MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation
Abstract
1. Introduction
2. Neutrophil Extracellular Traps
2.1. NET Formation
2.2. NETs and Pathology
3. MicroRNA Biology
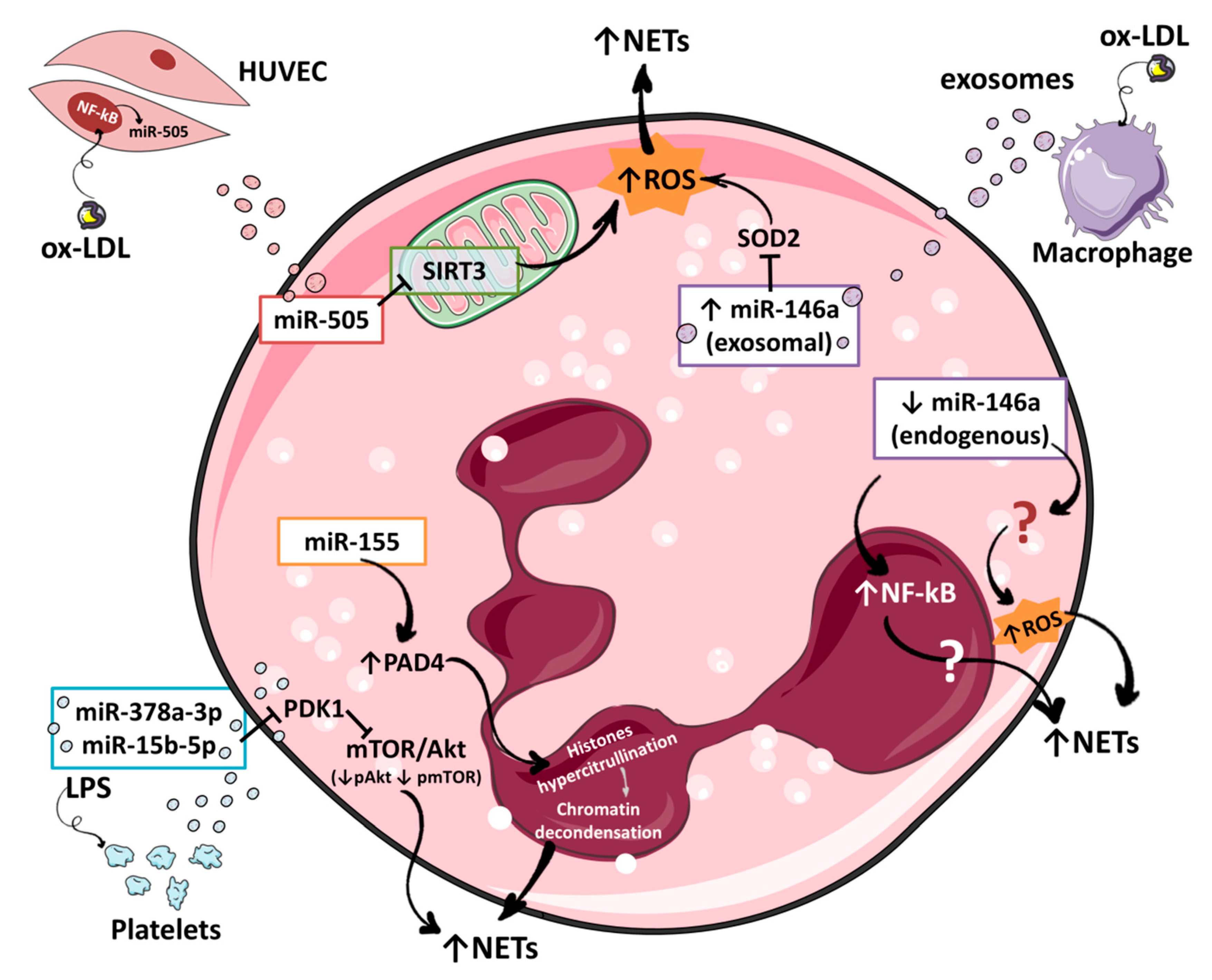
4. Regulation of NETosis by miRNAs
4.1. miR-146a
4.2. miR-155
4.3. miR-505
4.4. miR-378a-3p and miR-15b-5p
4.5. miR-1696 and miR-16-5p
5. Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGO2 | Argonaut protein 2 |
| CAD | Coronary artery disease |
| cfDNA | Cell free DNA |
| Cxcr1 | C-X-C Motif Chemokine Receptor 1 |
| GPx3 | Glutathione peroxidase 3 |
| HMGB1 | High mobility group box 1 protein |
| H2S | Hydrogen sulfide |
| IL | Interleukin |
| IRAK1 | Interleukin 1 Receptor Associated Kinase 1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| microRNAs | miRNAs |
| MPO | Myeloperoxidase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NE | Neutrophil elastase |
| NETs | Neutrophil extracellular traps |
| ox-LDL | Oxidized low-density lipoprotein |
| PAD4 | Protein-arginine deiminase IV |
| PDK1 | Phosphoinositide-dependent kinase-1 |
| PI3K/Akt | Phosphatidylinositol 3-kinase pathway |
| PIK3R1 | Phosphoinositide-3-Kinase Regulatory Subunit 1 |
| PKCα | Protein kinase C alpha |
| PMA | Phorbol-12-myristate-13-acetate |
| RAF1 | Raf-1 Proto-Oncogene Serine/Threonine Kinase |
| ROS | Reactive oxygen species |
| SIRT3 | NAD-dependent deacetylase sirtuin-3 |
| SOD2 | Superoxide dismutase 2 |
| STEMI | ST-Elevation Myocardial Infarction |
| TLR | Toll like receptor |
| TNFα | Tumor necrosis factor alpha |
| TRAF6 | Tumor Necrosis Factor Receptor Associated Factor 6 |
| 3′UTR | 3′ untranslated region |
| XPO5 | Exportin 5 |
References
- Liew, P.X.; Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Hoffman, H.M.; Kubes, P.; Cassatella, M.A.; Zychlinsky, A.; Hedrick, C.C.; Catz, S.D. Neutrophils: New insights and open questions. Sci. Immunol. 2018, 3, eaat4579. [Google Scholar] [CrossRef]
- Dąbrowska, D.; Jabłońska, E.; Iwaniuk, A.; Garley, M. Many ways–one destination: Different types of neutrophils death. Int. Rev. Immunol. 2019, 38, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, A.T.; Martinez, E.N.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2010, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Eleshner, M.; Ewang, S.; Elewis, C.; Ezheng, H.; Chen, X.A.; Esanty, L.; Ewang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Tait, J.F.; Tewari, M.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Linhares-Lacerda, L.; Temerozo, J.R.; Ribeiro-Alves, M.; Azevedo, E.P.; Mojoli, A.; Nascimento, M.T.C.; Silva-Oliveira, G.; Savino, W.; Foguel, D.; Bou-Habib, D.C.; et al. Neutrophil extracellular trap-enriched supernatants carry microRNAs able to modulate TNF-α production by macrophages. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The emerging role of neutrophil extracellular traps (Nets) in tumor progression and metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil extracellular traps: Villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef]
