Lipin1 by ROS-Dependent SREBP-2 Activation. Toxicol. Res. 2017, 33, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Cortés, V.; Costales, P.; Bernués, J.; Camino-Lopez, S.; Badimon, L. Sterol regulatory element-binding protein-2 negatively regulates low density lipoprotein receptor-related protein transcription. J. Mol. Biol. 2006, 359, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Cortés, V.; Royo, T.; Otero-Viñas, M.; Berrozpe, M.; Badimon, L. Sterol regulatory element binding proteins downregulate LDL receptor-related protein (LRP1) expression and LRP1-mediated aggregated LDL uptake by human macrophages. Cardiovasc. Res. 2007, 74, 526–536. [Google Scholar] [CrossRef]
- Akkaya, B.G.; Zolnerciks, J.K.; Ritchie, T.K.; Bauer, B.; Hartz, A.M.; Sullivan, J.A.; Linton, K.J. The multidrug resistance pump ABCB1 is a substrate for the ubiquitin ligase NEDD4–1. Mol. Membr. Biol. 2015, 32, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Pieper, R.O.; Li, D.; Wei, P.; Liu, M.; Woo, S.Y.; Aldape, K.D.; Sawaya, R.; Xie, K.; Huang, S. FoxM1B regulates NEDD4–1 expression, leading to cellular transformation and full malignant phenotype in immortalized human astrocytes. Cancer Res. 2010, 70, 2951–2961. [Google Scholar] [CrossRef]
- Kwak, Y.D.; Wang, B.; Li, J.J.; Wang, R.; Deng, Q.; Diao, S.; Chen, Y.; Xu, R.; Masliah, E.; Xu, H.; et al. Upregulation of the E3 ligase NEDD4–1 by oxidative stress degrades IGF-1 receptor protein in neurodegeneration. J. Neurosci. 2012, 32, 10971–10981. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.J.; Carr, J.R.; Wang, Z.; Nogueira, V.; Hay, N.; Tyner, A.L.; Lau, L.F.; Costa, R.H.; Raychaudhuri, P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009, 28, 2908–2918. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, X.; Qiu, C.; Zheng, J. FoxM1 is regulated by both HIF-1α and HIF- 2α and contributes to gastrointestinal stromal tumor progression. Gastric Cancer 2019, 22, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, L.; Zhu, Q.; Yi, F.; Zhang, F.; Li, P.L.; Li, N. Hypoxia-inducible factor-1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011, 79, 300–310. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-kappaB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef]
- Yan, L.; Xie, Y.; Satyanarayanan, S.K.; Zeng, H.; Liu, Q.; Huang, M.; Ma, Y.; Wan, J.B.; Yao, X.; Su, K.P.; et al. Omega-3 polyunsaturated fatty acids promote brain-to-blood clearance of β-Amyloid in a mouse model with Alzheimer’s disease. Brain Behav. Immun. 2020, 85, 35–45. [Google Scholar] [CrossRef]
- Chan, J.P.; Wong, B.H.; Chin, C.F.; Galam, D.L.A.; Foo, J.C.; Wong, L.C.; Ghosh, S.; Wenk, M.R.; Cazenave-Gassiot, A.; Silver, D.L. The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol. 2018, 16, e2006443. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, G.; Ma, M.; Qiu, N.; Zhu, L.; Li, J. Fatty acids modulate the expression levels of key proteins for cholesterol absorption in Caco-2 monolayer. Lipids Health Dis. 2018, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Dacks, P.A.; Shineman, D.W.; Fillit, H.M. Current evidence for the clinical use of long-chain polyunsaturated n-3 fatty acids to prevent age-related cognitive decline and Alzheimer’s disease. J. Nutr. Health Aging 2013, 17, 240–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef]
