Immunotherapy with 4-1BBL-Expressing iPS Cell‐Derived Myeloid Lines Amplifies Antigen-Specific T Cell Infiltration in Advanced Melanoma
Abstract
1. Introduction
2. Results
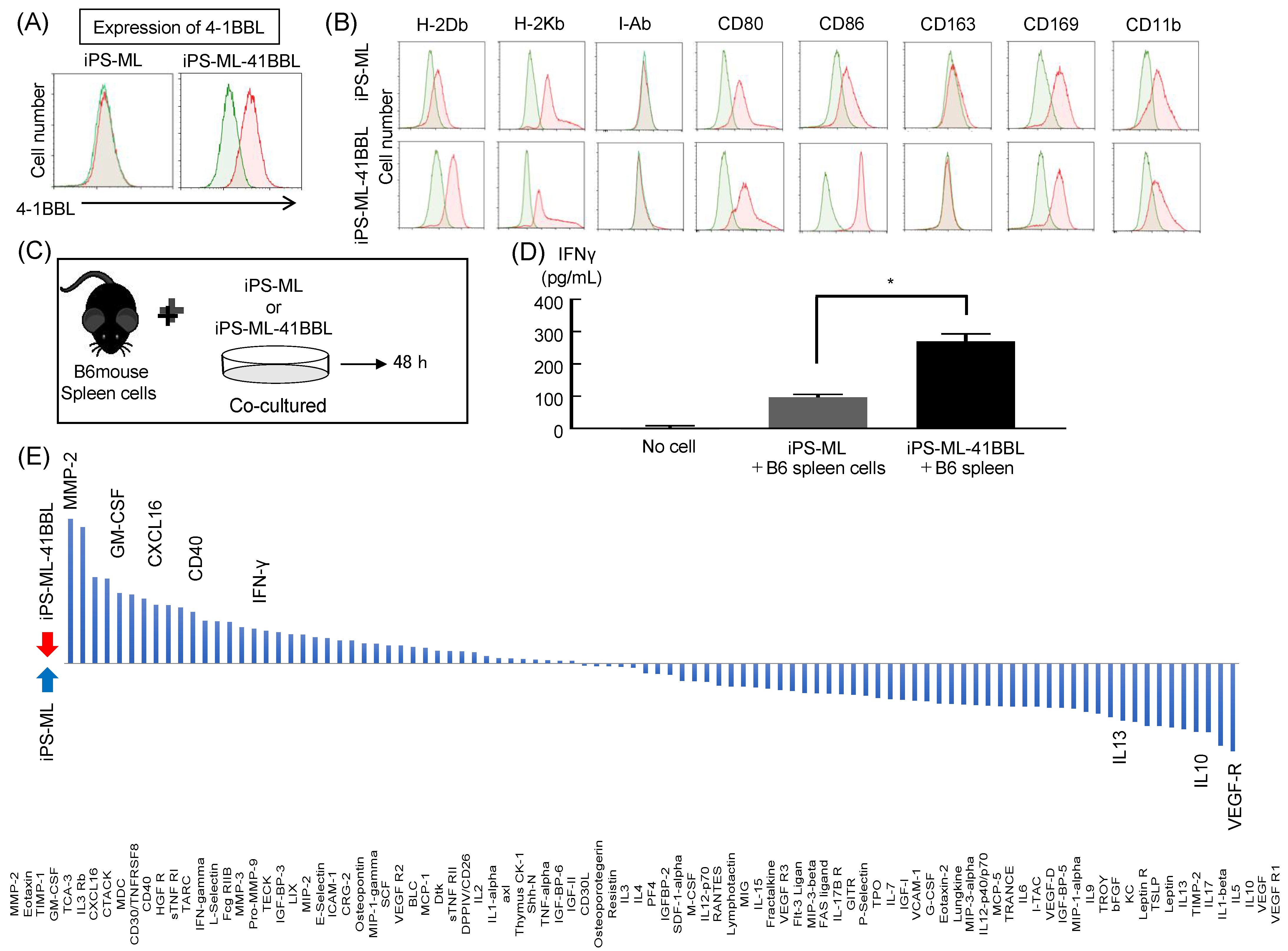
2.1. Characterization of Mouse iPS-ML and iPS-ML-41BBL
2.2. Cytokine Analysis after Coculture of iPS-ML and Mouse Spleen Cells
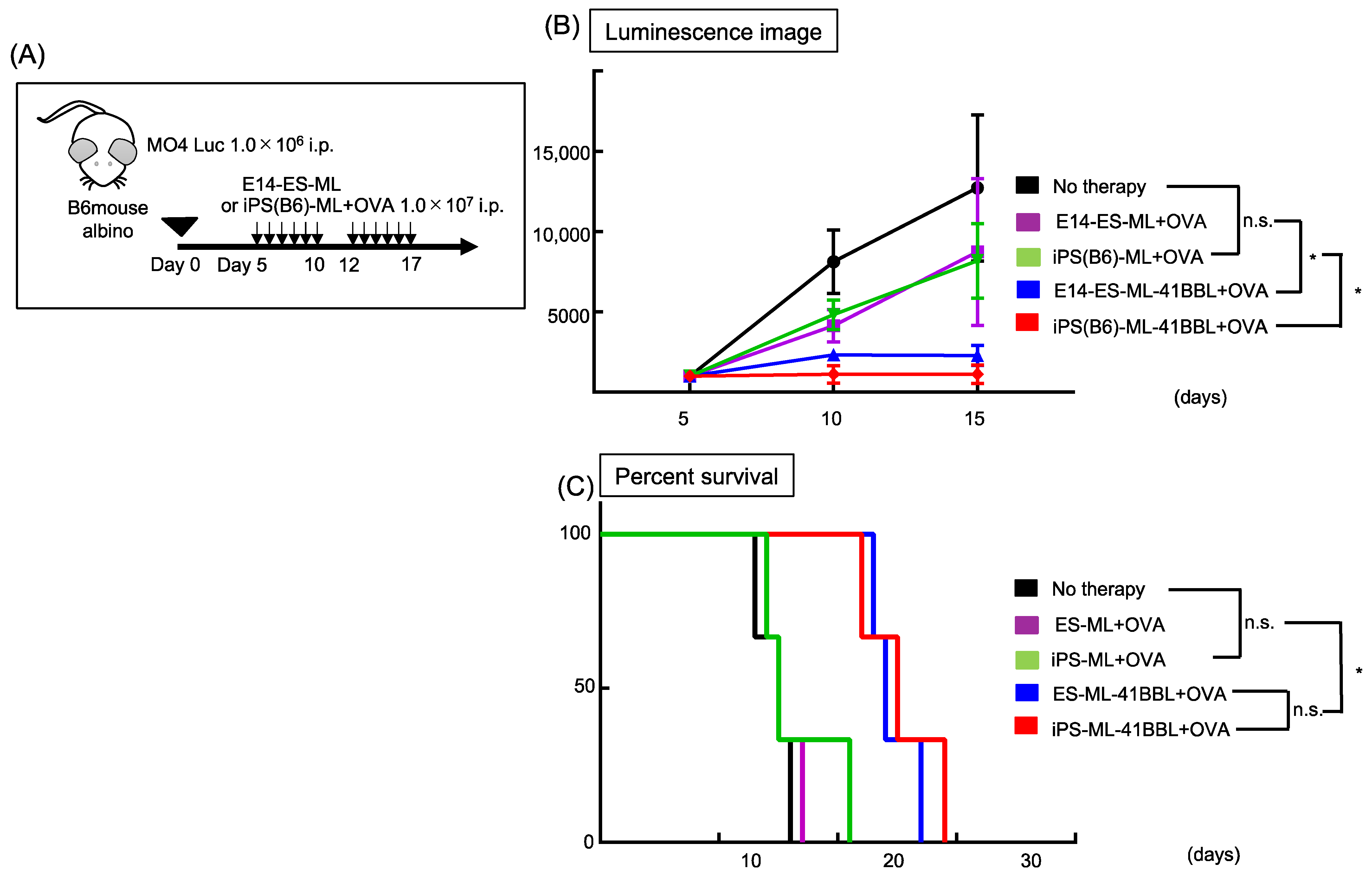
2.3. iPS-ML-41BBL Inhibits Peritoneal Dissemination of Mouse Melanoma
2.4. iPS-ML-41BBL Induce Changes in TILs
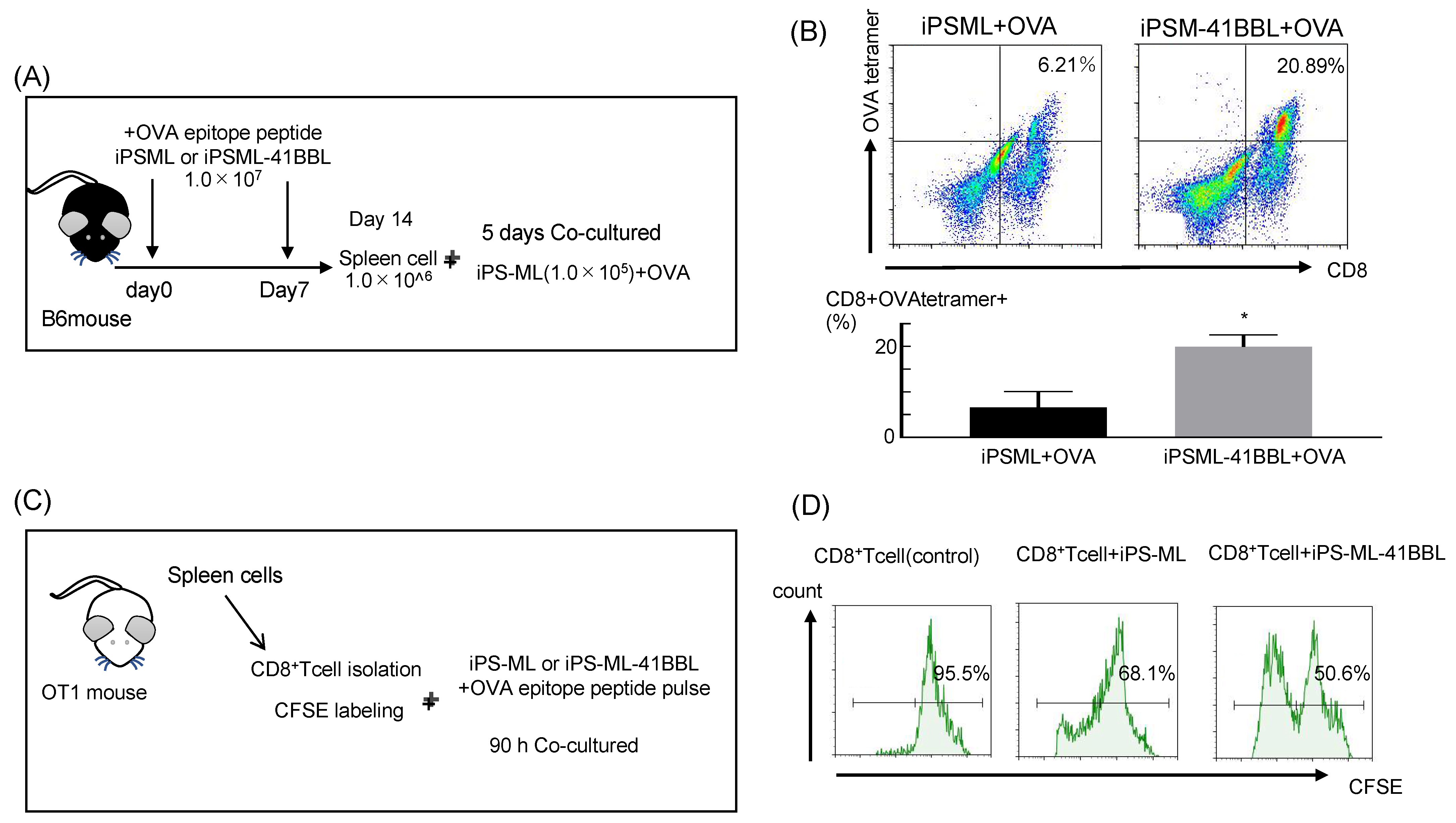
2.5. In Vivo Priming of iPS-ML-41BBL Therapy
2.6. Antigen Presenting Ability of the iPS-ML-41BBL
2.7. Infiltration of iPS-ML in Established Tumor Tissue
2.8. ES-ML (129)-41BBL Suppresses the Peritoneal Dissemination of MO4 Melanoma
2.9. Autoimmune Reactions after iPS-ML-41BBL Therapy
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Conditions
4.2. Mice
4.3. Flow Cytometry (FCM)
4.4. Generation of Genetically Modified 4-1BBL-Expressing iPS-ML (iPS-ML-41BBL)
4.5. Cytokine Production, ELISA, and Cytokine Array of Cocultured iPS-ML and the Mouse Spleen Cells
4.6. In Vivo Antitumor Activity of iPS-ML against MO4
4.7. OVA Tetramer Assay
4.8. Functional Analysis of TILs
4.9. Immunofluorescence Staining
4.10. PKH67 Labeling
4.11. Antigen-Presentation Assays
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Phan, G.Q.; Rosenberg, S.A. Adoptive cell transfer for patients with metastatic melanoma: The potential and promise of cancer immunotherapy. Cancer Control 2013, 20, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Tashiro, H.; Tsend-Ayush, G.; Haruta, M.; Dashdemberel, N.; Komohara, Y.; Tsuboki, J.; Takaishi, K.; Ohba, T.; Nishimura, Y.; et al. Novel therapeutic strategies for advanced ovarian cancer by using induced pluripotent stem cell-derived myelomonocytic cells producing interferon beta. Cancer Sci. 2018, 109, 3403–3410. [Google Scholar] [CrossRef]