- Podolska, M.J.; Mahajan, A.; Knopf, J.; Hahn, J.; Boeltz, S.; Munoz, L.; Bilyy, R.; Herrmann, M. Autoimmune, rheumatic, chronic inflammatory diseases: Neutrophil extracellular traps on parade. Autoimmunity 2018, 51, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Libby, P.; Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases: Recent experimental and clinical insights. Circ. Res. 2020, 126, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2012, 13, 34–45. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Rao, A.N.; Kazzaz, N.M.; Knight, J.S. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J. Cardiol. 2015, 7, 829–842. [Google Scholar] [CrossRef]
- Montecucco, F.; Liberale, L.; Bonaventura, A.; Vecchiè, A.; Dallegri, F.; Carbone, F. The role of inflammation in cardiovascular outcome. Curr. Atheroscler. Rep. 2017, 19, 11. [Google Scholar] [CrossRef]
- Carbone, F.; Mach, F.; Montecucco, F. Update on the role of neutrophils in atherosclerotic plaque vulnerability. Curr. Drug Targets 2015, 16, 321–333. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, immunity, and infection in atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Döring, Y.; Soehnlein, O.; Weber, C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 2017, 120, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Luo, W.; Kaplan, M.J.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Massberg, S.; Grahl, L.; Von Bruehl, M.-L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Joosen, I.A.; Crijns, H.J.; Wagner, D.D.; Kietselaer, B.L.J.H.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; et al. Elevated levels of circulating dna and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arter. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.; Wang, X.; Zhu, Z.; Wang, T.; Ma, A.; Liu, P. Neutrophil extracellular traps and dsDNA predict outcomes among patients with ST-elevation myocardial infarction. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Mangold, A.; Scherz, T.; Seidl, V.; Panzenböck, A.; Ondracek, A.S.; Müller, J.; Schneider, M.; Binder, T.; Hell, L.; et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction. Basic Res. Cardiol. 2019, 114, 1–15. [Google Scholar] [CrossRef]
- Mangold, A.; Alias, S.; Mascherbauer, J.; Winter, M.-P.; Distelmaier, K.; Adlbrecht, C.; Preissner, K.T.; Lang, I.M.; Scherz, T.; Hofbauer, T.M.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in st-elevation acute coronary syndrome are predictors of st-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Quillard, T.; Araújo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: Implications for superficial erosion. Eur. Heart J. 2015, 36, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Langseth, M.S.; Opstad, T.B.; Bratseth, V.; Solheim, S.; Arnesen, H.; Pettersen, A.Å.; Seljeflot, I.; Helseth, R. Markers of neutrophil extracellular traps are associated with adverse clinical outcome in stable coronary artery disease. Eur. J. Prev. Cardiol. 2018, 25, 762–769. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Rassenti, L.; Kipps, T.; Negrini, M.; Bullrich, F.; Croce, C.M.; Shimizu, M.; Bichi, R.; Zupo, S.; et al. Nonlinear partial differential equations and applications: Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Faggioni, A.; Trivedi, P.; Slack, F.J. The nefarious nexus of noncoding RNAs in cancer. Int. J. Mol. Sci. 2018, 19, 2072. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Hendrickson, D.G.; Hogan, D.J.; McCullough, H.L.; Myers, J.W.; Herschlag, D.; Ferrell, J.E.; Brown, P.O. Concordant regulation of translation and Mrna abundance for hundreds of targets of a human microrna. PLoS Biol. 2009, 7, e1000238. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Silverman, E.K.; Schmidt, H.H.H.W.; Di Costanzo, A.; Farina, L.; Fiscon, G.; Gatto, L.; Gentili, M.; Loscalzo, J.; Marchese, C.; Napoli, C.; et al. Molecular networks in network medicine: Development and applications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1489. [Google Scholar] [CrossRef]
