Formyl Peptide Receptor 2 Alleviates Hepatic Fibrosis in Liver Cirrhosis by Vascular Remodeling
Abstract
1. Introduction
2. Results
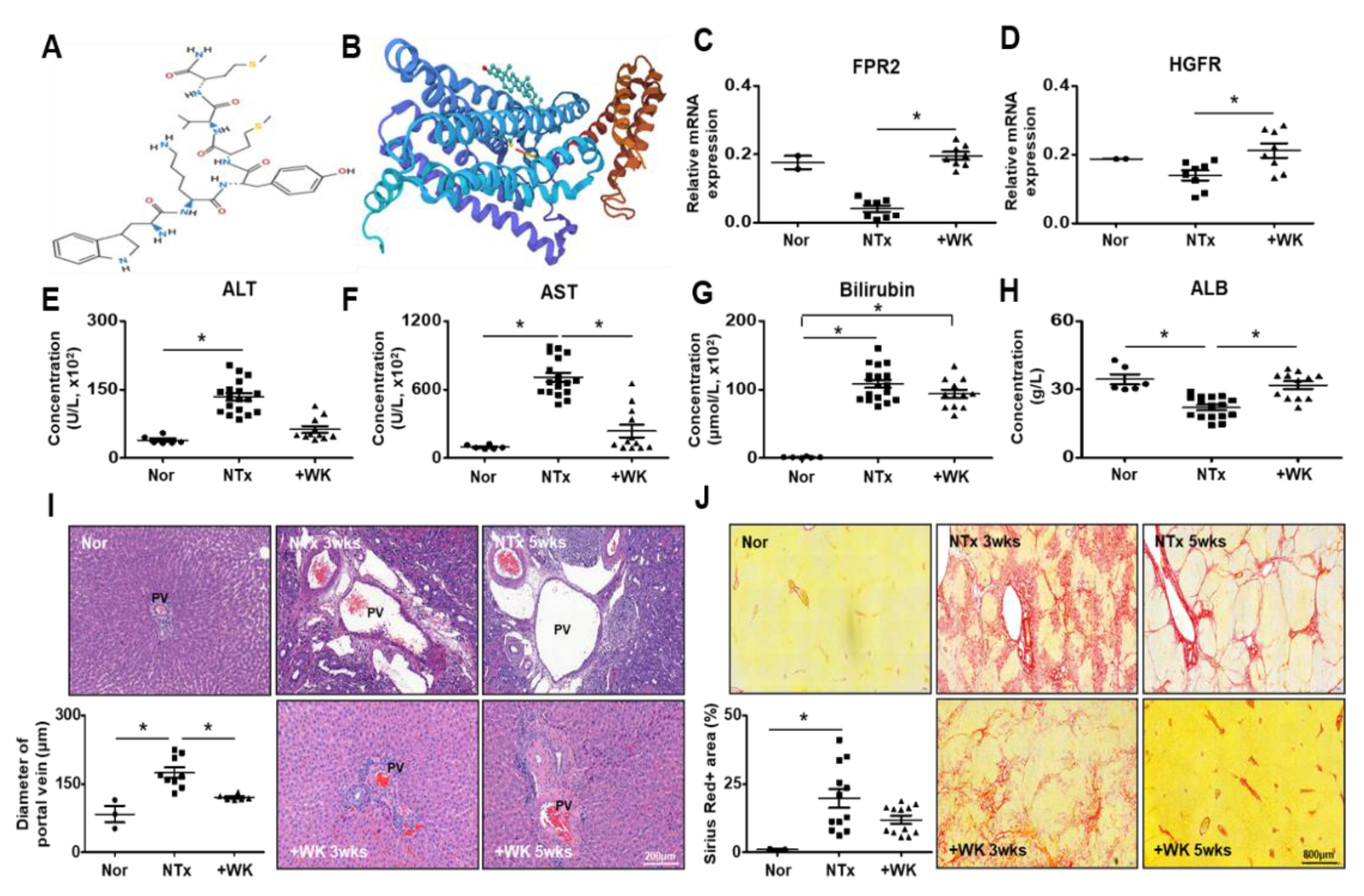
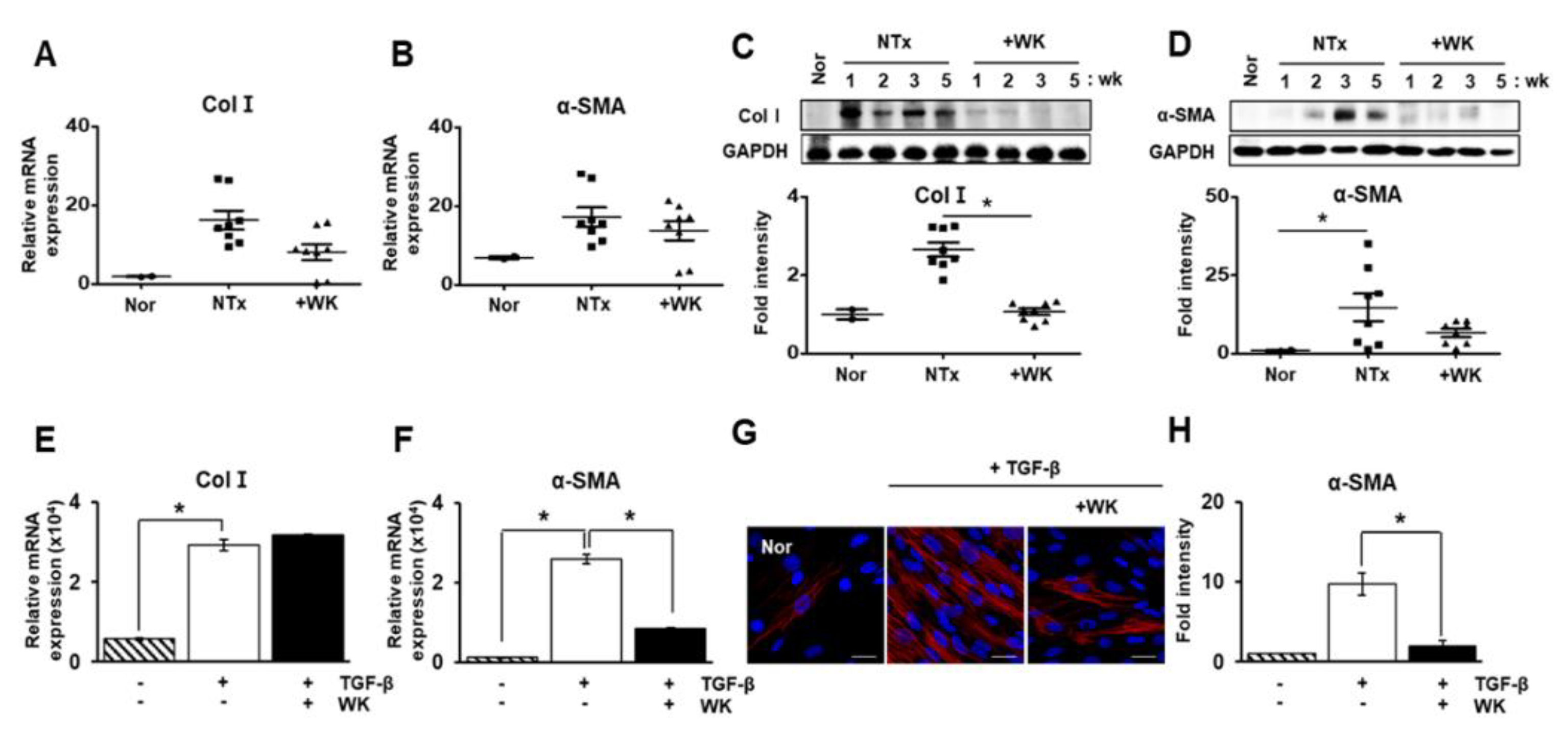
2.1. WKYMVm Attenuates Liver Fibrosis in the BDL Rat Model
2.2. WKYMVm Inhibits Hepatic Stellate Cell Activation Induced by Transforming Growth Factor-Beta (TGF-β)
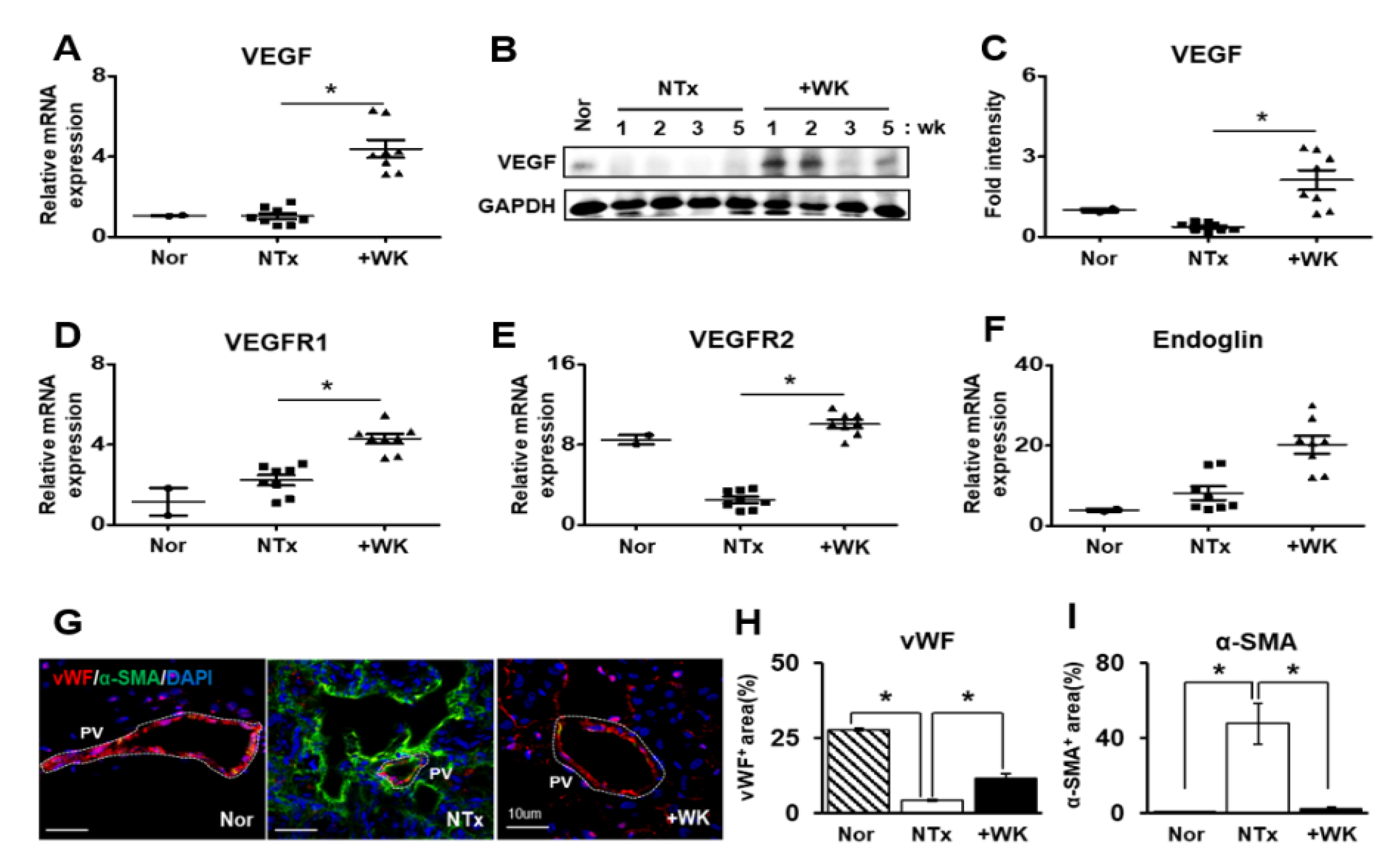
2.3. WKYMVm Promotes Vascular Remodeling in the BDL Rat Liver
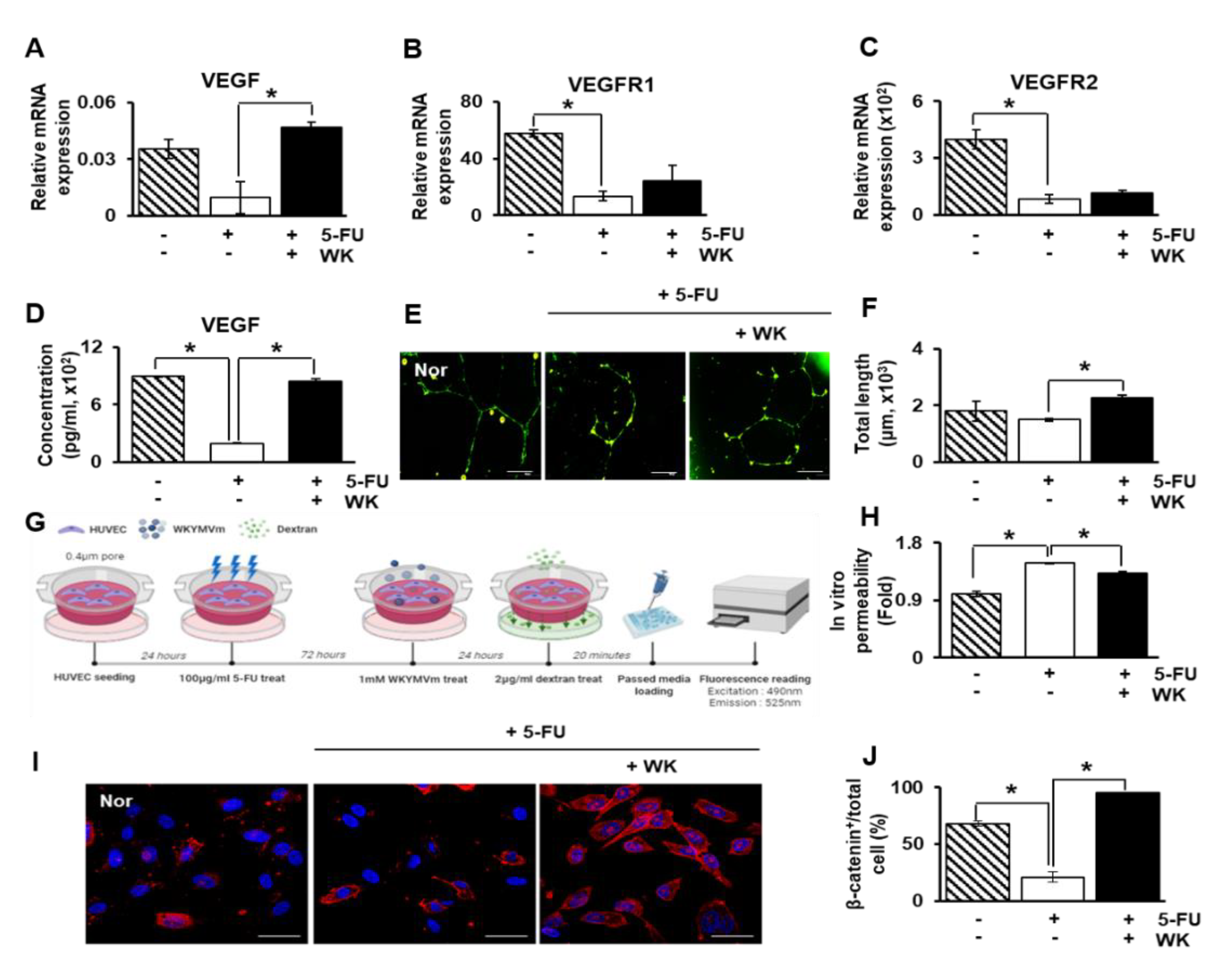
2.4. WKYMVm Enhances Angiogenic Activity in HUVECs
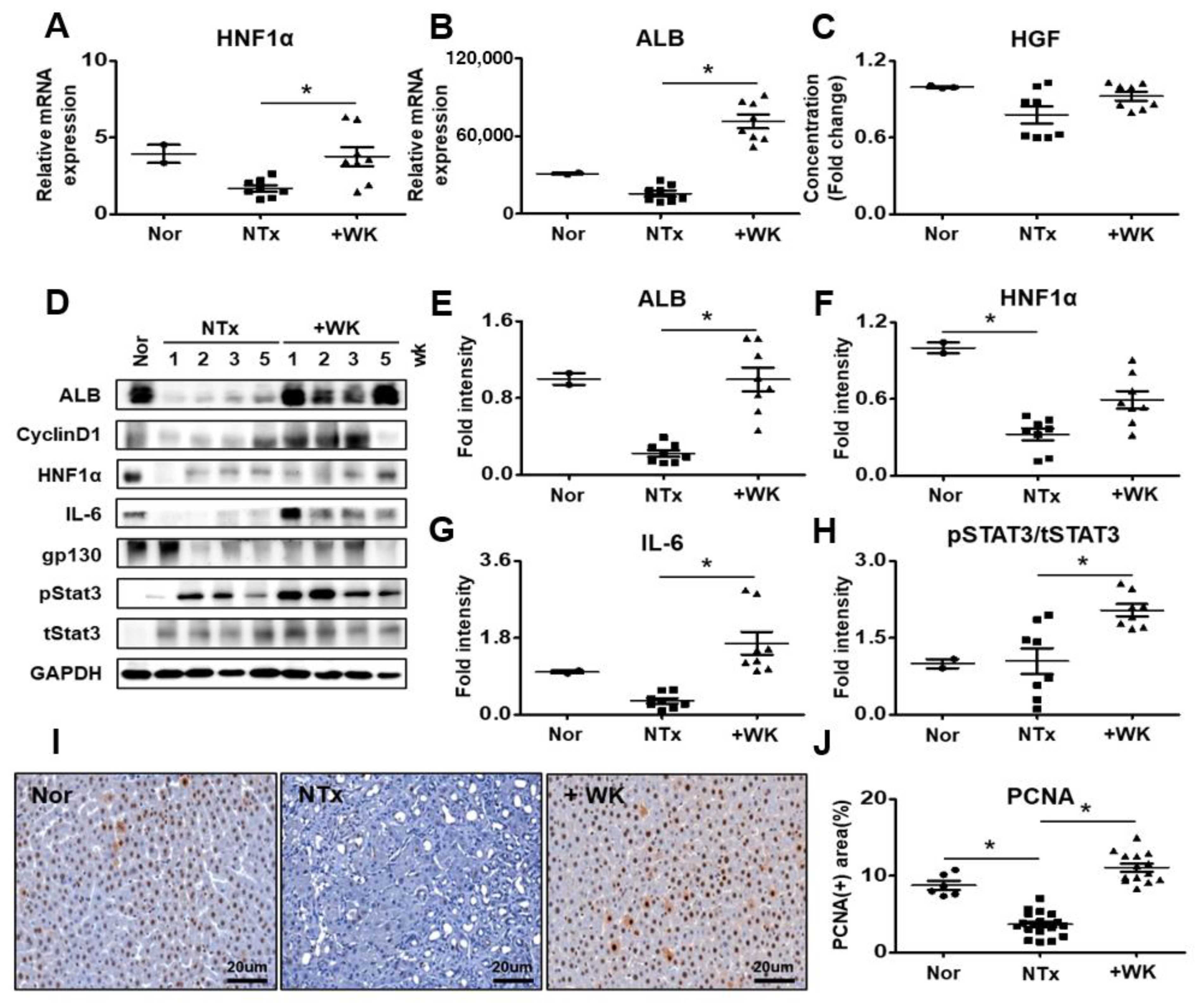
2.5. WKYMVm Improves Hepatic Regeneration in the BDL Rat Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Serum Biochemistry Analysis
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Cell Culture
4.6. Sirius Red Staining
4.7. Immunohistochemistry
4.8. Western Blot
4.9. Quantitative Reverse Transcription PCR
4.10. Immunofluorescence
4.11. Tube Formation Assay
4.12. Dextran Permeability Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo, V.; Moreau, R.; Kamath, P.S.; Jalan, R.; Gines, P.; Nevens, F.; Fernandez, J.; To, U.; Garcia-Tsao, G.; Schnabl, B. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Primers 2016, 2, 16041. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Fernandez, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in liver disease. J. Hepatol. 2009, 50, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Barrera, A.; Barranco-Fragoso, B.; Mendez-Sanchez, N. Management strategies for liver fibrosis. Ann. Hepatol. 2017, 16, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cassiman, D.; Libbrecht, L.; Desmet, V.; Denef, C.; Roskams, T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J. Hepatol. 2002, 36, 200–209. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar] [CrossRef]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Anita, K. Angiogenesis in liver regeneration and fibrosis: “A double-edged sword”. Hepatol. Int. 2013, 7, 959–968. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Yang, L.; Kwon, J.; Popov, Y.; Gajdos, G.B.; Ordog, T.; Brekken, R.A.; Mukhopadhyay, D.; Schuppan, D.; Bi, Y.; Simonetto, D.; et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology 2014, 146, 1339–1350.e1331. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, E.; Sakisaka, S.; Matsuo, K.; Tanikawa, K.; Sata, M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J. Histochem. Cytochem. 2001, 49, 121–130. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- DeLeve, L.D. Liver sinusoidal endothelial cells and liver regeneration. J. Clin. Invest. 2013, 123, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ye, T.; Sun, Y.; Ji, G.; Shido, K.; Chen, Y.; Luo, L.; Na, F.; Li, X.; Huang, Z.; et al. Targeting the vascular and perivascular niches as a regenerative therapy for lung and liver fibrosis. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Hanai, J.; Dhanabal, M.; Karumanchi, S.A.; Albanese, C.; Waterman, M.; Chan, B.; Ramchandran, R.; Pestell, R.; Sukhatme, V.P. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. J. Biol. Chem. 2002, 277, 16464–16469. [Google Scholar] [CrossRef]
- Rattner, A.; Wang, Y.; Zhou, Y.; Williams, J.; Nathans, J. The role of the hypoxia response in shaping retinal vascular development in the absence of Norrin/Frizzled4 signaling. Invest. Ophthalmol. Vis. Sci. 2014, 55, 8614–8625. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Tojais, N.; Peghaire, C.; Franzl, N.; Larrieu-Lahargue, F.; Jaspard, B.; Reynaud, A.; Moreau, C.; Couffinhal, T.; Duplaa, C.; Dufourcq, P. Frizzled7 controls vascular permeability through the Wnt-canonical pathway and cross-talk with endothelial cell junction complexes. Cardiovasc. Res. 2014, 103, 291–303. [Google Scholar] [CrossRef]
- Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Ziegler, N.; Godet, M.; Guilbert, T.; Liebner, S.; Couraud, P.O. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J. Cereb. Blood Flow Metab. 2014, 34, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gavins, F.N. Are formyl peptide receptors novel targets for therapeutic intervention in ischaemia-reperfusion injury? Trends Pharmacol. Sci. 2010, 31, 266–276. [Google Scholar] [CrossRef]
- Heo, S.C.; Kwon, Y.W.; Jang, I.H.; Jeong, G.O.; Yoon, J.W.; Kim, C.D.; Kwon, S.M.; Bae, Y.S.; Kim, J.H. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells 2014, 32, 779–790. [Google Scholar] [CrossRef]
- Heo, S.C.; Kwon, Y.W.; Jang, I.H.; Jeong, G.O.; Lee, T.W.; Yoon, J.W.; Shin, H.J.; Jeong, H.C.; Ahn, Y.; Ko, T.H.; et al. Formyl Peptide Receptor 2 Is Involved in Cardiac Repair After Myocardial Infarction Through Mobilization of Circulating Angiogenic Cells. Stem Cells 2017, 35, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.W.; Heo, S.C.; Jang, I.H.; Jeong, G.O.; Yoon, J.W.; Mun, J.H.; Kim, J.H. Stimulation of cutaneous wound healing by an FPR2-specific peptide agonist WKYMVm. Wound Repair Regen. 2015, 23, 575–582. [Google Scholar] [CrossRef]
