Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
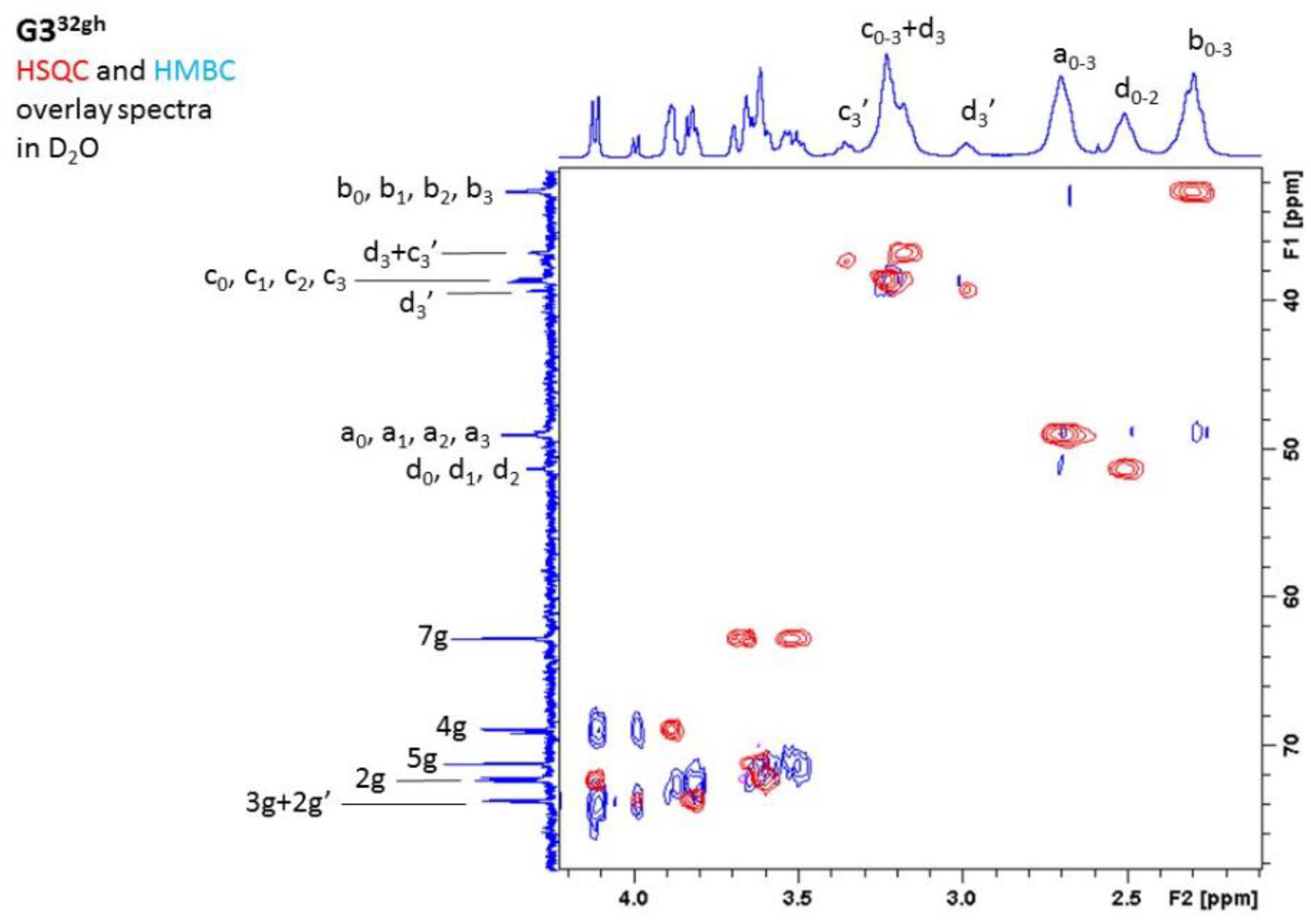
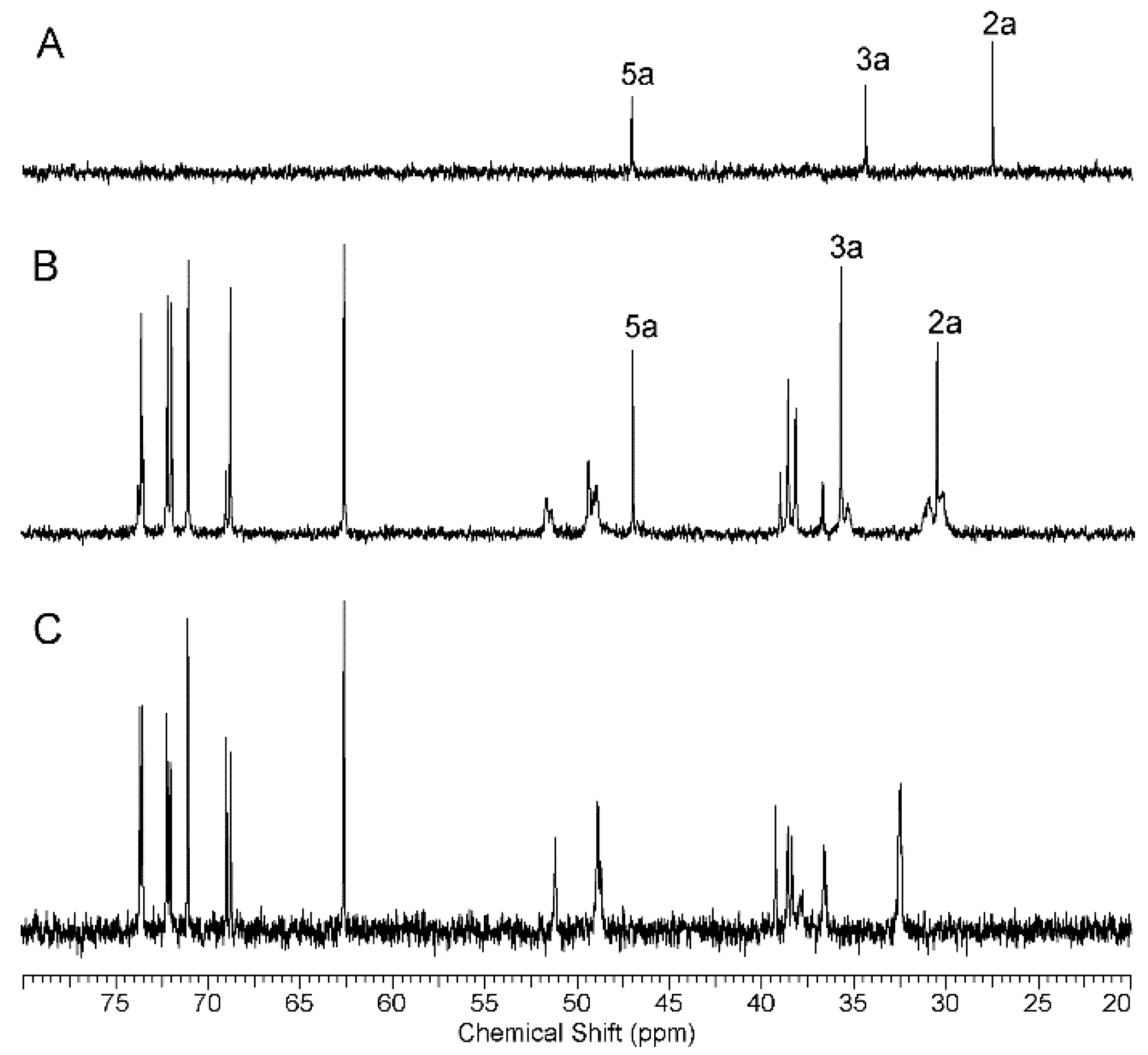
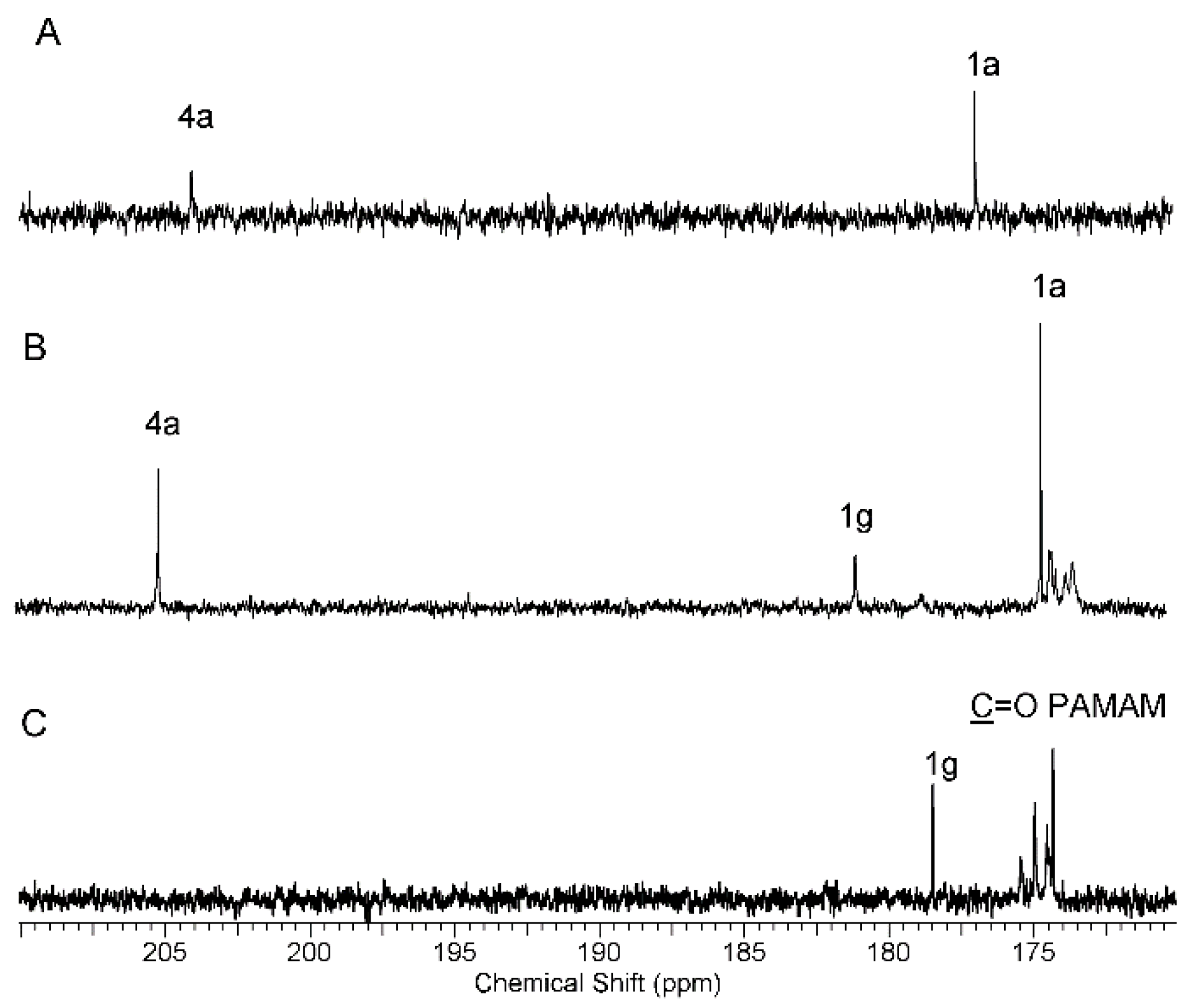
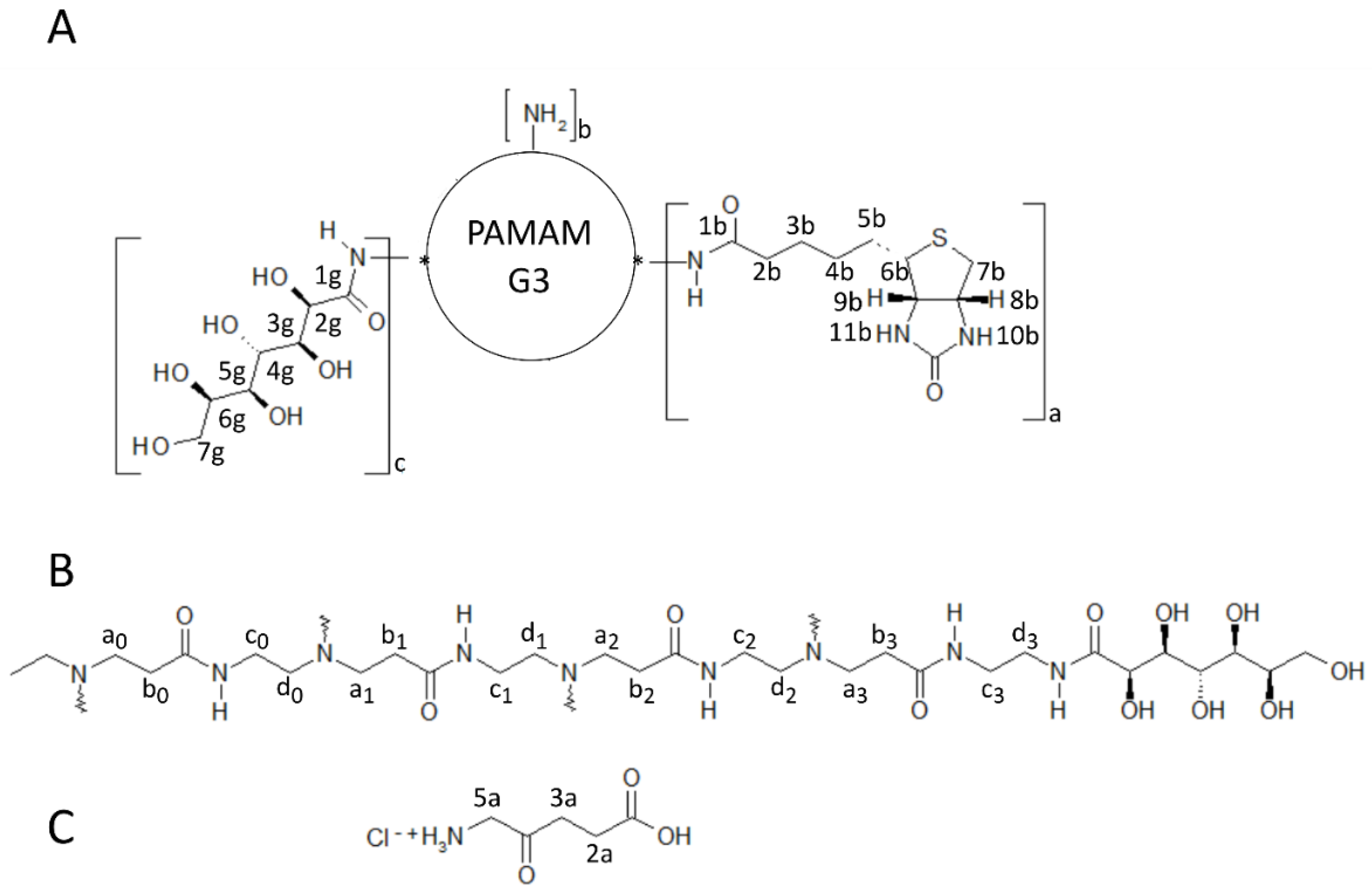
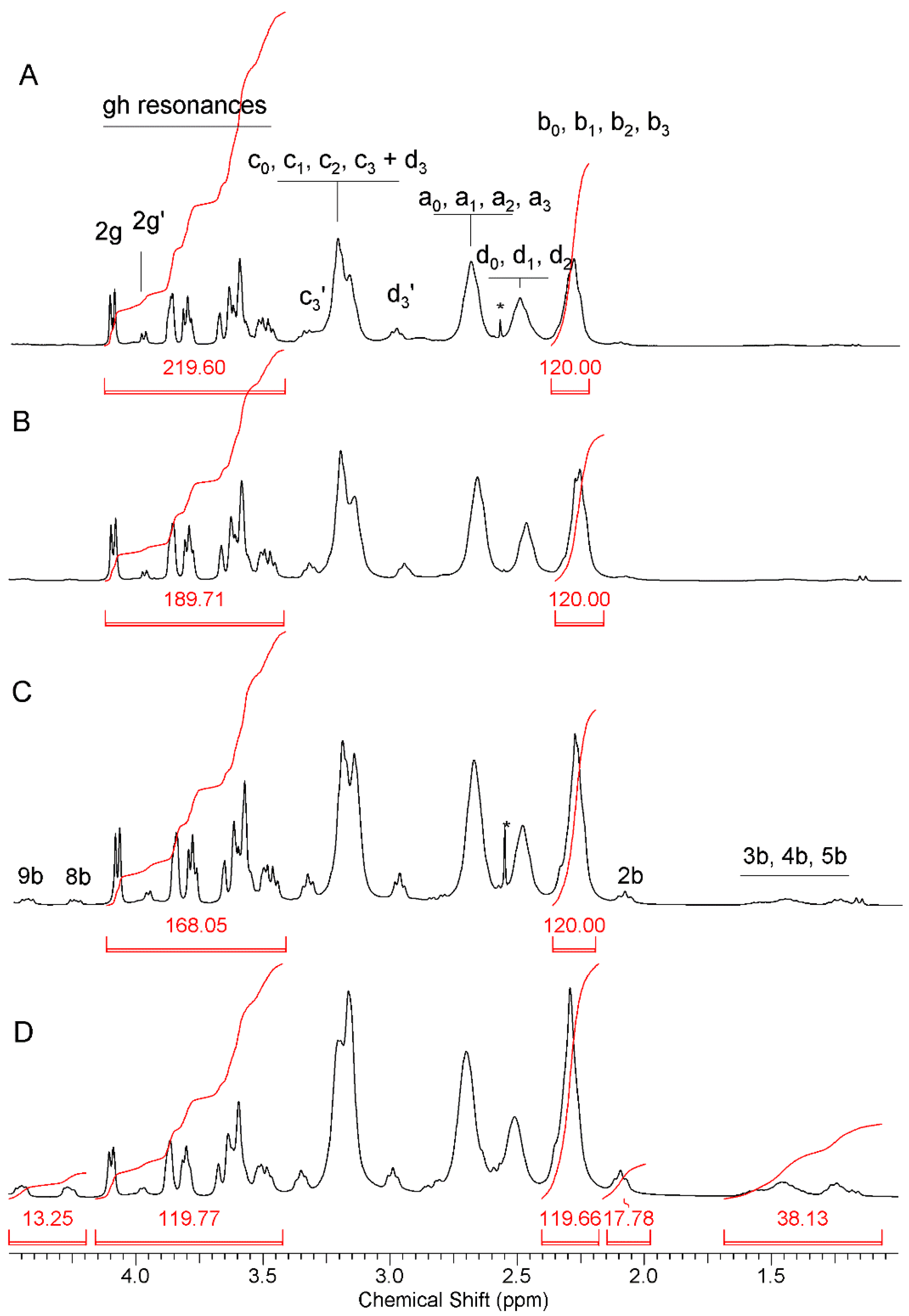
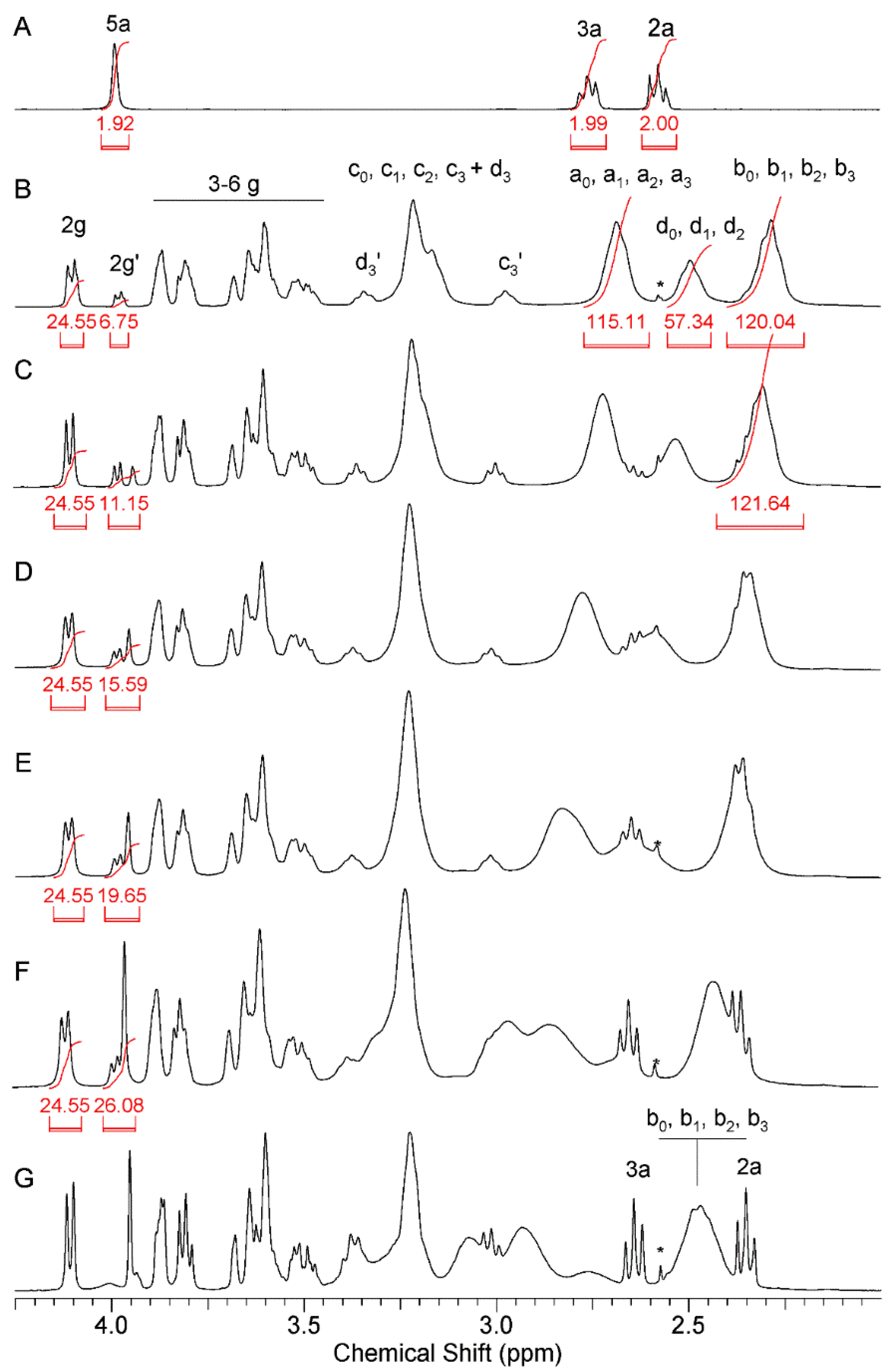
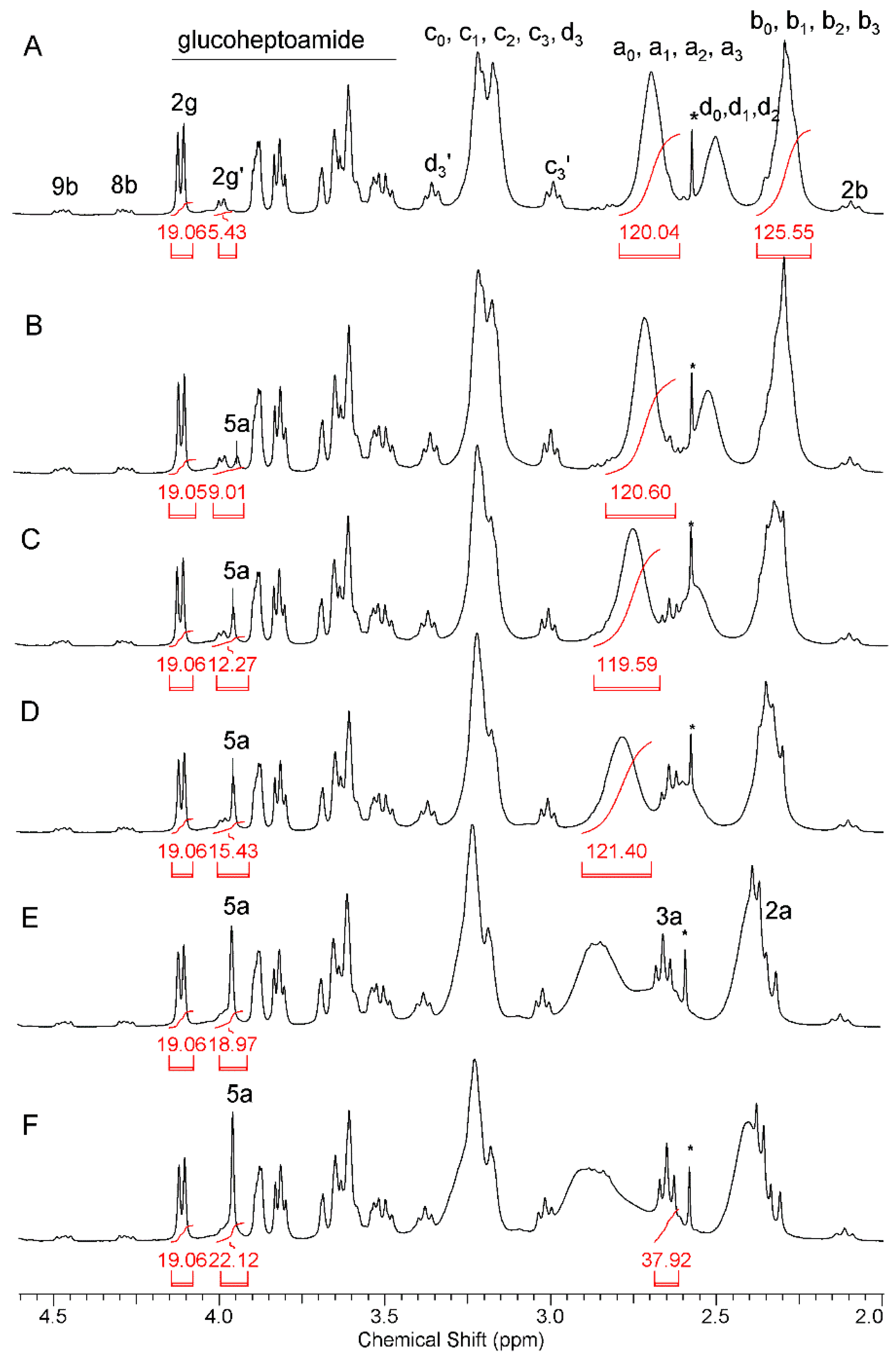
2.1.1. Synthesis and Characterization of Modified PAMAM G3 Dendrimers
2.1.2. Interaction of 5-Aminolevulinic Acid with G3Bgh Conjugates; Stability of ALA@G3gh Encapsulates
2.2. Biological Studies
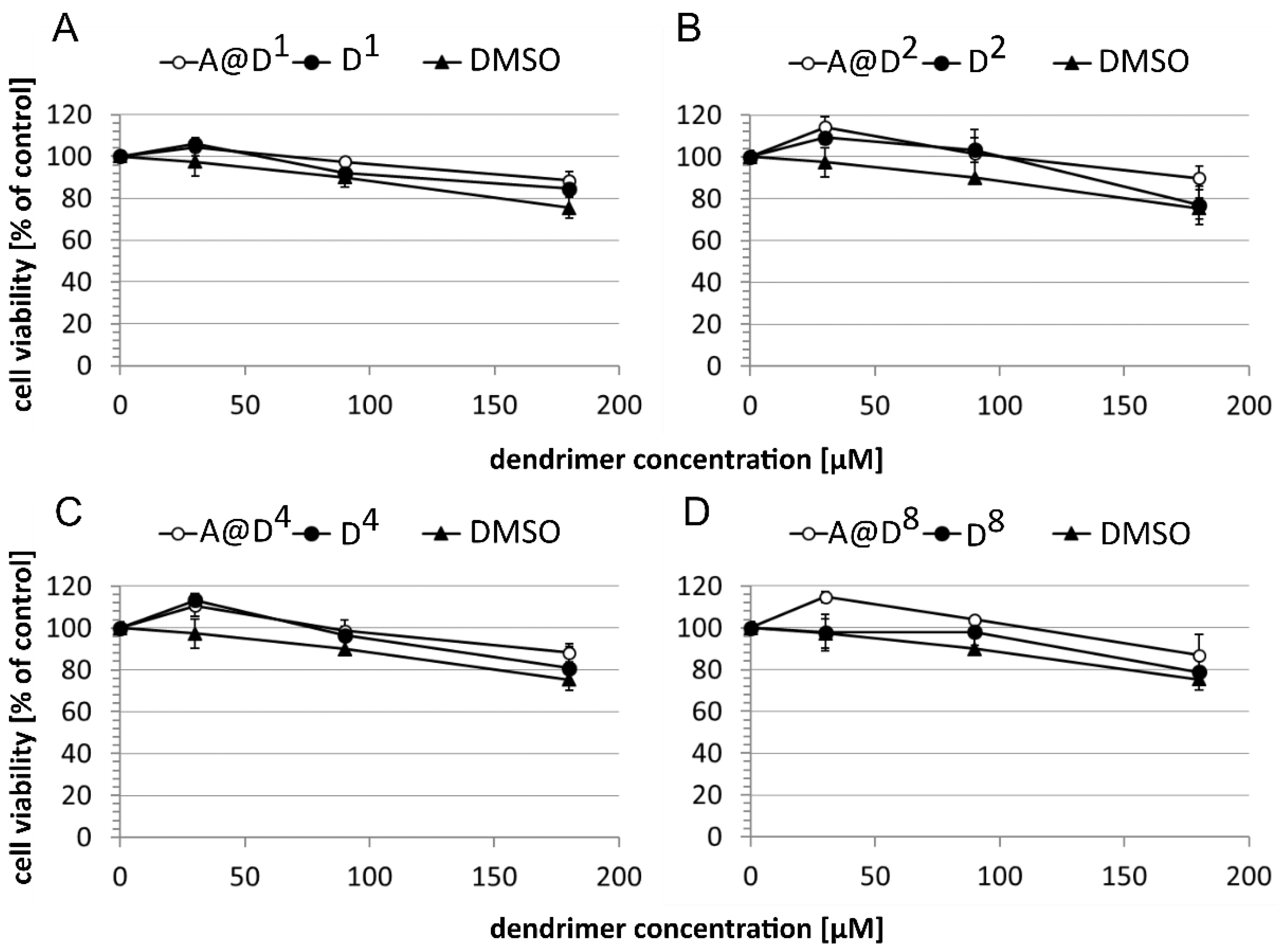
2.2.1. Cytotoxicity of Glucoheptoamidated PAMAM-Biotin Conjugates and ALA Encapsulates
2.2.2. Accumulation of Protoporphyrin IX in Caco-2 Cells
2.2.3. Intracellular Reactive Oxygen Species Level
3. Materials and Methods
3.1. Reagents and Methods
3.2. Chemical Syntheses
3.2.1. PAMAM G3 Substituted with Biotin
3.2.2. PAMAM G3 Substituted with Biotin and Glucoheptoamide
3.2.3. Encapsulation of ALA in the G3Bgh Conjugates
3.3. Cell Culture Conditions and Materials
3.4. Toxicity Studies
3.5. Accumulation of Protoporphyrin IX in Caco-2 Cells
3.6. Intracellular Reactive Oxygen Species Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Nguyen, M.; Sandhu, S.S.; Sivamani, R.K. Clinical utility of daylight photodynamic therapy in the treatment of actinic keratosis—A review of the literature. Clin. Cosmet. Investig. Dermatol. 2019, 12, 427–435. [Google Scholar] [CrossRef]
- Hasegawa, T.; Suga, Y.; Mizuno, Y.; Haruna, K.; Ogawa, H.; Ikeda, S. Efficacy of photodynamic therapy with topical 5- aminolevulinic acid using intense pulsed light for Bowen’s disease. J. Dermatol. 2010, 37, 623–628. [Google Scholar] [CrossRef]
