Validation of a Three-Dimensional Head and Neck Spheroid Model to Evaluate Cameras for NIR Fluorescence-Guided Cancer Surgery
Abstract
1. Introduction
2. Results
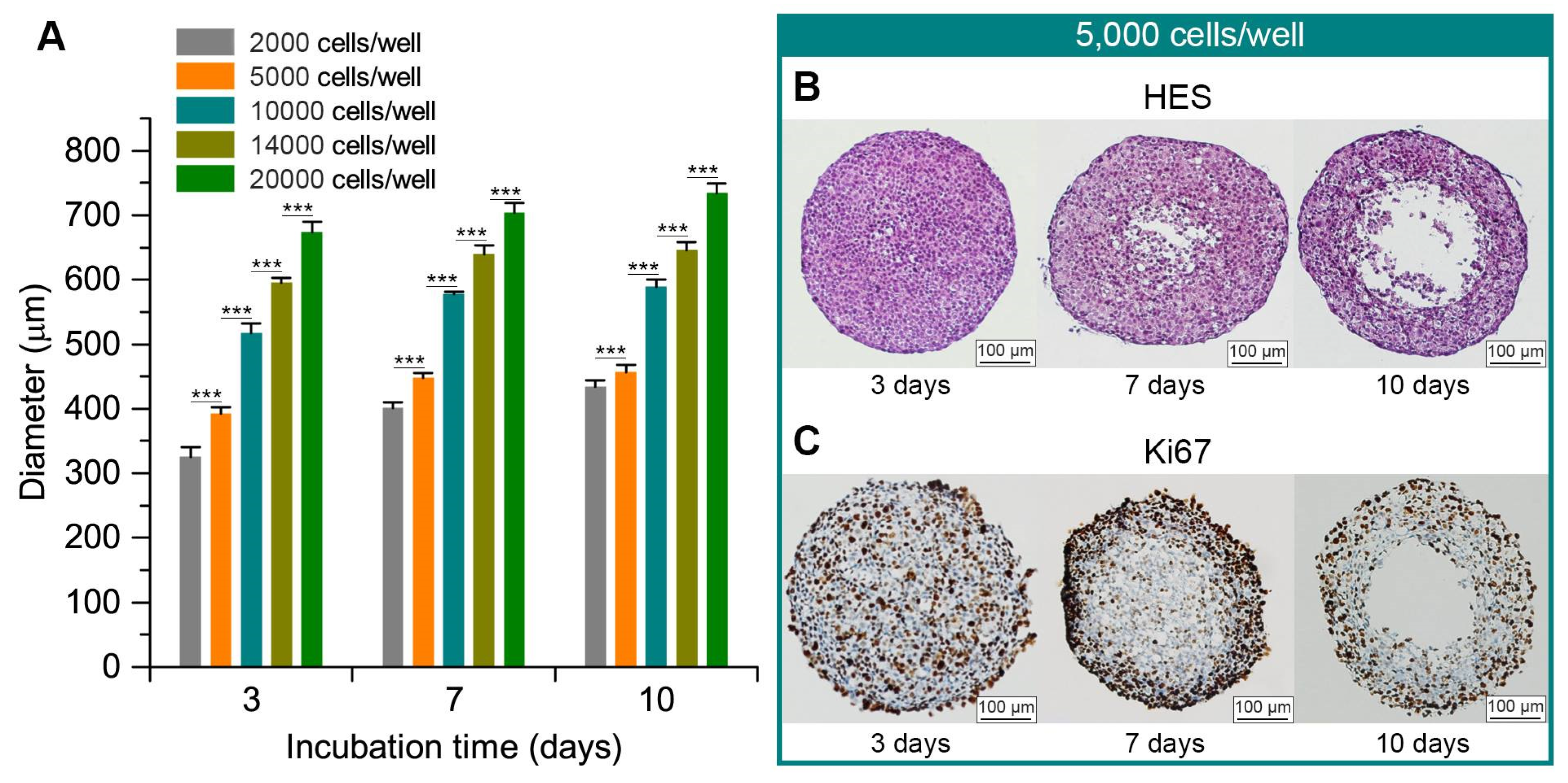
2.1. FaDu Spheroid Model
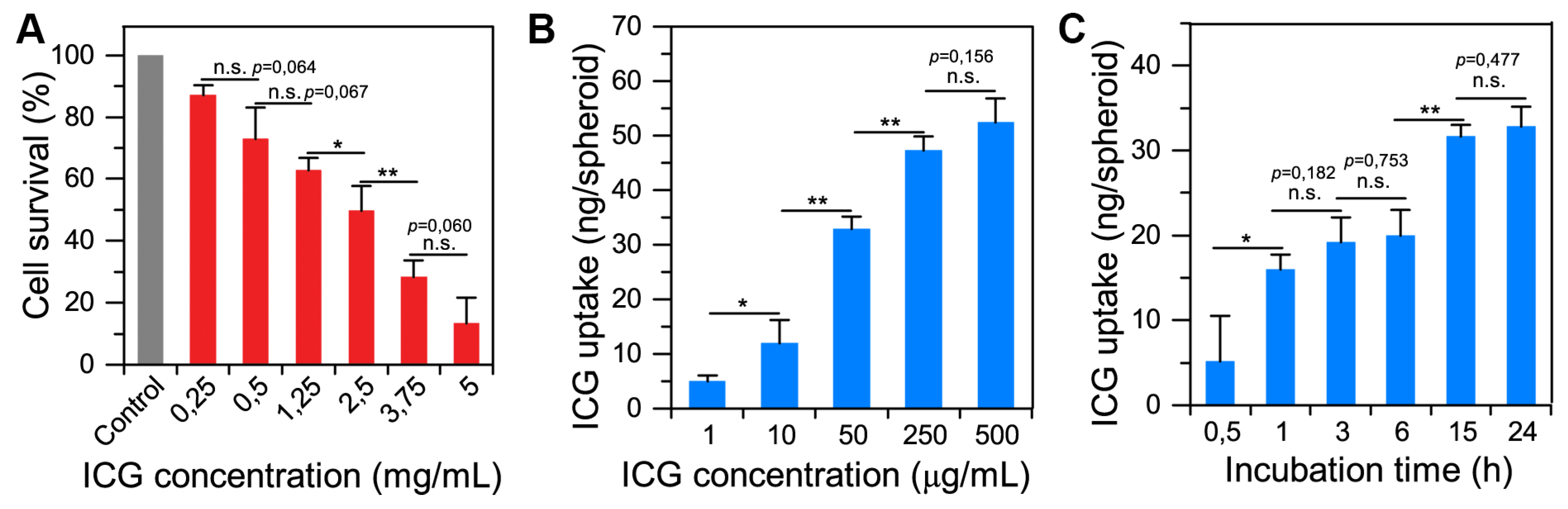
2.2. Spheroid–Indocyanine Green Interaction
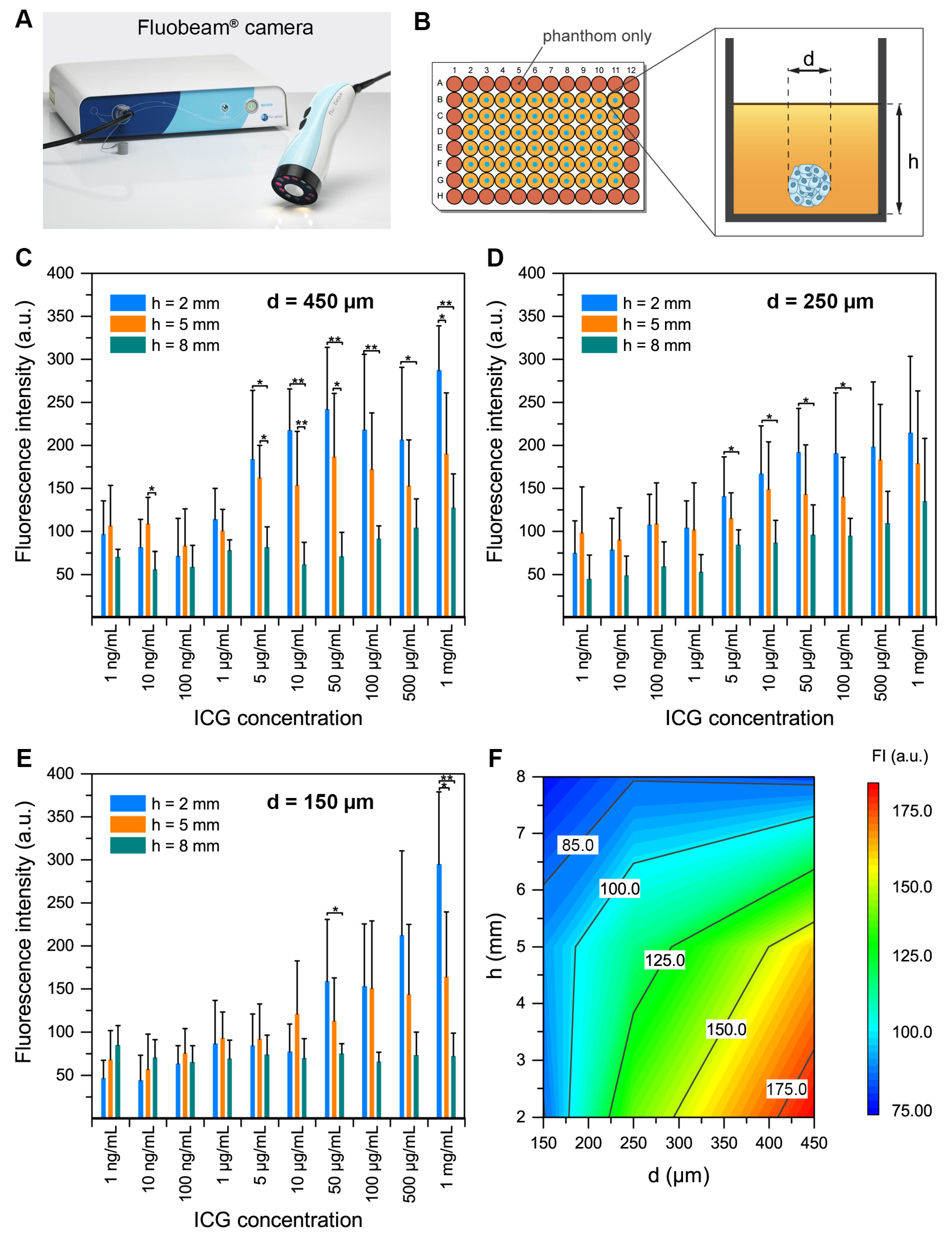
2.3. Spheroid Detection in Tissue-Like Phantoms
3. Discussion
4. Materials and Methods
4.1. Cell Line
4.2. Near-Infrared Camera System and Probe
4.3. FaDu Spheroid Model
4.4. Spheroid–Indocyanine Green Interaction
4.4.1. Indocyanine Green Cytotoxicity: Clonogenic Assays
4.4.2. Uptake and Chemical Extraction of ICG in Spheroids
4.4.3. Diffusion of ICG into Spheroids
4.5. Spheroid Detection in Tissue-Like Phantoms
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Atallah, I.; Milet, C.; Henry, M.; Josserand, V.; Reyt, E.; Coll, J.-L.; Hurbin, A.; Righini, C.A. Near-infrared fluorescence imaging-guided surgery improves recurrence-free survival rate in novel orthotopic animal model of head and neck squamous cell carcinoma. Head. Neck 2016, 38 (Suppl. 1), E246–E255. [Google Scholar] [CrossRef]
- Lau, O.D.; Hu, D.; Wang, L.; Wang, G.; Schaue, D.; Zhu, L.; Huang, M.; Lin, Y.; Dennis, M.; Abemayor, E.; et al. A novel modular polymer platform for the treatment of head and neck squamous cell carcinoma in an animal model. Arch. Otolaryngol. Head. Neck Surg. 2012, 138, 412–417. [Google Scholar] [CrossRef]
- Mehanna, H.; Paleri, V.; West, C.M.L.; Nutting, C. Head and neck cancer—Part 1: Epidemiology, presentation, and prevention. BMJ 2010, 341, c4684. [Google Scholar] [CrossRef]