- Nock, T.G.; Chouinard-Watkins, R.; Plourde, M. Carriers of an apolipoprotein E epsilon 4 allele are more vulnerable to a dietary deficiency in omega-3 fatty acids and cognitive decline. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1068–1078. [Google Scholar] [CrossRef]
- Santhanam, A.V.; d’Uscio, L.V.; He, T.; Das, P.; Younkin, S.G.; Katusic, Z.S. Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice. J. Neurochem. 2015, 134, 1129–1138. [Google Scholar] [CrossRef]
- Milstien, S.; Katusic, Z. Oxidation of tetrahydrobiopterin by peroxynitrite: Implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999, 263, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.P.; Druhan, L.J.; Guzman, J.E.; Parinandi, N.; Zhang, L.; Green-Church, K.B.; Cardounel, A.J. Mechanism of 4-HNE mediated inhibition of hDDAH-1: Implications in no regulation. Biochemistry 2008, 47, 1819–1826. [Google Scholar] [CrossRef]
- Dragovich, M.A.; Chester, D.; Fu, B.M.; Wu, C.; Xu, Y.; Goligorsky, M.S.; Zhang, X.F. Mechanotransduction of the endothelial glycocalyx mediates nitric oxide production through activation of TRP channels. Am. J. Physiol. Cell Physiol. 2016, 311, C846–C853. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Santos, R.M.; Barbosa, R.M.; Cadenas, E.; Radi, R.; Laranjinha, J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic. Biol Med. 2014, 73, 421–429. [Google Scholar] [CrossRef]
- Michetti, M.; Salamino, F.; Melloni, E.; Pontremoli, S. Reversible inactivation of calpain isoforms by nitric oxide. Biochem. Biophys. Res. Commun. 1995, 207, 1009–1014. [Google Scholar] [CrossRef]
- Samengo, G.; Avik, A.; Fedor, B.; Whittaker, D.; Myung, K.H.; Wehling-Henricks, M.; Tidball, J.G. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell 2012, 11, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, Y.; Wang, M.; Zhou, G.; Zhang, W. Effect of protein S-nitrosylation on autolysis and catalytic ability of μ-calpain. Food Chem. 2016, 213, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.A.; Katusic, Z.S. Loss of Endothelial Nitric Oxide Synthase Promotes p25 Generation and Tau Phosphorylation in a Murine Model of Alzheimer’s Disease. Circ. Res. 2016, 119, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Won, J.S.; Annamalai, B.; Choi, S.; Singh, I.; Singh, A.K. S-nitrosoglutathione reduces tau hyper-phosphorylation and provides neuroprotection in rat model of chronic cerebral hypoperfusion. Brain Res. 2015, 1624, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Waugh, W.H.; Daeschner, C.W., III; Files, B.A.; McConnell, M.E.; Strandjord, S.E. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: Early phase two results. J. Natl. Med. Assoc. 2001, 93, 363–371. [Google Scholar] [PubMed]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Selley, M.L. Increased concentrations of homocysteine and asymmetric dimethylarginine and decreased concentrations of nitric oxide in the plasma of patients with Alzheimer’s disease. Neurobiol. Aging 2003, 24, 903–907. [Google Scholar] [CrossRef]
