Intestinal Goblet Cell Loss during Chorioamnionitis in Fetal Lambs: Mechanistic Insights and Postnatal Implications
Abstract
1. Introduction
2. Results
2.1. Intestinal Inflammation
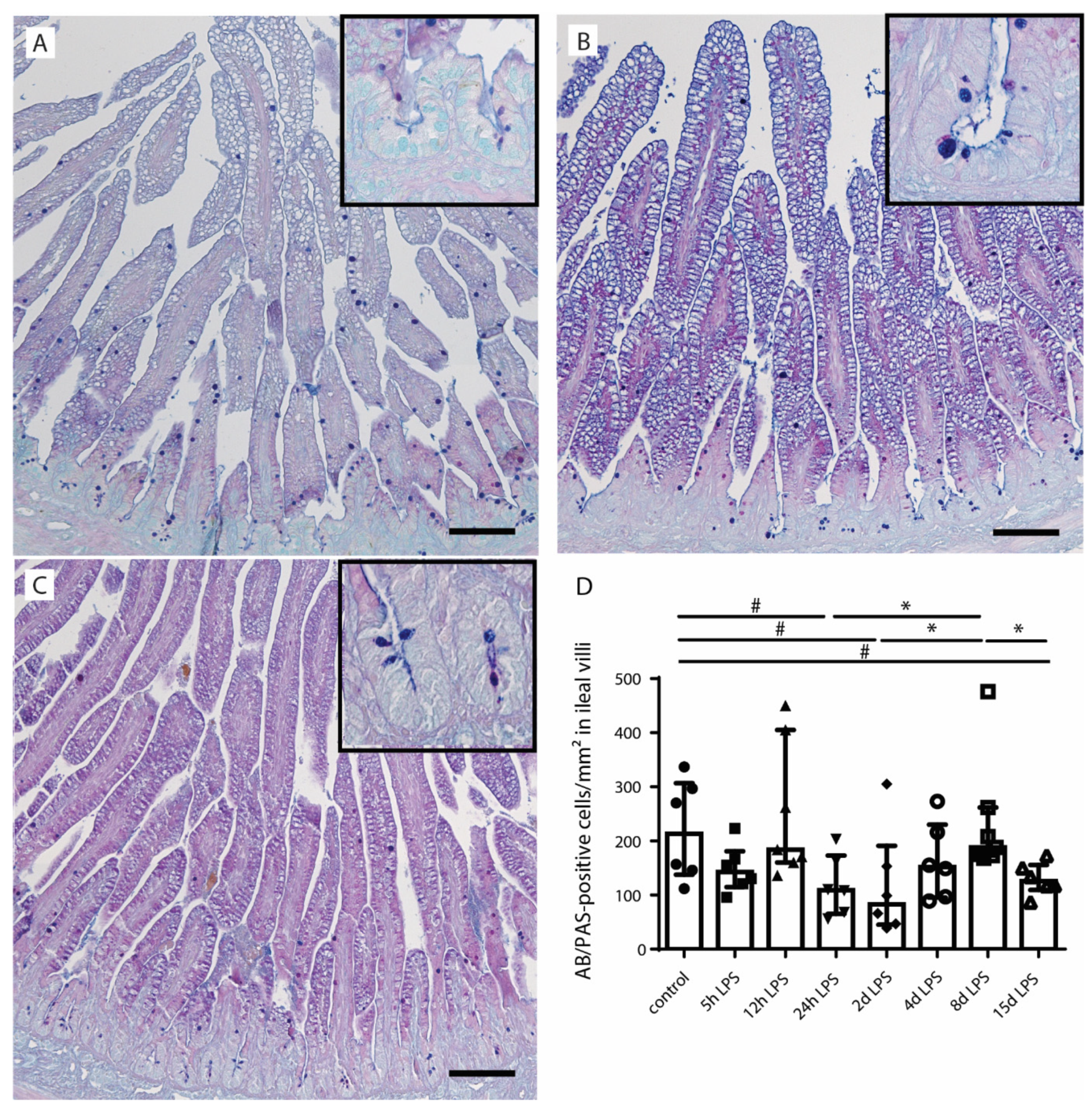