- Zaar, O.; Fougelberg, J.; Hermansson, A.; Gillstedt, M.; Wennberg-Larko, A.M.; Paoli, J. Effectiveness of photodynamic therapy in Bowen’s disease: A retrospective observational study in 423 lesions. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1289–1294. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.; Hamblin, M. Photodynamic therapy for localized infections−State of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Lv, T.; Tu, Q.; Huang, Z.; Wang, X. Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice. Exp. Dermatol. 2013, 22, 362–363. [Google Scholar] [CrossRef]
- de Albuquerque, I.O.; Nunes, J.; Longo, J.P.; Muehlmann, L.A.; de Azevedo, R.B. Photodynamic therapy in superficial basal cell carcinoma treatment. Photodiagn. Photodyn. Ther. 2019, 27, 428–432. [Google Scholar] [CrossRef]
- Chizenga, E.P.; Chandran, R.; Abrahamse, H. Photodynamic therapy of cervical cancer by eradication of cervical cancer cells and cervical cancer stem cells. Oncotarget 2019, 10, 4380–4396. [Google Scholar] [CrossRef]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anti-cancer photodynamic therapy properties of sulphur-doped graphene quantum dot and methylene blue preparations in MCF-7 breast cancer cell culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, L.; Chi, D.; Cong, D.; Zhou, P.; Jin, J.; Ji, H.; Liang, B.; Gao, S.; Hu, S. Photodynamic therapy combined with temozolomide inhibits C6 glioma migration and invasion and promotes mitochondrial-associated apoptosis by inhibiting sodium-hydrogen exchanger isoform 1. Photodiagn. Photodyn. Ther. 2019, 26, 405–412. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Sun, Y.; Wang, L.; Ding, L.; Zhu, W.