- Arlt, S.; Schulze, F.; Eichenlaub, M.; Maas, R.; Lehmbeck, J.T.; Schwedhelm, E.; Jahn, H.; Böger, R.H. Asymmetrical dimethylarginine is increased in plasma and decreased in cerebrospinal fluid of patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 26, 58–64. [Google Scholar] [CrossRef]
- Ginguay, A.; Regazzetti, A.; Laprevote, O.; Moinard, C.; De Bandt, J.P.; Cynober, L.; Billard, J.M.; Allinquant, B.; Dutar, P. Citrulline prevents age-related LTP decline in old rats. Sci. Rep. 2019, 9, 20138. [Google Scholar] [CrossRef] [PubMed]
- Haul, S.; Gödecke, A.; Schrader, J.; Haas, H.L.; Luhmann, H.J. Impairment of neocortical long-term potentiation in mice deficient of endothelial nitric oxide synthase. J. Neurophysiol. 1999, 81, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.G.; Hardingham, N.R.; Fox, K. Postsynaptic action potentials are required for nitric-oxide-dependent long-term potentiation in CA1 neurons of adult GluR1 knock-out and wild-type mice. J. Neurosci. 2008, 28, 14031–14041. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Palencia, C.A.; Pahan, K. Fibrillar amyloid-beta peptides activate microglia via TLR2: Implications for Alzheimer’s disease. J. Immunol. 2008, 181, 7254–7262. [Google Scholar] [CrossRef] [PubMed]
- Gambuzza, M.E.; Sofo, V.; Salmeri, F.M.; Soraci, L.; Marino, S.; Bramanti, P. Toll-like receptors in Alzheimer’s disease: A therapeutic perspective. CNS Neurol. Disord. Drug Targets 2014, 13, 1542–1558. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Hao, W.; Wolf, L.; Kiliaan, A.J.; Penke, B.; Rübe, C.E.; Walter, J.; Heneka, M.T.; Hartmann, T.; et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J. Immunol. 2012, 188, 1098–10107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, Y.; Li, Q.; Chen, C.; Chen, H.; Song, Y.; Hua, F.; Zhang, Z. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci. Lett. 2020, 736, 135279. [Google Scholar] [CrossRef]
- Balducci, C.; Frasca, A.; Zotti, M.; La Vitola, P.; Mhillaj, E.; Grigoli, E.; Iacobellis, M.; Grandi, F.; Messa, M.; Colombo, L.; et al. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav. Immun. 2017, 60, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Parajuli, B.; Sonobe, Y.; Horiuchi, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Oligomeric amyloid β induces IL-1β processing via production of ROS: Implication in Alzheimer’s disease. Cell Death Dis. 2013, 4, e975. [Google Scholar] [CrossRef] [PubMed]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 Inflammasome Is Expressed and Functional in Mouse Brain Microglia but Not in Astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhang, J.; Yang, G. Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer’s Disease. Neurochem. Res. 2020, 45, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, G.; Wang, B.; Yin, H.; Fang, X.; He, F.; Zhao, D.; Liu, Q.; Shi, L. IL-1R(-/-) alleviates cognitive deficits through microglial M2 polarization in AD mice. Brain Res. Bull. 2020, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Cheng, D.; Tsukamoto, M.R.; Koike, M.A.; Wes, P.D.; Vasilevko, V.; Cribbs, D.H.; LaFerla, F.M. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 2011, 187, 6539–6549. [Google Scholar] [CrossRef]