- Esaba, R.; Sorensen, D.L.; Booth, S.A. MicroRNA-146a: A dominant, negative regulator of the innate immune response. Front. Immunol. 2014, 5, 578. [Google Scholar] [CrossRef]
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.-A.; Steinle, J.J. miR-146a attenuates inflammatory pathways mediated by TLR4/NF-κB and TNFαto protect primary human retinal microvascular endothelial cells grown in high glucose. Mediat. Inflamm. 2016, 2016, 3958453. [Google Scholar] [CrossRef]
- Boldin, M.P.; Taganov, K.D.; Rao, D.S.; Yang, L.; Zhao, J.L.; Kalwani, M.; Garcia-Flores, Y.; Luong, M.; Devrekanli, A.; Xu, J.; et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011, 208, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Nahid, A.; Pauley, K.M.; Satoh, M.; Chan, E.K.L. miR-146a Is Critical for Endotoxin-induced tolerance. J. Biol. Chem. 2009, 284, 34590–34599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Rao, D.S.; Boldin, M.P.; Taganov, K.D.; O’Connell, R.M.; Baltimore, D. NF-κB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 9184–9189. [Google Scholar] [CrossRef]
- Löfgren, S.E.; Frostegård, J.; Truedsson, L.; Pons-Estel, B.A.; D’Alfonso, S.; Witte, T.; Lauwerys, B.R.; Endreffy, E.; Kovács, L.; Vasconcelos, C.C.F.; et al. Genetic association of miRNA-146a with systemic lupus erythematosus in Europeans through decreased expression of the gene. Genes Immun. 2012, 13, 268–274. [Google Scholar] [CrossRef]
- Stickel, N.; Hanke, K.; Brossart, P.; Wolf, D.; Von Bubnoff, N.; Finke, J.; Duyster, J.; Ferrara, J.; Salzer, U.; Zeiser, R.; et al. MicroRNA-146a reduces MHC-II expression via targeting JAK/STAT signaling in dendritic cells after stem cell transplantation. Leukemia 2017, 31, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Bastami, M.; Choupani, J.; Saadatian, Z.; Vahed, S.Z.; Mansoori, Y.; Daraei, A.; Kafil, H.S.; Masotti, A.; Nariman-Saleh-Fam, Z. miRNA polymorphisms and risk of cardio-cerebrovascular diseases: A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Marschner, D.; Falk, M.; Javorniczky, N.R.; Hanke-Müller, K.; Rawluk, J.; Schmitt-Graeff, A.; Simonetta, F.; Haring, E.; Dicks, S.; Ku, M.; et al. MicroRNA-146a regulates immune-related adverse events caused by immune checkpoint inhibitors. JCI Insight 2020, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.B.; Reyes-García, A.M.D.L.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldán, V.; Vicente, V.; Martínez, C.; González-Conejero, R. MiR-146a regulates neutrophil extracellular trap formation that predicts adverse cardiovascular events in patients with atrial fibrillation. Arter. Thromb. Vasc. Biol. 2018, 38, 892–902. [Google Scholar] [CrossRef]