- Jones, A.S.; Bin Hanafi, Z.; Nadapalan, V.; Roland, N.J.; Kinsella, A.; Helliwell, T.R. Do positive resection margins after ablative surgery for head and neck cancer adversely affect prognosis? A study of 352 patients with recurrent carcinoma following radiotherapy treated by salvage surgery. Br. J. Cancer 1996, 74, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Atallah, I.; Milet, C.; Coll, J.-L.; Reyt, E.; Righini, C.A.; Hurbin, A. Role of near-infrared fluorescence imaging in head and neck cancer surgery: From animal models to humans. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Kerrien, E.; Yakavets, I.; Meilender, R.; Mastronicola, R.; Renard, S. ICG-induced NIR fluorescence mapping in patients with head & neck tumors after the previous radiotherapy. Photodiagnosis Photodyn. Ther. 2020, 31, 101838. [Google Scholar]
- van Driel PB a, A.; van de Giessen, M.; Boonstra, M.C.; Snoeks, T.J.A.; Keereweer, S.; Oliveira, S. Characterization and evaluation of the artemis camera for fluorescence-guided cancer surgery. Mol. Imaging Biol. 2015, 17, 413–423. [Google Scholar] [CrossRef][Green Version]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; Van Der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; Van De Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.T.; Rainaldi, G. Three-dimensional spheroid model in tumor biology. Pathobiology 1999, 67, 148–157. [Google Scholar] [CrossRef]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical cancer models in tumor biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug delivery to solid tumors: The predictive value of the multicellular tumor spheroid model for nanomedicine screening. Int. J. Nanomed. 