- Simãµes, A.P.; Duarte, J.A.; Agasse, F.; Canas, P.M.; Tomé, A.R.; Agostinho, P.; Cunha, R.A. Blockade of adenosine A2A receptors prevents interleukin-1β-induced exacerbation of neuronal toxicity through a p38 mitogen-activated protein kinase pathway. J. Neuroinflammation 2012, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, L.; Hayashi, Y.; Liu, X.; Koyama, S.; Wu, Z.; Nakanishi, H. Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1beta. Neurobiol. Dis. 2010, 38, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ruan, X.; Zhang, S.; Sun, X. Effect of interleukin-1 beta on the elevation of cytoplasmic free calcium of the cultured hippocampal neurons induced by L-glutamate. J. Tongji Med. Univ. 1999, 19, 120–123. [Google Scholar] [PubMed]
- Viviani, B.; Bartesaghi, S.; Gardoni, F.; Vezzani, A.; Behrens, M.M.; Bartfai, T.; Binaglia, M.; Corsini, E.; Di Luca, M.; Galli, C.L.; et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003, 23, 8692–8700. [Google Scholar] [CrossRef]
- Zhang, R.; Yamada, J.; Hayashi, Y.; Wu, Z.; Koyama, S.; Nakanishi, H. Inhibition of NMDA-induced outward currents by interleukin-1beta in hippocampal neurons. Biochem. Biophys. Res. Commun. 2008, 372, 816–820. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, C.Y.; Fujita, T.; Luo, S.W.; Kumamoto, E. Enhancement by interleukin-1β of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal horn neurons. Mol. Pain 2013, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Trapp, B.D. Microglia and neuroprotection. J. Neurochem. 2016, 136 (Suppl. 1), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M.F. HMGB1-Mediated Neuroinflammatory Responses in Brain Injuries: Potential Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 4609. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Lerner, A. The Second Phase of Brain Trauma Can Be Controlled by Nutraceuticals that Suppress DAMP-Mediated Microglial Activation. Expert Rev. Neurother. 2021. in review process. [Google Scholar]
- Grimm, M.O.W.P.; Thiel, A.; Lauer, A.A.; Winkler, J.; Lehmann, J.; Regner, L.; Nelke, C.; Janitschke, D.; Benoist, C.; Streidenberger, O.; et al. Vitamin D and Its Analogues Decrease Amyloid- β (Aβ) Formation and Increase Aβ-Degradation. Int. J. Mol. Sci. 2017, 18, 2764. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Zhou, H.Y.; Gui, Y.R.; Yang, Y.H.; Wu, M.J.; Xiao, Y.F.; Shang, J.T.; Long, G.F.; Shu, X.J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of diets containing curcumin, epigallocatechin-3-gallate, docosahexaenoic acid and α-lipoic acid on amyloid load and inflammation in a male transgenic mouse model of Alzheimer’s disease: Are combinations more effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimers Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Han, K.; Kong, J.J.; Zhang, X.M.; Sha, S.; Ren, G.R.; Cao, Y.P. (-)-Epigallocatechin-3-gallate alleviates spatial memory impairment in APP/PS1 mice by restoring IRS-1 signaling defects in the hippocampus. Mol. Cell. Biochem. 2013, 380, 211–218. [Google Scholar] [CrossRef]