- Roldán, V.; Arroyo, A.B.; Salloum-Asfar, S.; Manzano-Fernández, S.; García-Barberá, N.; Marín, F.; Vicente, V.; González-Conejero, R.; Martínez, C. Prognostic role of MIR146A polymorphisms for cardiovascular events in atrial fibrillation. Thromb. Haemost. 2014, 112, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.B.; Fernández-Pérez, M.P.; Vicente, V.; Menéndez, R.; Andrés, V.; González-Conejero, R.; Martínez, C.; Del Monte, A.; Águila, S.; Méndez, R.; et al. miR-146a is a pivotal regulator of neutrophil extracellular trap formation promoting thrombosis. Haematologica 2020. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, W.; Kolaczkowska, E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018, 371, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Song, Y.; Guo, X.-L.; Miao, R.-Y.; Fu, Y.-Q.; Miao, C.-F.; Zhang, C. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle 2019, 18, 2672–2682. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, A.S.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. miR-146 and miR-155: Two key modulators of immune response and tumor development. Non-Coding RNA 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Mehta, A.; Zhao, J.L.; Lee, K.; Marinov, G.K.; Garcia-Flores, Y.; Lu, L.-F.; Rudensky, A.Y.; Baltimore, D. An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat. Commun. 2017, 8, 851. [Google Scholar] [CrossRef]
- Hawez, A.; Al-Haidari, A.; Madhi, R.; Rahman, M.; Thorlacius, H. MiR-155 regulates PAD4-dependent formation of neutrophil extracellular traps. Front. Immunol. 2019, 10, 2462. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Amulic, B.; Zychlinsky, A. Neutrophil extracellular trap formation is independent of de novo gene expression. PLoS ONE 2016, 11, e0157454. [Google Scholar] [CrossRef]
- Tatsiy, O.; McDonald, P.P. Physiological stimuli induce PAD4-dependent, ROS-independent NETosis, with early and late events controlled by discrete signaling pathways. Front. Immunol. 2018, 9, 2036. [Google Scholar] [CrossRef]
- Xie, K.; Ma, H.; Liang, C.; Wang, C.; Qin, N.; Shen, W.; Gu, Y.; Yan, C.; Zhang, K.; Dai, N.; et al. A functional variant in miR-155 regulation region contributes to lung cancer risk and survival. Oncotarget 2015, 6, 42781–42792. [Google Scholar] [CrossRef]
- Zhao, P.; Guan, H.; Dai, Z.; Ma, Y.; Zhao, Y.; Liu, D. Long noncoding RNA DLX6-AS1 promotes breast cancer progression via miR-505-3p/RUNX2 axis. Eur. J. Pharmacol. 2019, 865, 172778. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Lu, T.; Shen, J.; Wang, J. LncRNA ZEB1-AS1 promotes pancreatic cancer progression by regulating miR-505-3p/TRIB2 axis. Biochem. Biophys. Res. Commun. 2020, 528, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Escate, R.; Mata, P.; Cepeda, J.M.; Padreó, T.; Badimon, L. miR-505-3p controls chemokine receptor up-regulation in macrophages: Role in familial hypercholesterolemia. FASEB J. 2017, 32, 601–612. [Google Scholar] [CrossRef]
- Chen, L.; Hu, L.; Li, Q.; Ma, J.; Li, H. Exosome-encapsulated miR-505 from ox-LDL-treated vascular endothelial cells aggravates atherosclerosis by inducing NET formation. Acta Biochim. Biophys. Sin. 2019, 51, 1233–1241. [Google Scholar] [CrossRef]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, S.; Zhang, T.; Ma, X.; Wu, Z.; Jiang, K.; Zhang, X.; Guo, X.; Deng, G. MiR-505 as an anti-inflammatory regulator suppresses HMGB1/NF-κB pathway in lipopolysaccharide-mediated endometritis by targeting HMGB1. Int. Immunopharmacol. 2020, 88, 106912. [Google Scholar] [CrossRef]