2017, 12, 7993–8007. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Sun, C.-H.; Tromberg, B.J.; Cristini, V.; De Magalhães, N.; Hirschberg, H. Multicell tumor spheroids in photodynamic therapy. Lasers Surg. Med. 2006, 38, 555–564. [Google Scholar] [CrossRef]
- Braunholz, D.; Saki, M.; Niehr, F.; Öztürk, M.; Puértolas, B.B.; Konschak, R.; Budach, V.; Tinhofer, I. Spheroid Culture of Head and Neck Cancer Cells Reveals an Important Role of EGFR Signalling in Anchorage Independent Survival. PLoS ONE 2016, 11, e0163149. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Walenta, S.; Mueller-Klieser, W. Efficacy of catumaxomab in tumor spheroid killing is mediated by its trifunctional mode of action. Cancer Immunol. Immunother. 2010, 59, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Hussien, K.; Muschel, R.J. 3D tumour spheroids as a model to assess the suitability of [18F]FDG-PET as an early indicator of response to PI3K inhibition. Nucl. Med. Biol. 2012, 39, 986–992. [Google Scholar] [CrossRef]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.-P. NIR fluorescence-guided tumor surgery: New strategies for the use of indocyanine green. Int. J. Nanomed. 2019, 14, 7823–7838. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Yokoyama, J.; Fujimaki, M.; Ohba, S.; Anzai, T.; Yoshii, R.; Ito, S.; Kojima, M.; Ikeda, K. A feasibility study of NIR fluorescent image-guided surgery in head and neck cancer based on the assessment of optimum surgical time as revealed through dynamic imaging. OncoTargets Ther. 2013, 6, 325–330. [Google