- Durairajan, S.S.; Liu, L.F.; Lu, J.H.; Chen, L.L.; Yuan, Q.; Chung, S.K.; Huang, L.; Li, X.S.; Huang, J.D.; Li, M. Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging 2012, 33, 2903–2919. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.N.; Cai, C.Z.; Wu, M.Y.; Su, H.X.; Li, M.; Lu, J.H. Neuroprotective effects of berberine in animal models of Alzheimer’s disease: A systematic review of pre-clinical studies. BMC Complement. Altern. Med. 2019, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N., Jr.; Frautschy, S.A.; Cole, G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Perez, S.E.; Berg, B.M.; Moore, K.A.; He, B.; Counts, S.E.; Fritz, J.J.; Hu, Y.S.; Lazarov, O.; Lah, J.J.; Mufson, E.J. DHA diet reduces AD pathology in young APPswe/PS1 Delta E9 transgenic mice: Possible gender effects. J. Neurosci. Res. 2010, 88, 1026–1040. [Google Scholar] [PubMed]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid- β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Shin, S.J.; Kim, H.S.; Hong, S.B.; Kim, S.; Nam, Y.; Kim, J.J.; Lim, K.; Kim, J.S.; Kim, J.I.; et al. Omega-3 Fatty Acid-Type Docosahexaenoic Acid Protects against Aβ -Mediated Mitochondrial Deficits and Pathomechanisms in Alzheimer’s Disease-Related Animal Model. Int. J. Mol. Sci. 2020, 21, 3879. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: Evidence from meta-analysis. Nutr. J. 2015, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Teixeira, A.L.; Diniz, B.S. Association of Vitamin D Levels with Incident All-Cause Dementia in Longitudinal Observational Studies: A Systematic Review and Meta-analysis. J. Prev. Alzheimers Dis. 2020, 7, 14–20. [Google Scholar]
- Yang, K.; Chen, J.; Li, X.; Zhou, Y. Vitamin D concentration and risk of Alzheimer disease: A meta-analysis of prospective cohort studies. Medicine 2019, 98, e16804. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- O’Day, D.H.; Eshak, K.; Myre, M.A. Calmodulin Binding Proteins and Alzheimer’s Disease. J. Alzheimers Dis. 2015, 46, 553–569. [Google Scholar]
- Malmendal, A.; Linse, S.; Evenäs, J.; Forsén, S.; Drakenberg, T. Battle for the EF-hands: Magnesium-calcium interference in calmodulin. Biochemistry 1999, 38, 11844–11850. [Google Scholar] [CrossRef]
- Grabarek, Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim. Biophys. Acta 2011, 1813, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary Mineral Intake and Risk of Mild Cognitive Impairment: The PATH through Life Project. Front. Aging Neurosci. 2014, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, N.S.; Chung, C.H.; Lin, F.H.; Huang, C.F.; Yeh, C.B.; Huang, S.Y.; Lu, R.B.; Chang, H.A.; Kao, Y.C.; Yeh, H.W.; et al. Magnesium oxide use and reduced risk of dementia: A retrospective, nationwide cohort study in Taiwan. Curr. Med. Res. Opin. 2018, 34, 163–169. [Google Scholar] [CrossRef]
- Li, W.; Yu, J.; Liu, Y.; Huang, X.; Abumaria, N.; Zhu, Y.; Huang, X.; Xiong, W.; Ren, C.; Liu, X.G.; et al. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer’s disease mouse model. Mol. Brain 2014, 7, 65. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Xun, P.; He, K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: Meta-analysis and systematic review. Eur. J. Clin. Nutr. 2014, 68, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendia, L.E.; Sahebkar, A.; Rodriguez-Moran, M.; Zambrano-Galvan, G.; Guerrero-Romero, F. Effect of Magnesium Supplementation on Plasma C-reactive Protein Concentrations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2017, 23, 4678–4686. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, M.; Li, N.; Wang, C.; Zheng, Y.; Cao, X. CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood 2008, 112, 4961–4970. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, X.; Wu, X.; Huang, S. Ketamine inhibits LPS-mediated BV2 microglial inflammation via NMDA receptor blockage. Fundam. Clin. Pharmacol. 2020, 34, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Weinger, J.G.; Lu, Z.L.; Xue, F.; Sadeghpour, S. Efficacy and Safety of MMFS-01, a Synapse Density Enhancer, for Treating Cognitive Impairment in Older Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2016, 49, 971–990. [Google Scholar] [CrossRef] [PubMed]
- Wroolie, T.E.; Chen, K.; Watson, K.T.; Iagaru, A.; Sonni, I.; Snyder, N.; Lee, W.; Reiman, E.M.; Rasgon, N.L. An 8-week open label trial of L-threonic acid magnesium salt in patients with mild to moderate dementia. Pers. Med. Psychiatry 2017, 4–6, 7–12. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.H.; Gao, X.; Na, M.; Kris-Etherton, P.M.; Mitchell, D.C.; Jensen, G.L. Dietary Pattern, Diet Quality, and Dementia: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Alzheimers Dis. 2020, 78, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Guure, C.B.; Ibrahim, N.A.; Adam, M.B.; Said, S.M. Impact of Physical Activity on Cognitive Decline, Dementia, and Its Subtypes: Meta-Analysis of Prospective Studies. Biomed. Res. Int. 2017, 2017, 9016924. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.F.; Wan, Y.; Tan, C.C.; Yu, J.T.; Tan, L. Leisure time physical activity and dementia risk: A dose-response meta-analysis of prospective studies. BMJ Open 2017, 7, e014706. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, T.; Kunutsor, S.; Kauhanen, J.; Laukkanen, J.A. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing 2017, 46, 245–249. [Google Scholar] [CrossRef]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef] [PubMed]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, C.D.; Selbæk, G.; Kivipelto, M.; Moreira, J.B.N.; et al. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Castillo- García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Barnes, J.N.; Corkery, A.T. Exercise Improves Vascular Function, but does this Translate to the Brain? Brain Plast. 2018, 4, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.P.; Minett, G.M.; Gibson, O.R.; Kerr, G.K.; Stewart, I.B. Could Heat Therapy Be an Effective Treatment for Alzheimer’s and Parkinson’s Diseases? A Narrative Review. Front. Physiol. 2019, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Barua, S.; Jeong, Y.J.; Lee, J.E. Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6419. [Google Scholar] [CrossRef]
- McCarty, M.F. GCN2 and FGF21 are likely mediators of the protection from cancer, autoimmunity, obesity, and diabetes afforded by vegan diets. Med. Hypotheses 2014, 83, 365–371. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. The moderate essential amino acid restriction entailed by low-protein vegan diets may promote vascular health by stimulating FGF21 secretion. Horm. Mol. Biol. Clin. Investig. 2016, 30. [Google Scholar] [CrossRef]