-H.; Di, W.; Duan, Y.-R. Gold-caged copolymer nanoparticles as multimodal synergistic photodynamic/photothermal/ chemotherapy platform against lethality androgen-resistant prostate cancer. Biomaterials 2019, 212, 73–86. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Reis, R.L.; Oliverira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-J.; Shieh, M.-J.; Lin, F.-H.; Lu, P.-J.; Peng, C.-L.; Wei, M.-F.; Jao, C.-J.; Lai, P.-S.; Young, T.H. Colorectal cancer cell detection by 5-aminolaevulinic acid-loaded chitosan nano-particles. Cancer Lett. 2009, 273, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Sieroń, A. Photodynamic therapy in colorectal cancer treatment: The state of the art in clinical trials. Photodiagn. Photodyn. Ther. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Kawczyk-Krupka, A.; Czuba, Z.P.; Kwiatek, B.; Kwiatek, S.; Krupka, M.; Sieroń, K. The effect of ALA-PDT under normoxia and cobalt chloride (CoCl2)-induced hypoxia on adhesion molecules (ICAM-1, VCAM-1) secretion by colorectal cancer cells. Photodiagn. Photodyn. Ther. 2017, 19, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Simelane, N.W.N.; Kruger, C.A.; Abrahamse, H. Photodynamic diagnosis and photodynamic therapy of colorectal cancer in vitro and in vivo. RSC Adv. 2020, 10, 41560. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X.; Zhao, F.; Luan, H.; Tu, Q.; Huang, Z.; Wang, H.; Wang, H.W. In vitro evaluation of 5-aminolevulinic acid (ALA) loaded PLGA nanoparticles. Int. J. Nanomed. 2013, 8, 2669–2676. [Google Scholar] [CrossRef]