- Choi, Y.H.; Heo, S.C.; Kwon, Y.W.; Kim, H.D.; Kim, S.H.; Jang, I.H.; Kim, J.H.; Hwang, N.S. Injectable PLGA microspheres encapsulating WKYMVM peptide for neovascularization. Acta Biomater. 2015, 25, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Qu, B.; Liu, B.R.; Du, Y.J.; Chen, J.; Cheng, Y.Q.; Xu, W.; Wang, X.H. Wnt/beta-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Kim, J.Y.; Choi, J.H.; Lim, J.Y.; Kim, K.; Kim, G.J. Exosomes from Placenta-Derived Mesenchymal Stem Cells Are Involved in Liver Regeneration in Hepatic Failure Induced by Bile Duct Ligation. Stem Cells Int. 2020, 2020, 5485738. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Jung, J.; Kim, J.Y.; Hwang, S.G.; Bae, S.H.; Kim, G.J. Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver. Int. J. Stem Cells 2020. [Google Scholar] [CrossRef]
- Streetz, K.L.; Luedde, T.; Manns, M.P.; Trautwein, C. Interleukin 6 and liver regeneration. Gut 2000, 47, 309–312. [Google Scholar] [CrossRef]
- Costa, R.H.; Kalinichenko, V.V.; Holterman, A.X.; Wang, X. Transcription factors in liver development, differentiation, and regeneration. Hepatology 2003, 38, 1331–1347. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Taura, K.; De Minicis, S.; Seki, E.; Hatano, E.; Iwaisako, K.; Osterreicher, C.H.; Kodama, Y.; Miura, K.; Ikai, I.; Uemoto, S.; et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 2008, 135, 1729–1738. [Google Scholar] [CrossRef]