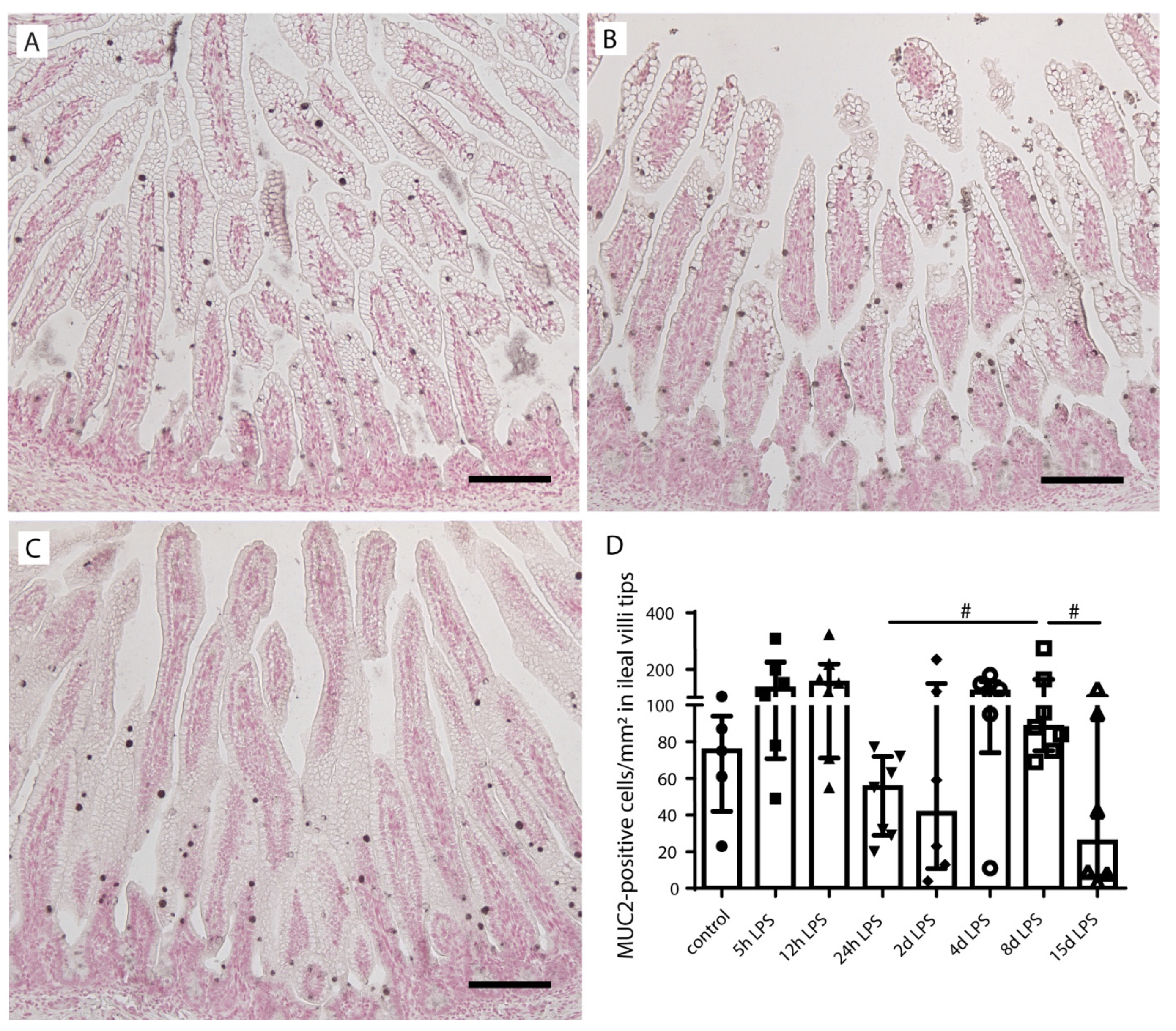
2.2. Goblet Cell Number and Distribution