- Miyashita, A.; Fukushima, S.; Nakahara, S.; Kubo, Y.; Tokuzumi, A.; Yamashita, J.; Aoi, J.; Haruta, M.; Senju, S.; Nishimura, Y.; et al. Immunotherapy against Metastatic Melanoma with Human iPS Cell-Derived Myeloid Cell Lines Producing Type I Interferons. Cancer Immunol. Res. 2016, 4, 248–258. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Zhang, R.; Iwama, T.; Ueda, N.; Liu, T.; Tatsumi, M.; Sasaki, Y.; Shimoda, R.; Osako, Y.; Sawada, Y.; et al. Type I Interferon Delivery by iPSC-Derived Myeloid Cells Elicits Antitumor Immunity via XCR1. Cell Rep. 2019, 29, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Tomita, Y.; Yuno, A.; Matsumura, K.; Ikeda, T.; Takamatsu, K.; Haga, E.; Koba, C.; Nishimura, Y.; Senju, S. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther. 2013, 20, 504–513. [Google Scholar] [CrossRef]
- Alderson, M.R.; Smith, C.A.; Tough, T.W.; Davis-Smith, T.; Armitage, R.J.; Falk, B.; Roux, E.; Baker, E.; Sutherland, G.R.; Din, W.S. Molecular and biological characterization of human 4-1BB and its ligand. Eur. J. Immunol. 1994, 24, 2219–2227. [Google Scholar] [CrossRef]
- Langstein, J.; Michel, J.; Fritsche, J.; Kreutz, M.; Andreesen, R.; Schwarz, H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J. Immunol. 1998, 160, 2488–2494. [Google Scholar] [PubMed]