- Li, L.; Lv, G.; Wang, B.; Ma, H. Long Noncoding RNA LINC00525 Promotes the Aggressive Phenotype of Chordoma Through Acting as a microRNA-505-3p Sponge and Consequently Raising HMGB1 Expression. OncoTargets Ther. 2020, 13, 9015–9027. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Lee, J.-K. Role of HMGB1 in the interplay between NETosis and Thrombosis in ischemic stroke: A review. Cells 2020, 9, 1794. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, W.; Wang, W.; Tong, X.; Xia, R.; Fan, J.; Du, J.; Zhang, C.; Shi, X. Platelet-derived exosomes promote neutrophil extracellular trap formation during septic shock. Crit. Care 2020, 24, 1–18. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and autophagy-related diseases: A review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in neutrophils: From granulopoiesis to neutrophil extracellular traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, B. Autophagy-mediated regulation of neutrophils and clinical applications. Burn. Trauma 2020, 8, tkz001. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Yin, K.; Zhang, Q.; Li, S. MiR-1696/GPx3 axis is involved in oxidative stress mediated neutrophil extracellular traps inhibition in chicken neutrophils. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Yin, K.; Cui, Y.; Qu, Y.; Zhang, J.; Zhang, H.; Lin, H. Hydrogen sulfide upregulates miR-16-5p targeting PiK3R1 and RAF1 to inhibit neutrophil extracellular trap formation in chickens. Ecotoxicol. Environ. Saf. 2020, 194, 110412. [Google Scholar] [CrossRef]
- Remijsen, Q.; Berghe, T.V.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2010, 21, 290–304. [Google Scholar] [CrossRef]
- Sollberger, G.; Tilley, D.O.; Zychlinsky, A. Neutrophil extracellular traps: The biology of chromatin externalization. Dev. Cell 2018, 44, 542–553. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Wang, G.-D.; Wang, H.-S.; Huang, Y.; Liu, X.-M.; Cai, X.-H. miR-16 inhibits cell proliferation by targeting IGF1R and the Raf1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS Lett. 2013, 587, 1366–1372. [Google Scholar] [CrossRef]
- Sorrentino, D.; Frentzel, J.; Espinos, E.; Chiarle, R.; Giuriato, S.; Mitou, G.; Blasco, R.B.; Torossian, A.; Hoareau-Aveilla, C.; Pighi, C.; et al. High levels of miR-7-5p potentiate crizotinib-induced cytokilling and autophagic flux by targeting RAF1 in NPM-ALK positive lymphoma cells. Cancers 2020, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, J. CircAGFG1 promotes cervical cancer progression via miR-370-3p/RAF1 signaling. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, Y.; Zhao, D.; Sun, Q.; Zeng, Z.; Dress, A.; Lin, M.C.; Kung, H.-F.; Rui, H.; Liu, L.-Z.; et al. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin. Cancer Res. 2011, 17, 1722–1730. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Huang, X.-P.; Zhu, J.-Y.; Chen, Z.-G.; Li, X.-J.; Zhang, X.-H.; Huang, S.; He, J.-B.; Lian, F.; Zhao, Y.-N.; et al. miR-128-3p suppresses hepatocellular carcinoma proliferation by regulating PIK3R1 and is correlated with the prognosis of HCC patients. Oncol. Rep. 2015, 33, 2889–2898. [Google Scholar] [CrossRef]