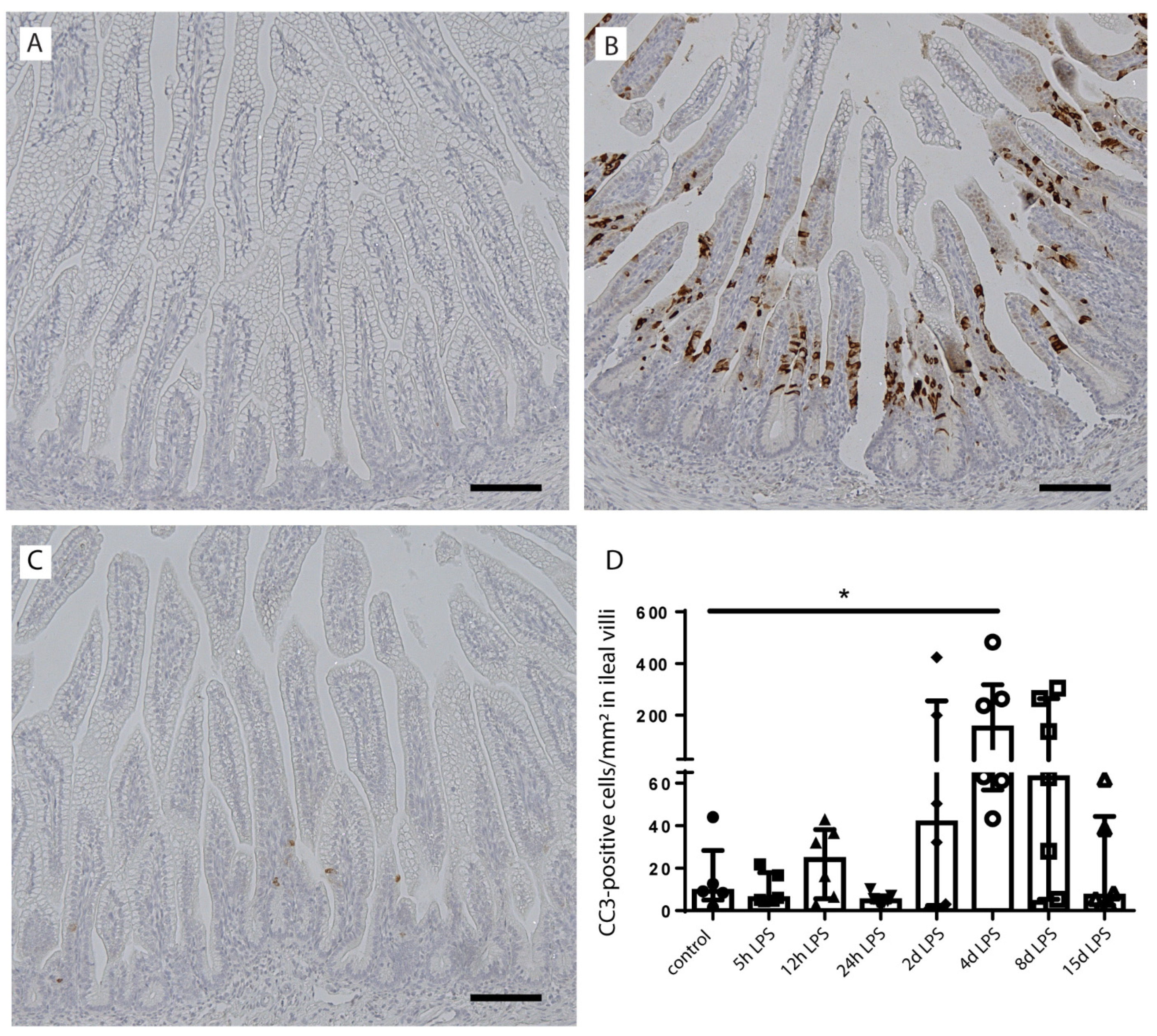
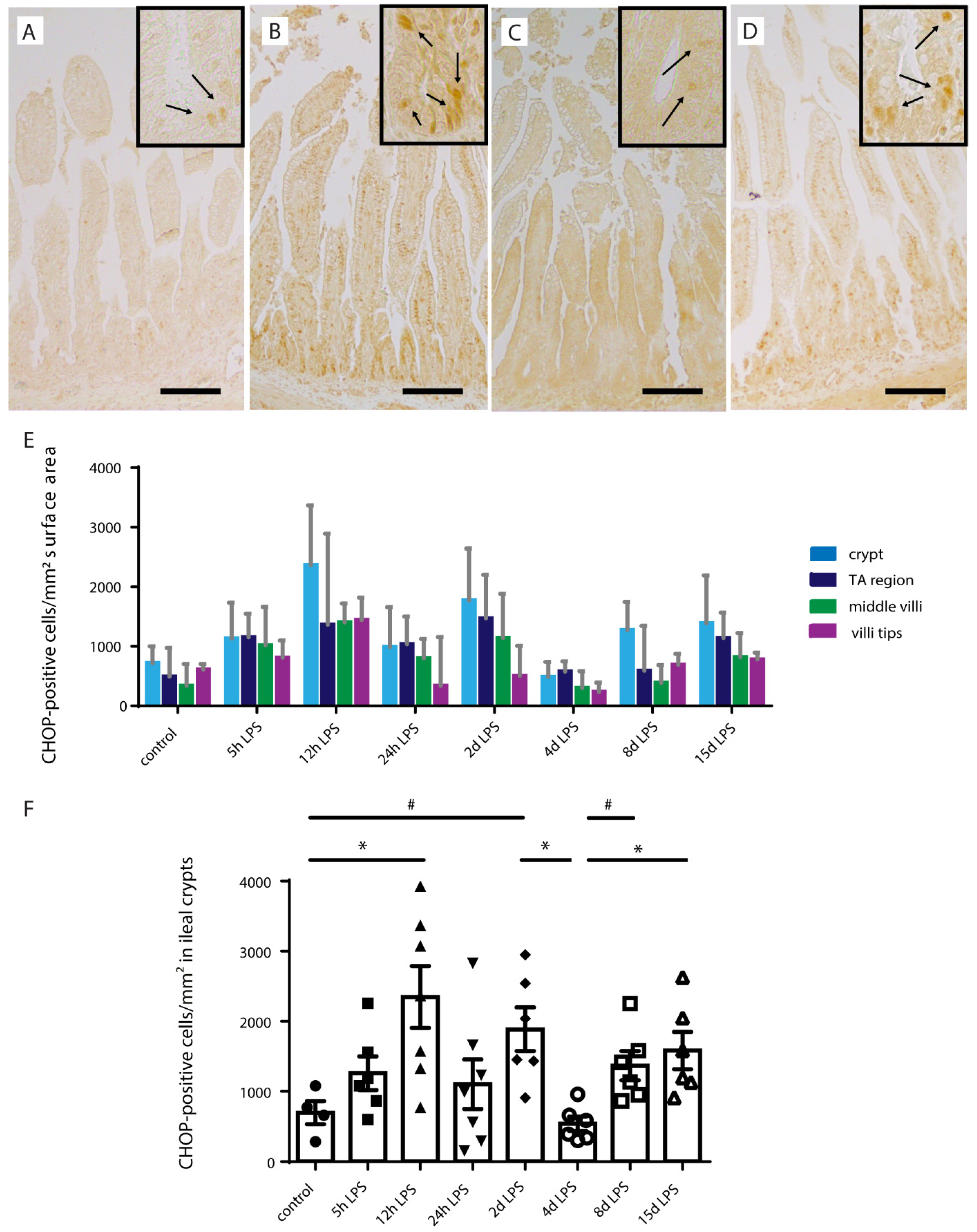
2.3. Intestinal Epithelial Cell Death