- Goodwin, R.G.; Din, W.S.; Davis-Smith, T.; Anderson, D.M.; Gimpel, S.D.; Sato, T.A.; Maliszewski, C.R.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: A member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 1993, 23, 2631–2641. [Google Scholar] [CrossRef]
- Hurtado, J.C.; Kim, Y.J.; Kwon, B.S. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997, 158, 2600–2609. [Google Scholar] [PubMed]
- Shuford, W.W.; Klussman, K.; Tritchler, D.D.; Loo, D.T.; Chalupny, J.; Siadak, A.W.; Brown, T.J.; Emswiler, J.; Raecho, H.; Larsen, C.P.; et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997, 186, 47–55. [Google Scholar] [CrossRef]
- Sakellariou-Thompson, D.; Forget, M.A.; Creasy, C.; Bernard, V.; Zhao, L.; Kim, Y.U.; Hurd, M.W.; Uraoka, N.; Parra, E.R.; Kang, Y.; et al. 4-1BB Agonist Focuses CD8. Clin. Cancer Res. 2017, 23, 7263–7275. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Shuford, W.W.; Newby, S.A.; Aruffo, A.; Ledbetter, J.A.; Hellström, K.E.; Mittler, R.S.; Chen, L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997, 3, 682–685. [Google Scholar] [CrossRef]
- Houot, R.; Goldstein, M.J.; Kohrt, H.E.; Myklebust, J.H.; Alizadeh, A.A.; Lin, J.T.; Irish, J.M.; Torchia, J.A.; Kolstad, A.; Chen, L.; et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood 2009, 114, 3431–3438. [Google Scholar] [CrossRef]
- Curran, M.A.; Kim, M.; Montalvo, W.; Al-Shamkhani, A.; Allison, J.P. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS ONE 2011, 6, e19499. [Google Scholar] [CrossRef] [PubMed]
- Belcaid, Z.; Phallen, J.A.; Zeng, J.; See, A.P.; Mathios, D.; Gottschalk, C.; Nicholas, S.; Kellett, M.; Ruzevick, J.; Jackson, C.; et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE 2014, 9, e101764. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, E.; Etxeberria, I.; Rodriguez-Ruiz, M.E.; Melero, I. Anti-CD137 and PD-1/PD-L1 Antibodies En Route toward Clinical Synergy. Clin. Cancer Res. 2017, 23, 5326–5328. [Google Scholar] [CrossRef] [PubMed]