- Drixler, T.A.; Vogten, M.J.; Ritchie, E.D.; van Vroonhoven, T.J.; Gebbink, M.F.; Voest, E.E.; BorelRinkes, I.H. Liver regeneration is an angiogenesis- associated phenomenon. Ann. Surg. 2002, 236, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, X.; Chen, Y.; Han, X.; Li, L.; Yang, Z.; Duan, L.; Lu, H.; He, Q. The protective effect of WKYMVm peptide on inflammatory osteolysis through regulating NF-kappaB and CD9/gp130/STAT3 signalling pathway. J. Cell Mol. Med. 2020, 24, 1893–1905. [Google Scholar] [CrossRef]
- Jung, J.; Moon, J.W.; Choi, J.H.; Lee, Y.W.; Park, S.H.; Kim, G.J. Epigenetic Alterations of IL-6/STAT3 Signaling by Placental Stem Cells Promote Hepatic Regeneration in a Rat Model with CCl4-induced Liver Injury. Int. J. Stem Cells 2015, 8, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.J. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl. Med. 2016, 5, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Kim, Y.K.; Lee, H.Y.; Kim, Y.S.; Jeon, S.G.; Baek, S.H.; Song, D.K.; Ryu, S.H.; Bae, Y.S. The agonists of formyl peptide receptors prevent development of severe sepsis after microbial infection. J. Immunol. 2010, 185, 4302–4310. [Google Scholar] [CrossRef]