- Wang, Z.; Gai, S.; Wang, C.; Yang, G.; Zhong, C.; Dai, Y.; He, F.; Yang, D.; Yang, P. Self-assembled zinc phthalocyanine nanoparticles as excellent photothermal/photodynamic synergistic agent for antitumor treatment. Chem. Eng. J. 2019, 361, 117–128. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-mediated x-ray induced photodynamic therapy for deep-seated tumors: From concept to biomedical applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef]
- Namikawa, T.; Yatabe, T.; Inoue, K.; Shuin, T.; Hanazaki, K. Clinical applications of 5-aminolevulinic acid-mediated fluorescence for gastric cancer. World J. Gastroenterol. 2015, 21, 8769–8775. [Google Scholar] [CrossRef]
- Fotinos, N.; Campo, M.A.; Popowycz, F.; Gurny, R.; Lange, N. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem. Photobiol. 2006, 82, 994–1015. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Abdel Gaber, S.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J. Photochem. Photobiol. B Biol. 2020, 210, 111963. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ogawa, T.; Yanagihara, C.; Tamai, I. Kinetic Evaluation of Determinant Factors for Cellular Accumulation of Protoporphyrin IX Induced by External 5-Aminolevulinic Acid for Photodynamic Cancer Therapy. J. Pharm. Sci. 2015, 104, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fujiwara, T.; Ota, U.; Hatta, S.; Ichikawa, S.; Kobayashi, M.; Okitsu, Y.; Fukuhara, N.; Onishi, Y.; Ishizuka, M.; et al. Dynamics of absorption, metabolism, and excretion of 5-aminolevulinic acid in human intestinal Caco-2 cells. Biochem. Biophys. Rep. 2017, 11, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sourdon, A.; Gary-Bobo, M.; Maynadier, M.; Garcia, M.; Majoral, J.-P.; Caminade, A.-M.; Mongin, O.; Blanchard-Desce, M. Dendrimeric Nanoparticles for Two-Photon Photodynamic Therapy and Imaging: Synthesis, Photophysical Properties, Innocuousness in Daylight and Cytotoxicity under Two-Photon Irradiation in the NIR. Chem. Eur. J. 2019, 25, 3637–3649. [Google Scholar] [CrossRef]