- Tau, G.Z.; Cowan, S.N.; Weisburg, J.; Braunstein, N.S.; Rothman, P.B. Regulation of IFN-gamma signaling is essential for the cytotoxic activity of CD8(+) T cells. J. Immunol. 2001, 167, 5574–5582. [Google Scholar] [CrossRef]
- Du, R.; Petritsch, C.; Lu, K.; Liu, P.; Haller, A.; Ganss, R.; Song, H.; Vandenberg, S.; Bergers, G. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008, 10, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Umehara, H.; Nakayama, T.; Yoneda, O.; Hieshima, K.; Kakizaki, M.; Dohmae, N.; Yoshie, O.; Imai, T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J. Immunol. 2002, 168, 6173–6180. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.R.; Barnden, M.; Kurts, C.; Carbone, F.R.; Miller, J.F.; Heath, W.R. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 2000, 78, 110–117. [Google Scholar] [CrossRef]
- Mendes, O.; Kim, H.T.; Stoica, G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin. Exp. Metastasis 2005, 22, 237–246. [Google Scholar] [CrossRef]
- Nishida, M.; Okumura, Y.; Ozawa, S.; Shiraishi, I.; Itoi, T.; Hamaoka, K. MMP-2 inhibition reduces renal macrophage infiltration with increased fibrosis in UUO. Biochem. Biophys. Res. Commun. 2007, 354, 133–139. [Google Scholar] [CrossRef]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012, 4, 127ra137. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Sułkowski, M.; Konieczny, P.; Chlebanowska, P.; Majka, M. Introduction of Exogenous HSV-TK Suicide Gene Increases Safety of Keratinocyte-Derived Induced Pluripotent Stem Cells by Providing Genetic “Emergency Exit” Switch. Int. J. Mol. Sci. 2018, 19, 197. [Google Scholar] [CrossRef]