- Standish, R.A.; Cholongitas, E.; Dhillon, A.; Burroughs, A.K.; Dhillon, A.P. An appraisal of the histopathological assessment of liver fibrosis. Gut 2006, 55, 569–578. [Google Scholar] [CrossRef]
| Gene | Sequence | Accession Number |
|---|---|---|
| FPR2 | F: 5′-ACTGTTGAAGAAGTGCTGGA-3′ | XM_001073508.5 |
| R: 5′-AACAAGTGCTCTTCTGTGGA-3′ | ||
| HGFR | F: 5′-ACCCAGATTGTTTTCCTTGT-3′ | XM_032906920.1 |
| R: 5′-ATTGTCAGGAGGAAGGACAT-3′ | ||
| VEGF | F: 5′-ACGGACAGACAGACAGACAC-3′ | NM_001287114.1 |
| R: 5′-CTTCTGGGCTCTTCTCTCTC-3′ | ||
| VEGFR1 | F: 5′-CCACACCTGAAATCTACCAA-3′ | NM_019306.2 |
| R: 5′-TGGGGACTGAGTATGTGAAG-3′ | ||
| VEGFR2 | F: 5′-AAGCAAATGCTCAGCAGGAT-3′ | NM_013062.1 |
| R: 5′-TAGGCAGGGAGAGTCCAGAA-3′ | ||
| Endoglin | F: 5′-AAGGTGTGACTGTACACAAG-3′ | NM_001010968.2 |
| R: 5′-CCAGATCTGCATATTGTGGT-3′ | ||
| Col Ⅰ | F: 5′-CATGTTCAGCTTTGTGGACC-3′ | NM_053304.1 |
| R: 5′-GCAGCTGACTTCAGGGATGT-3′ | ||
| α-SMA | F: 5′-AACTGGTATTGTGCTGGACTCT-3′ | NM_031004.2 |
| R: 5′-CTCAGCAGTAGTCACGAAGGA-3′ | ||
| HNF1α | F: 5′-AAGATGACACGGATGACGATGG-3′ | NM_012669.1 |
| R: 5′-GGTTGAGACCCGTAGTGTCC-3′ | ||
| ALB | F: 5′-CTT CAA GCC TGG GCAGTAG-3′ | XM_032916218.1 |
| R: 5′-GCACTGGCTTATCACAGCAA-3′ | ||
| GAPDH | F: 5′-TCCCTCAAGATTGTCAGCAA-3′ | NM_017008.4 |