- Pandey, P.K.; Maheshwari, R.; Raval, N.; Gondaliya, P.; Kalia, K.; Tekade, R.K. Nanogold-core multifunctional dendrimer for pulsatile chemo-, photothermal- and photodynamic- therapy of rheumatoid arthritis. J. Colloid Interface Sci. 2019, 15, 61–77. [Google Scholar] [CrossRef]
- Zhou, T.; Battah, S.; Mazzacuva, F.; Hider, R.C.; Dobbin, P.; MacRobert, A.J. Design of Bifunctional Dendritic 5-Aminolevulinic Acid and Hydroxypyridinone Conjugates for Photodynamic Therapy. Bioconjug. Chem. 2018, 29, 3411–3428. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.B.; Wood, R.C.; Salas, P.J.I.; Zea, D.L.; Ramsauer, V.P. Early Response to ErbB2 Over-Expression in Polarized Caco-2 Cells Involves Partial Segregation from ErbB3 by Relocalization to the Apical Surface and Initiation of Survival Signaling. J. Cell Biochem. 2010, 111, 643–652. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Filipowicz, A.; Zaręba, M.; Wałajtys-Rode, E.; Wołowiec, S. Cellular uptake of glucoheptoamidated poly(amidoamine) PAMAM G3 dendrimer with amide-conjugated biotin, a potential carrier of anticancer drugs. Bioorg. Med. Chem. 2017, 25, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Elfsson, B.; Wallin, I.; Eksborg, S.; Rudaeusa, K.; Ros, A.M.; Ehrsson, H. Stability of 5-aminolevulinic acid in aqueous solution. Eur. J. Pharm. Sci. 1998, 7, 87–91. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Tutka, P.; Pyda, M.; Walczak, M.; Uram, Ł.; Misiorek, M.; Chmiel, E.; Wołowiec, S. Stepwise glucoheptoamidation of poly(amidoamine) dendrimer G3 to tune physicochemical properties of the potential drug carrier; in vitro tests for cytisine conjugates. Pharmaceutics 2020, 12, 473. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, H.-M.; Chang, F.-P.; Shen, C.-R.; Liu, C.-L.; Mao, W.-Y.; Lin, C.-C.; Lee, H.-S.; Shen, C.-N. Protoporphyrin IX accumulation disrupts mitochondrial dynamics and function in ABCG2-deficient hepatocytes. FEBS Lett. 2013, 587, 3202–3209. [Google Scholar] [CrossRef]
- Wirth, D.J.; Sibai, M.; Wilson, B.C.; Roberts, D.W.; Paulsen, K. First experience with spatial frequency domain imaging and red-light excitation of protoporphyrin IX fluorescence during tumor resection. Biomed. Opt. Express 2020, 11, 4306–4315. [Google Scholar] [CrossRef]
- Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Czarnik-Kwaśniak, J.; Kwaśniak, K.; Tutaj, K.; Filiks, I.; Uram, Ł.; Stompor, M.; Wołowiec, S. Glucoheptoamidated polyamidoamine PAMAM G3 dendrimer as a vehicle for succinate linked doxorubicin; enhanced toxicity of DOX against grade IV glioblastoma U-118 MG cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101424. [Google Scholar] [CrossRef]
- Yang, S.-J.; Lin, F.-H.; Tsai, K.-C.; Wei, M.-F.; Tsai, H.-M.; Wong, J.-M.; Shieh, M.-J. Folic Acid-Conjugated Chitosan Nanoparticles Enhanced Protoporphyrin IX Accumulation in Colorectal Cancer Cells. Bioconjug. Chem. 2010, 21, 679–689. [Google Scholar] [CrossRef]
- Avci, P.; Erdem, S.S.; Hamblin, M.R. Photodynamic Therapy: One Step Ahead with Self-Assembled Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Laskowska, B.; Magoń, A.; Myśliwiec, B.; Pyda, M.; Wołowiec, S. PAMAM dendrimers as solubilizers and hosts for 8-methoxypsoralene enabling transdermal diffusion of the guest. Int. J. Pharm. 2010, 398, 185–189. [Google Scholar] [CrossRef]