- Tian, F.; Wang, J.; Ouyang, T.; Lu, N.; Lu, J.; Shen, Y.; Bai, Y.; Xie, X.; Ge, Q. MiR-486-5p serves as a good biomarker in nonsmall cell lung cancer and suppresses cell growth with the involvement of a target PIK3R1. Front. Genet. 2019, 10, 688. [Google Scholar] [CrossRef]
- Huang, C.-K.; Kafert-Kasting, S.; Thum, T. Preclinical and clinical development of noncoding RNA therapeutics for cardiovascular disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef]
- Li, K.; Ching, D.; Luk, F.S.; Raffai, R.L. Apolipoprotein E enhances MicroRNA-146a in monocytes and macrophages to suppress nuclear factor- B-driven inflammation and atherosclerosis. Circ. Res. 2015, 117, e1–e11. [Google Scholar] [CrossRef]
- Cheng, H.S.; Besla, R.; Khyzha, N.; Li, T.; MacParland, S.A.; Husain, M.; Cybulsky, M.I.; Boulanger, C.M.; Temel, R.E.; Schober, A.; et al. Paradoxical suppression of atherosclerosis in the absence of microRNA-146a. Circ. Res. 2017, 121, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L.; Liang, X.; Zhu, G. MicroRNA-155 promotes atherosclerosis inflammation via targeting SOCS1. Cell. Physiol. Biochem. 2015, 36, 1371–1381. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Slack, F.J. Malicious exosomes. Science 2014, 346, 1459–1460. [Google Scholar] [CrossRef]

| miRNAs | miR-146a | miR-155 | miR-505 | miR-378a-3p | miR-15b-5p | miR-1696 | miR-16-5p |
|---|---|---|---|---|---|---|---|
| Data in mammalian models | Yes | Yes | Yes | Yes | Yes | No data | No data |
| Data in CVD | Yes | Yes | Yes | No data | No data | No data | No data |
| Genetic regulation of miRNA levels | Yes | Yes | No data | No data | No data | No data | No data |
| Exogenous source | Yes | No data | Yes | Yes | Yes | No data | No data |
| Mammalian miRNA | Yes | Yes | Yes | Yes | Yes | No data | Yes |
| Interaction with ncRNAs | No data | No data | No data | No data | No data | No data | No data |
| Therapeutic potential in CVD | Yes | Yes | No data | No data | No data | No data | No data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Águila, S.; de los Reyes-García, A.M.; Fernández-Pérez, M.P.; Reguilón-Gallego, L.; Zapata-Martínez, L.; Ruiz-Lorente, I.; Vicente, V.; González-Conejero, R.; Martínez, C. MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation. Int. J. Mol. Sci. 2021, 22, 2116. https://doi.org/10.3390/ijms22042116
Águila S, de los Reyes-García AM, Fernández-Pérez MP, Reguilón-Gallego L, Zapata-Martínez L, Ruiz-Lorente I, Vicente V, González-Conejero R, Martínez C. MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation. International Journal of Molecular Sciences. 2021; 22(4):2116. https://doi.org/10.3390/ijms22042116
Chicago/Turabian StyleÁguila, Sonia, Ascensión M. de los Reyes-García, María P. Fernández-Pérez, Laura Reguilón-Gallego, Laura Zapata-Martínez, Inmaculada Ruiz-Lorente, Vicente Vicente, Rocío González-Conejero, and Constantino Martínez. 2021. "MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation" International Journal of Molecular Sciences 22, no. 4: 2116. https://doi.org/10.3390/ijms22042116
APA StyleÁguila, S., de los Reyes-García, A. M., Fernández-Pérez, M. P., Reguilón-Gallego, L., Zapata-Martínez, L., Ruiz-Lorente, I., Vicente, V., González-Conejero, R., & Martínez, C. (2021). MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation. International Journal of Molecular Sciences, 22(4), 2116. https://doi.org/10.3390/ijms22042116