- Castaño-Martinez, T.; Schumacher, F.; Schumacher, S.; Kochlik, B.; Weber, D.; Grune, T.; Biemann, R.; McCann, A.; Abraham, K.; Weikert, C.; et al. Methionine restriction prevents onset of type 2 diabetes in NZO mice. FASEB J. 2019, 33, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Giem, P.; Beeson, W.L.; Fraser, G.E. The incidence of dementia and intake of animal products: Preliminary findings from the Adventist Health Study. Neuroepidemiology 1993, 12, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, S.T.; Sun, Y.; Xu, Z.; Wang, Y.; Yao, S.Y.; Yao, W.B.; Gao, X.D. Fibroblast growth factor 21 ameliorates neurodegeneration in rat and cellular models of Alzheimer’s disease. Redox Biol. 2019, 22, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Chan, K.H. Potential Neuroprotective Effects of Adiponectin in Alzheimer’s Disease. Int. J. Mol. Sci. 2017, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, H.C.; Ogunniyi, A.; Hall, K.S.; Baiyewu, O.; Unverzagt, F.W.; Gureje, O.; Gao, S.; Evans, R.M.; Ogunseyinde, A.O.; Adeyinka, A.O.; et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA 2001, 285, 739–747. [Google Scholar] [CrossRef]
- Hendrie, H.C.; Murrell, J.; Gao, S.; Unverzagt, F.W.; Ogunniyi, A.; Hall, K.S. International studies in dementia with particular emphasis on populations of African origin. Alzheimer Dis. Assoc. Disord. 2006, 20, S42–S46. [Google Scholar] [CrossRef][Green Version]
- Parrella, E.; Maxim, T.; Maialetti, F.; Zhang, L.; Wan, J.; Wei, M.; Cohen, P.; Fontana, L.; Longo, V.D. Protein restriction cycles reduce IGF-1 and phosphorylated Tau, and improve behavioral performance in an Alzheimer’s disease mouse model. Aging Cell 2013, 12, 257–268. [Google Scholar] [CrossRef]
- Ruan, Y.; Tang, J.; Guo, X.; Li, K.; Li, D. Dietary Fat Intake and Risk of Alzheimer’s Disease and Dementia: A Meta-Analysis of Cohort Studies. Curr. Alzheimer Res. 2018, 15, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Cole, R.M.; Deems, N.P.; Belury, M.A.; Barrientos, R.M. Fatty food, fatty acids, and microglial priming in the adult and aged hippocampus and amygdala. Brain Behav. Immun. 2020, 89, 145–158. [Google Scholar] [CrossRef]
- Desale, S.E.; Chinnathambi, S. Role of dietary fatty acids in microglial polarization in Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.; Wang, F.; Liu, S.; Zhao, S.; Ling, E.A.; Hao, A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF- κB signalling. Br. J. Nutr. 2012, 107, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, Y.; Zhang, X.; Xu, J.; Lin, J.; Zhang, R.; Kang, K.; Liu, C.; Zhao, C.; Zhao, M. Low-Density Lipoprotein Cholesterol and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 5. [Google Scholar] [CrossRef]
- Proitsi, P.; Lupton, M.K.; Velayudhan, L.; Newhouse, S.; Fogh, I.; Tsolaki, M.; Daniilidou, M.; Pritchard, M.; Kloszewska, I.; Soininen, H.; et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: A Mendelian randomization analysis. PLoS Med. 2014, 11, e1001713. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Finan, C.; Schmidt, A.F.; Burgess, S.; Hingorani, A.D. Lipid lowering and Alzheimer disease risk: A mendelian randomization study. Ann. Neurol. 2020, 87, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer’s Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF- κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef]