- Borowska, K.; Wolowiec, S.; Rubaj, A.; Głowniak, K.; Sieniawska, E.; Radej, S. Effect of polyamidoamine dendrimer G3 and G4 on skin permeation of 8-methoxypsoralene—In vivo study. Int. J. Pharm. 2012, 426, 280–283. [Google Scholar] [CrossRef]
- Borowska, K.; Wolowiec, S.; Głowniak, K.; Sieniawska, E.; Radej, S. Transdermal delivery of 8-methoxypsoralene mediated by polyamidoamine dendrimer G2.5 and G3.5—In vitro and in vivo study. Int. J. Pharm. 2012, 436, 764–770. [Google Scholar] [CrossRef]
- Battah, S.; Balaratnam, S.; Casas, A.; O’Neill, S.; Edwards, C.; Batlle, A.; Dobbin, P.; MacRobert, A.J. Macromolecular delivery of 5-aminolaevulinic acid for photodynamic therapy using dendrimer conjugates. Mol. Cancer Ther. 2007, 6, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Szuster, M.; Uram, Ł.; Filipowicz-Rachwał, A.; Wołowiec, S.; Wałajtys-Rode, E. Evaluation of the localization and biological effects of PAMAM G3 dendrimer-biotin/pyridoxal conjugate as HaCaT keratinocyte targeted nanocarrier. Acta Biochim. Polon. 2019, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Wołowiec, S. Pol. Patent P.432139. 2020. Available online: https://uprp.gov.pl/sites/default/files/bup/2020/Wynalazki%20i%20wzory%20użytkowe/06_12-14/12/bup12_2020.pdf (accessed on 17 February 2021).

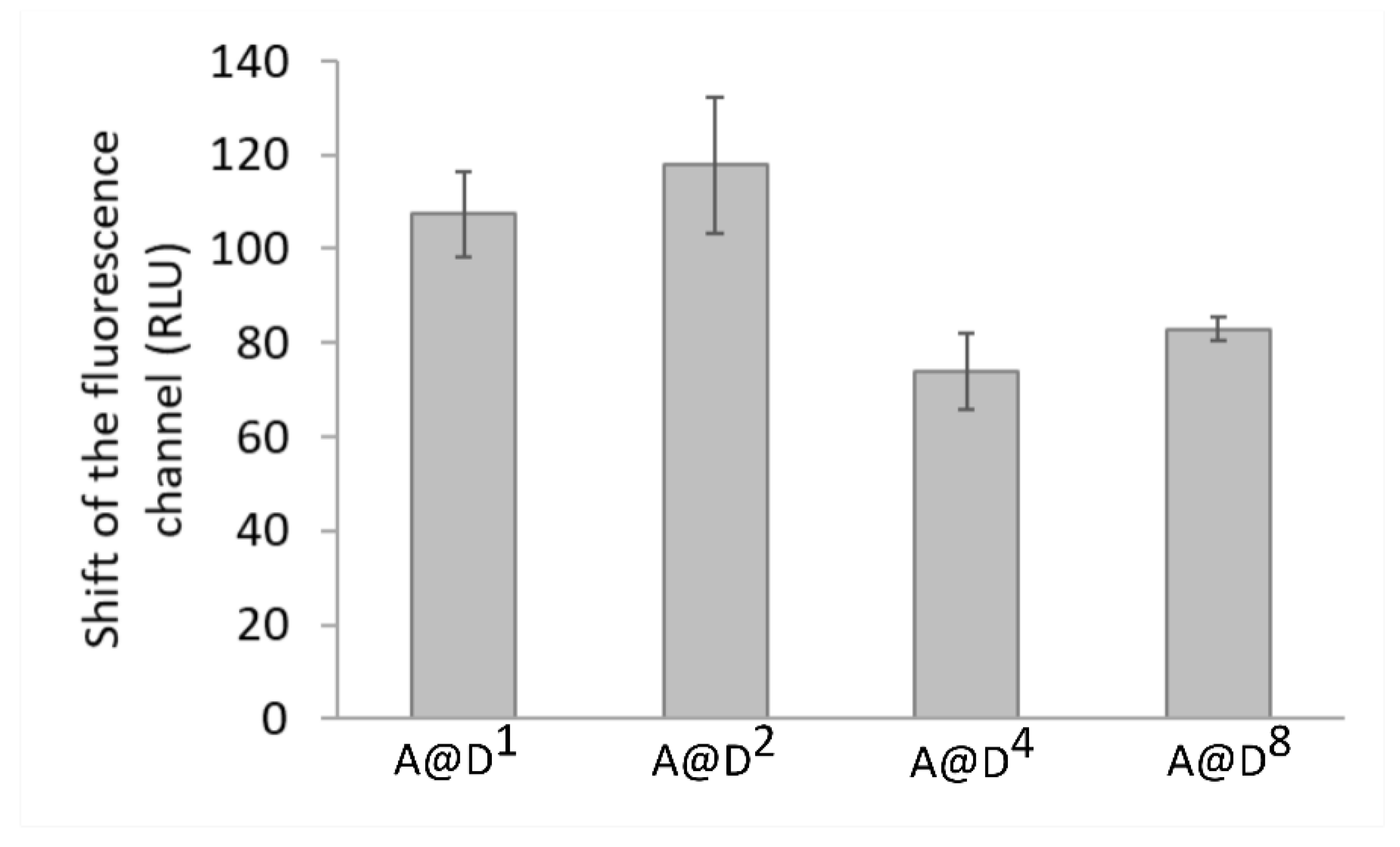
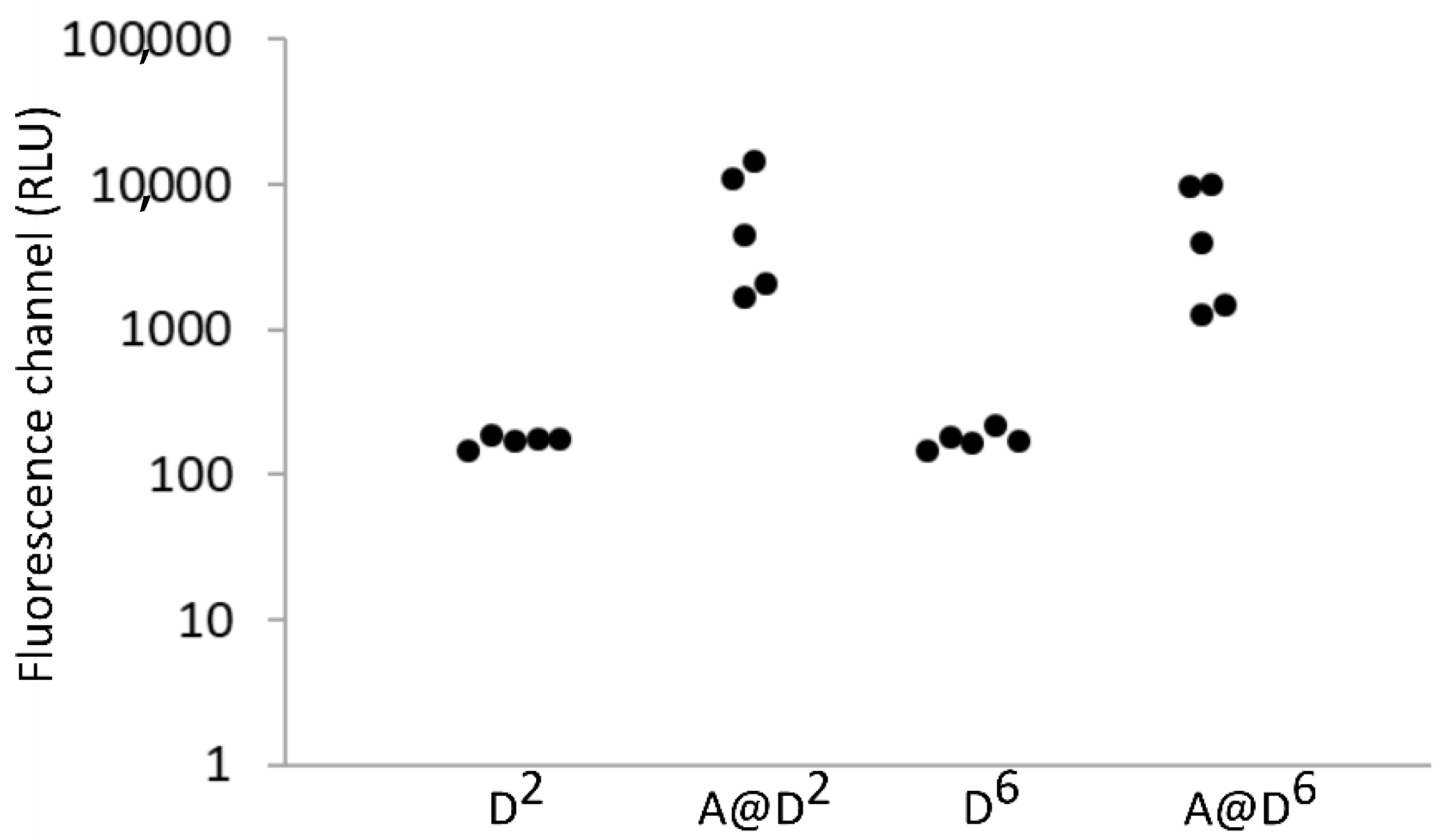
| Shift of the Fluorescence Channel of H2DCFDA (RLU) | ||||||
|---|---|---|---|---|---|---|
| H2O/DMSO Solution | in H2O (DMSO Excluded) | |||||