- Haga, E.; Endo, Y.; Haruta, M.; Koba, C.; Matsumura, K.; Takamatsu, K.; Ikeda, T.; Nishimura, Y.; Senju, S. Therapy of peritoneally disseminated colon cancer by TAP-deficient embryonic stem cell-derived macrophages in allogeneic recipients. J. Immunol. 2014, 193, 2024–2033. [Google Scholar] [CrossRef]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, T.Y.; Senju, S.; Haruta, M.; Hirosawa, N.; Suzuki, M.; Tatsumi, M.; Ueda, N.; Maki, H.; Nakatsuka, R.; et al. Generation of mouse pluripotent stem cell-derived proliferating myeloid cells as an unlimited source of functional antigen-presenting cells. Cancer Immunol. Res. 2015, 3, 668–677. [Google Scholar] [CrossRef]
- Hogquist, K.A.; Jameson, S.C.; Heath, W.R.; Howard, J.L.; Bevan, M.J.; Carbone, F.R. Pillars article: T cell receptor antagonist peptides induce positive selection. Cell 1994, 76, 17–27. J. Immunol. 2012, 188, 2046–2056. [Google Scholar]
- Thevenot, P.T.; Sierra, R.A.; Raber, P.L.; Al-Khami, A.A.; Trillo-Tinoco, J.; Zarreii, P.; Ochoa, A.C.; Cui, Y.; Del Valle, L.; Rodriguez, P.C. The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 2014, 41, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, H.; Yamane, F.; Fukushima, K.; Matsuki, T.; Kawasaki, T.; Ebina, I.; Kuniyoshi, K.; Tanaka, H.; Maruyama, K.; Maeda, K.; et al. Antitumor effect of Batf2 through IL-12 p40 up-regulation in tumor-associated macrophages. Proc. Natl. Acad. Sci. USA 2017, 114, E7331–E7340. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuriyama, H.; Fukushima, S.; Kimura, T.; Kanemaru, H.; Miyashita, A.; Okada, E.; Kubo, Y.; Nakahara, S.; Tokuzumi, A.; Nishimura, Y.; et al. Immunotherapy with 4-1BBL-Expressing iPS Cell‐Derived Myeloid Lines Amplifies Antigen-Specific T Cell Infiltration in Advanced Melanoma. Int. J. Mol. Sci. 2021, 22, 1958. https://doi.org/10.3390/ijms22041958
Kuriyama H, Fukushima S, Kimura T, Kanemaru H, Miyashita A, Okada E, Kubo Y, Nakahara S, Tokuzumi A, Nishimura Y, et al. Immunotherapy with 4-1BBL-Expressing iPS Cell‐Derived Myeloid Lines Amplifies Antigen-Specific T Cell Infiltration in Advanced Melanoma. International Journal of Molecular Sciences. 2021; 22(4):1958. https://doi.org/10.3390/ijms22041958
Chicago/Turabian StyleKuriyama, Haruka, Satoshi Fukushima, Toshihiro Kimura, Hisashi Kanemaru, Azusa Miyashita, Etsuko Okada, Yosuke Kubo, Satoshi Nakahara, Aki Tokuzumi, Yuki Nishimura, and et al. 2021. "Immunotherapy with 4-1BBL-Expressing iPS Cell‐Derived Myeloid Lines Amplifies Antigen-Specific T Cell Infiltration in Advanced Melanoma" International Journal of Molecular Sciences 22, no. 4: 1958. https://doi.org/10.3390/ijms22041958
APA StyleKuriyama, H., Fukushima, S., Kimura, T., Kanemaru, H., Miyashita, A., Okada, E., Kubo, Y., Nakahara, S., Tokuzumi, A., Nishimura, Y., Kajihara, I., Makino, K., Aoi, J., Masuguchi, S., Tsukamoto, H., Inozume, T., Zhang, R., Nakatsura, T., Uemura, Y., ... Ihn, H. (2021). Immunotherapy with 4-1BBL-Expressing iPS Cell‐Derived Myeloid Lines Amplifies Antigen-Specific T Cell Infiltration in Advanced Melanoma. International Journal of Molecular Sciences, 22(4), 1958. https://doi.org/10.3390/ijms22041958