Scholar] [CrossRef]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Bubb, K.J.; Tang, O.; Kizana, E.; Gentile, C. Vascularized Cardiac Spheroids as Novel 3D in vitro Models to Study Cardiac Fibrosis. Cells Tissues Organs 2017, 204, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, A.; Phan, D.T.T.; Datta, R.; Wang, X.; Hachey, S.J.; Romero-López, M.; Gratton, E.; Lee, A.P.; George, S.C.; Hughes, C.C.W. 3D microtumors in vitro supported by perfused vascular networks. Sci. Rep. 2016, 6, 31589. [Google Scholar] [CrossRef]
- De Grand, A.M.; Lomnes, S.J.; Lee, D.S.; Pietrzykowski, M.; Ohnishi, S.; Morgan, T.G.; Gogbashian, A.; Laurence, R.G.; Frangioni, J.V. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J. Biomed. Opt. 2006, 11, 014007. [Google Scholar] [CrossRef]
- Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Basile, N.; Castellano, P.; Orditura, M.; Galizia, G. Fluorescence-Guided Surgery in the Combined Treatment of Peritoneal Carcinomatosis from Colorectal Cancer: Preliminary Results and Considerations. World J. Surg. 2018, 42, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Modes of invasion during tumour dissemination. Mol. Oncol. 2017, 11, 5–27. [Google Scholar] [CrossRef]
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer Invasion: Patterns and Mechanisms. Acta Nat. 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K.B.; Annapragada, A. NIR-II fluorescence imaging using indocyanine green nanoparticles. Sci. Rep. 2018, 8, 14455. [Google Scholar] [CrossRef]
- Yakavets, I.; Millard, M.; Lamy, L.; Francois, A.; Scheglmann, D.; Wiehe, A.; Lassalle, H.-P.; Zorin, V.; Bezdetnaya, L. Matryoshka-Type Liposomes Offer the Improved Delivery of Temoporfin to Tumor Spheroids. Cancers 2019, 11, 1366. [Google Scholar] [CrossRef]