| Experiment | A@D2 | A@D6 | A@D1 | A@D2 | A@D4 | A@D8 |
| A (30 s illumination) | 263.28 | 443.29 | ||||
| B (60 s illumination) | −206.1 | 44.49 | −529.43 | −363.42 | −514.6 | −1084.1 |
| Compound | Dendrimer Concentration | ALA Concentration | DMSO Concentration |
|---|---|---|---|
| D1, D2, D4, D8 | 30 µM | 0 | 0.5% |
| 90 µM | 0 | 1.5% | |
| 180 µM | 0 | 3% | |
| A@D1, A@D2, A@D4, A@D8 | 30 µM | 180 µM | 0.5% |
| 90 µM | 540 µM | 1.5% | |
| 180 µM | 1.08 mM | 3% | |
| D2, D6 | 45 µM | 0 | – |
| 135 µM | 0 | ||
| 270 µM | 0 | ||
| A@D2, A@D6 | 45 µM | 180 µM | |
| 135 µM | 540 µM | ||
| 270 µM | 1.08 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczorowska, A.; Malinga-Drozd, M.; Kałas, W.; Kopaczyńska, M.; Wołowiec, S.; Borowska, K. Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System. Int. J. Mol. Sci. 2021, 22, 1982. https://doi.org/10.3390/ijms22041982
Kaczorowska A, Malinga-Drozd M, Kałas W, Kopaczyńska M, Wołowiec S, Borowska K. Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System. International Journal of Molecular Sciences. 2021; 22(4):1982. https://doi.org/10.3390/ijms22041982
Chicago/Turabian StyleKaczorowska, Aleksandra, Małgorzata Malinga-Drozd, Wojciech Kałas, Marta Kopaczyńska, Stanisław Wołowiec, and Katarzyna Borowska. 2021. "Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System" International Journal of Molecular Sciences 22, no. 4: 1982. https://doi.org/10.3390/ijms22041982
APA StyleKaczorowska, A., Malinga-Drozd, M., Kałas, W., Kopaczyńska, M., Wołowiec, S., & Borowska, K. (2021). Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System. International Journal of Molecular Sciences, 22(4), 1982. https://doi.org/10.3390/ijms22041982