- Anstey, K.J.; Mack, H.A.; Cherbuin, N. Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am. J. Geriatr. Psychiatry 2009, 17, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Riss, J.; Décordé, K.; Sutra, T.; Delage, M.; Baccou, J.C.; Jouy, N.; Brune, J.P.; Oréal, H.; Cristol, J.P.; Rouanet, J.M. Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J. Agric. Food Chem. 2007, 55, 7962–7967. [Google Scholar] [CrossRef]
- McCarty, M.F. NADPH Oxidase Activity in Cerebral Arterioles Is a Key Mediator of Cerebral Small Vessel Disease-Implications for Prevention. Healthcare 2015, 3, 233–251. [Google Scholar] [CrossRef]
- McCarty, M.F. Supplementation with Phycocyanobilin, Citrulline, Taurine, and Supranutritional Doses of Folic Acid and Biotin-Potential for Preventing or Slowing the Progression of Diabetic Complications. Healthcare 2017, 5, 15. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, L.; Yang, X.; Pan, Q.; Cheng, X. Antidiabetic potential of phycocyanin: Effects on KKAy mice. Pharm. Biol. 2013, 51, 539–544. [Google Scholar] [CrossRef] [PubMed]
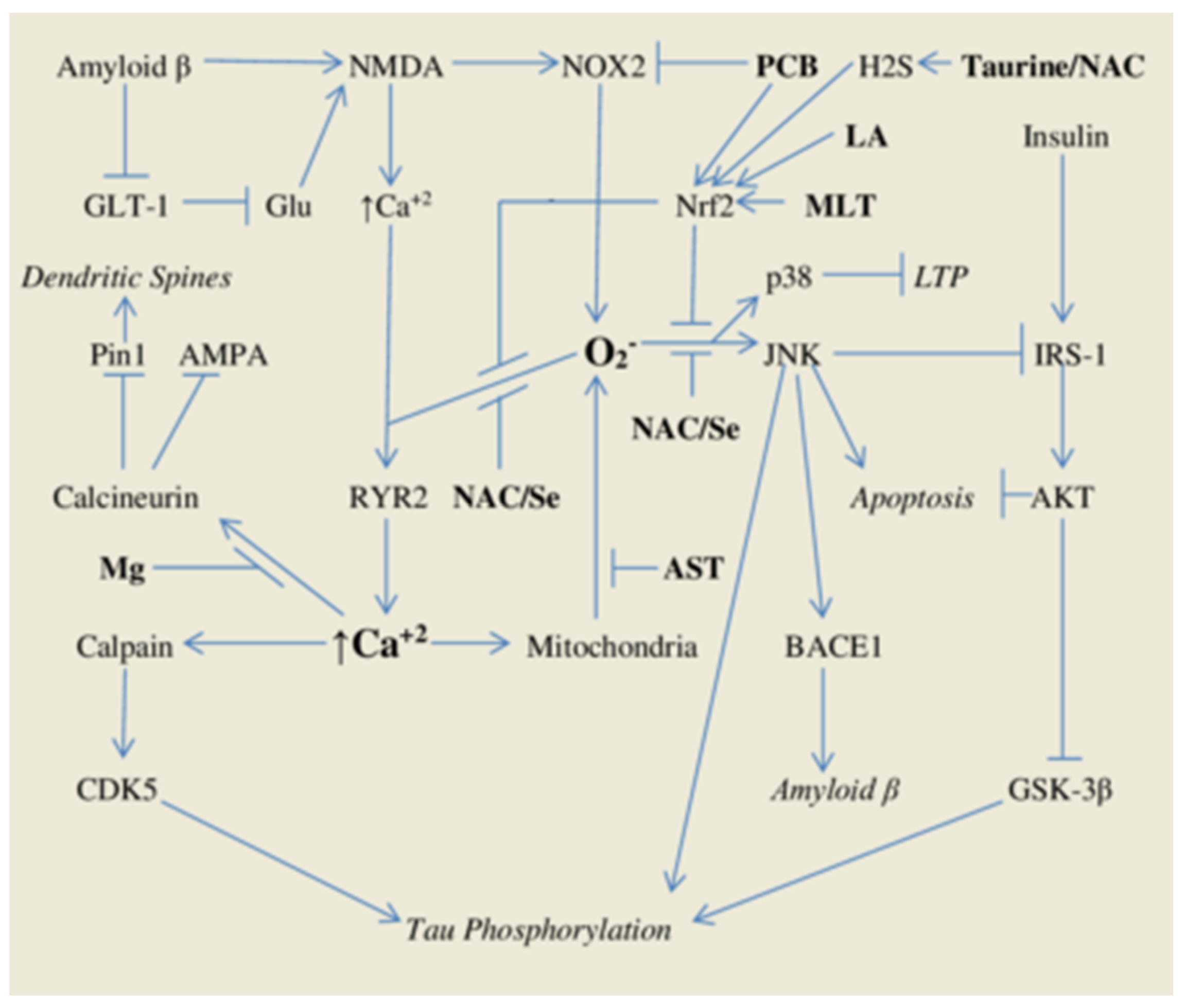

| Nutraceuticals | Function | Typical Supplemental Dose/Day |
|---|---|---|
| Phycocyanobilin | Algal chromophore antioxidant | 5–15 g spirulina |
| Lipoic Acid | Metabolic cofactor, phase two inducer | 600 mg X 2–3 |
| Melatonin | Neurohormone, phase two inducer | 3–20 mg at bedtime |
| Taurine | Antioxidant/osmoregulatory cofactor | 1–2 g X 2 |
| N-Acetylcysteine | Supplemental source of L-cysteine | 600 mg X 2–3 |
| Selenium | Essential mineral | 50–100 mcg |
| Astaxanthin | Natural carotenoid antioxidant | 12–20 mg |
| Biotin | B vitamin, activator of guanylate cyclase | 10–30 mg |
| Magnesium | Essential mineral | 100–400 mg |
| Citrulline | Precursor/delivery form for arginine | 2 g X 2 |
| DHA | Long-chain omega-3 fatty acid | 100–1000 mg |
| Vitamin D | Vitamin with anti-inflammatory activity | 1000–5000 IU |
| Berberine | Phytochemical which activates AMPK | 500 mg X 2–3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCarty, M.F.; DiNicolantonio, J.J.; Lerner, A. A Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis—And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder. Int. J. Mol. Sci. 2021, 22, 2140. https://doi.org/10.3390/ijms22042140
McCarty MF, DiNicolantonio JJ, Lerner A. A Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis—And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder. International Journal of Molecular Sciences. 2021; 22(4):2140. https://doi.org/10.3390/ijms22042140
Chicago/Turabian StyleMcCarty, Mark F., James J. DiNicolantonio, and Aaron Lerner. 2021. "A Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis—And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder" International Journal of Molecular Sciences 22, no. 4: 2140. https://doi.org/10.3390/ijms22042140
APA StyleMcCarty, M. F., DiNicolantonio, J. J., & Lerner, A. (2021). A Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis—And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder. International Journal of Molecular Sciences, 22(4), 2140. https://doi.org/10.3390/ijms22042140