- Yakavets, I.; Jenard, S.; Francois, A.; Maklygina, Y.; Loschenov, V.; Lassalle, H.-P. Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening. J. Clin. Med. 2019, 8, 1686. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egloff-Juras, C.; Yakavets, I.; Scherrer, V.; Francois, A.; Bezdetnaya, L.; Lassalle, H.-P.; Dolivet, G. Validation of a Three-Dimensional Head and Neck Spheroid Model to Evaluate Cameras for NIR Fluorescence-Guided Cancer Surgery. Int. J. Mol. Sci. 2021, 22, 1966. https://doi.org/10.3390/ijms22041966
Egloff-Juras C, Yakavets I, Scherrer V, Francois A, Bezdetnaya L, Lassalle H-P, Dolivet G. Validation of a Three-Dimensional Head and Neck Spheroid Model to Evaluate Cameras for NIR Fluorescence-Guided Cancer Surgery. International Journal of Molecular Sciences. 2021; 22(4):1966. https://doi.org/10.3390/ijms22041966
Chicago/Turabian StyleEgloff-Juras, Claire, Ilya Yakavets, Victoria Scherrer, Aurélie Francois, Lina Bezdetnaya, Henri-Pierre Lassalle, and Gilles Dolivet. 2021. "Validation of a Three-Dimensional Head and Neck Spheroid Model to Evaluate Cameras for NIR Fluorescence-Guided Cancer Surgery" International Journal of Molecular Sciences 22, no. 4: 1966. https://doi.org/10.3390/ijms22041966
APA StyleEgloff-Juras, C., Yakavets, I., Scherrer, V., Francois, A., Bezdetnaya, L., Lassalle, H.-P., & Dolivet, G. (2021). Validation of a Three-Dimensional Head and Neck Spheroid Model to Evaluate Cameras for NIR Fluorescence-Guided Cancer Surgery. International Journal of Molecular Sciences, 22(4), 1966. https://doi.org/10.3390/ijms22041966