| R: 5′-AGATCCACAACGGATACTT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, J.H.; Park, S.Y.; Park, S.; Park, H.J.; Kim, J.Y.; Park, G.T.; Bae, S.H.; Kim, J.H.; Kim, G.J. Formyl Peptide Receptor 2 Alleviates Hepatic Fibrosis in Liver Cirrhosis by Vascular Remodeling. Int. J. Mol. Sci. 2021, 22, 2107. https://doi.org/10.3390/ijms22042107
Jun JH, Park SY, Park S, Park HJ, Kim JY, Park GT, Bae SH, Kim JH, Kim GJ. Formyl Peptide Receptor 2 Alleviates Hepatic Fibrosis in Liver Cirrhosis by Vascular Remodeling. International Journal of Molecular Sciences. 2021; 22(4):2107. https://doi.org/10.3390/ijms22042107
Chicago/Turabian StyleJun, Ji Hye, Soo Young Park, Sohae Park, Hee Jung Park, Jae Yeon Kim, Gyu Tae Park, Si Hyun Bae, Jae Ho Kim, and Gi Jin Kim. 2021. "Formyl Peptide Receptor 2 Alleviates Hepatic Fibrosis in Liver Cirrhosis by Vascular Remodeling" International Journal of Molecular Sciences 22, no. 4: 2107. https://doi.org/10.3390/ijms22042107
APA StyleJun, J. H., Park, S. Y., Park, S., Park, H. J., Kim, J. Y., Park, G. T., Bae, S. H., Kim, J. H., & Kim, G. J. (2021). Formyl Peptide Receptor 2 Alleviates Hepatic Fibrosis in Liver Cirrhosis by Vascular Remodeling. International Journal of Molecular Sciences, 22(4), 2107. https://doi.org/10.3390/ijms22042107