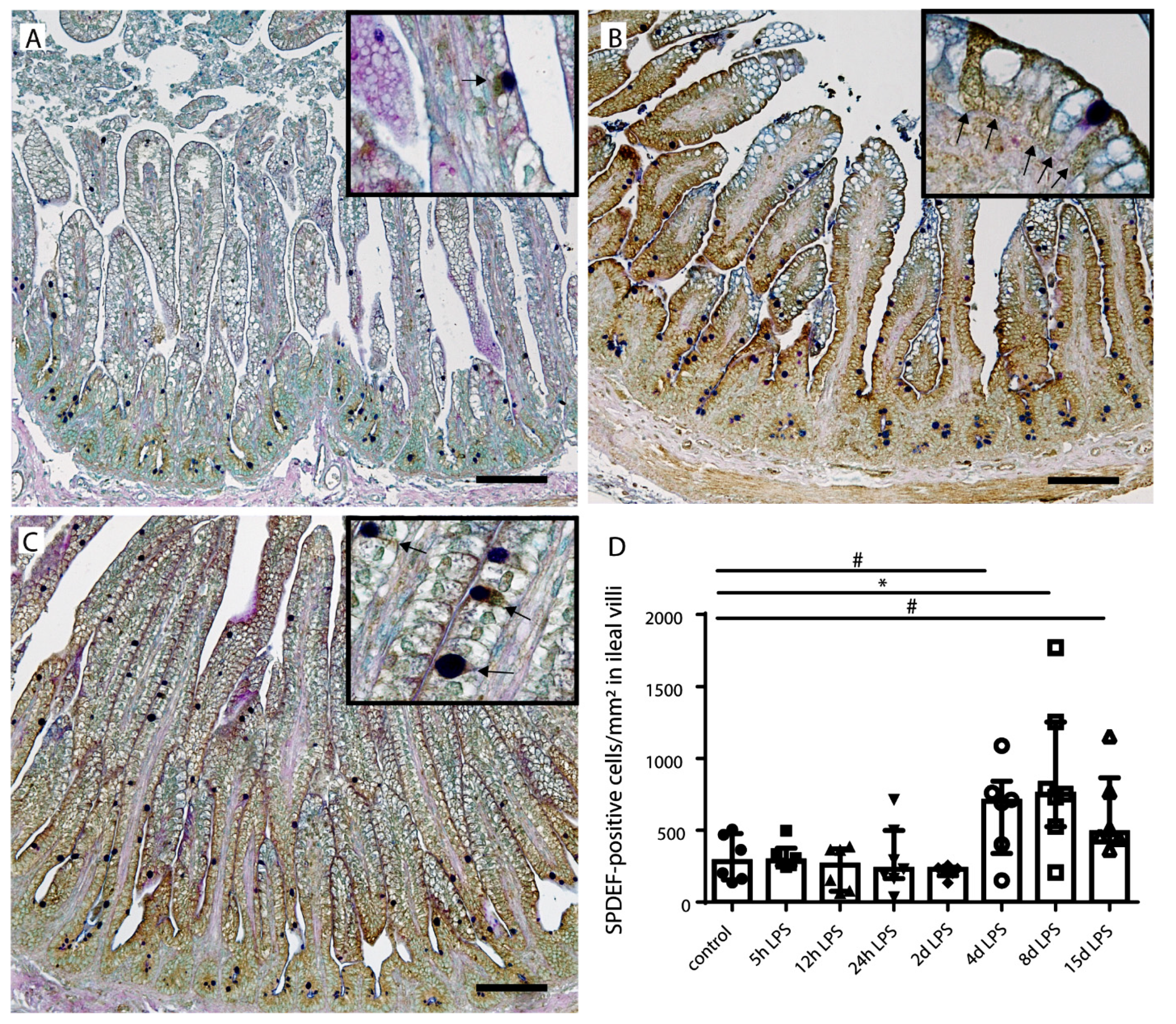
2.4. Goblet Cell Differentiation
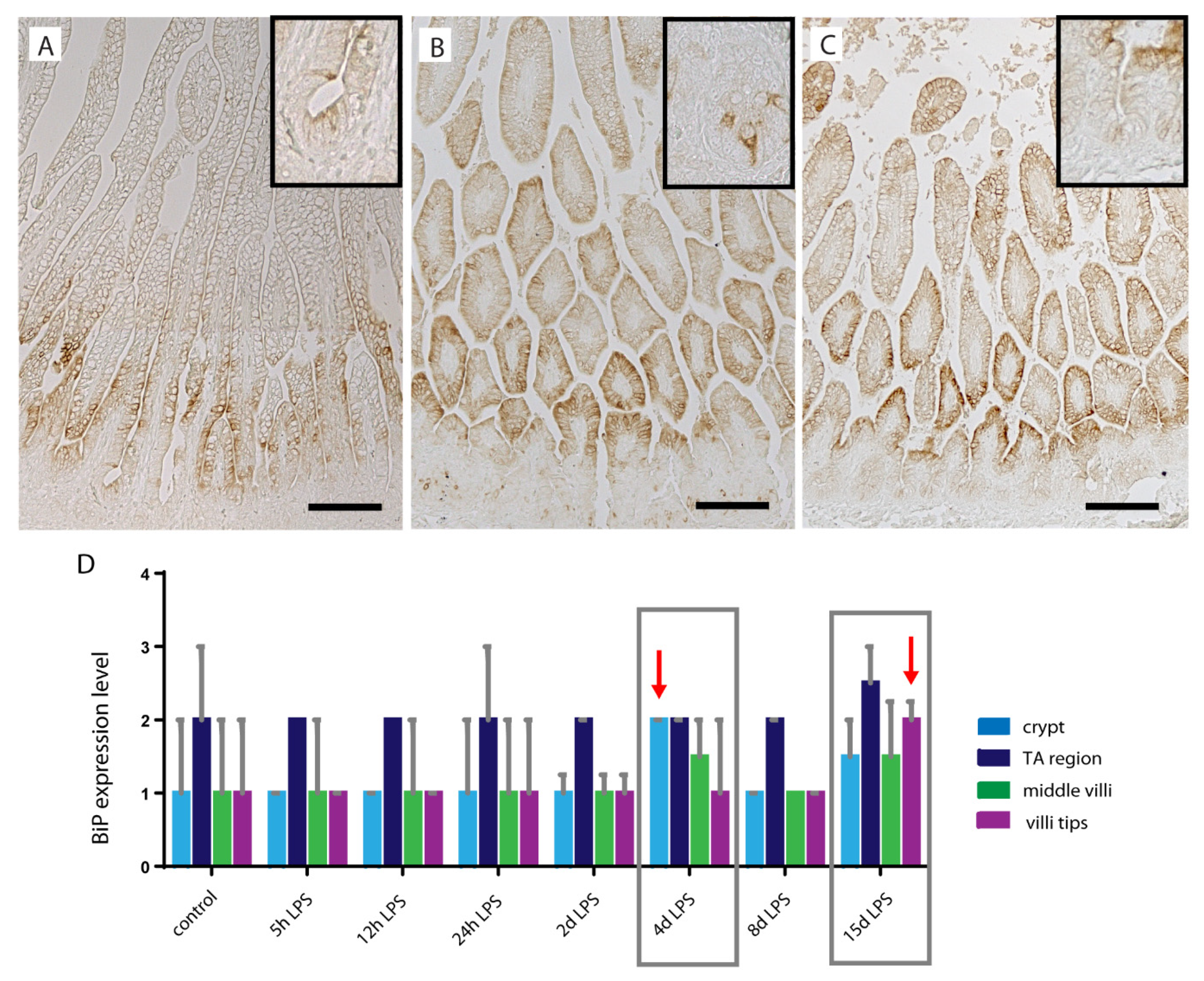
2.5. ER-Stress in Distal Ileum of Premature Lambs
3. Discussion
4. Material and Methods
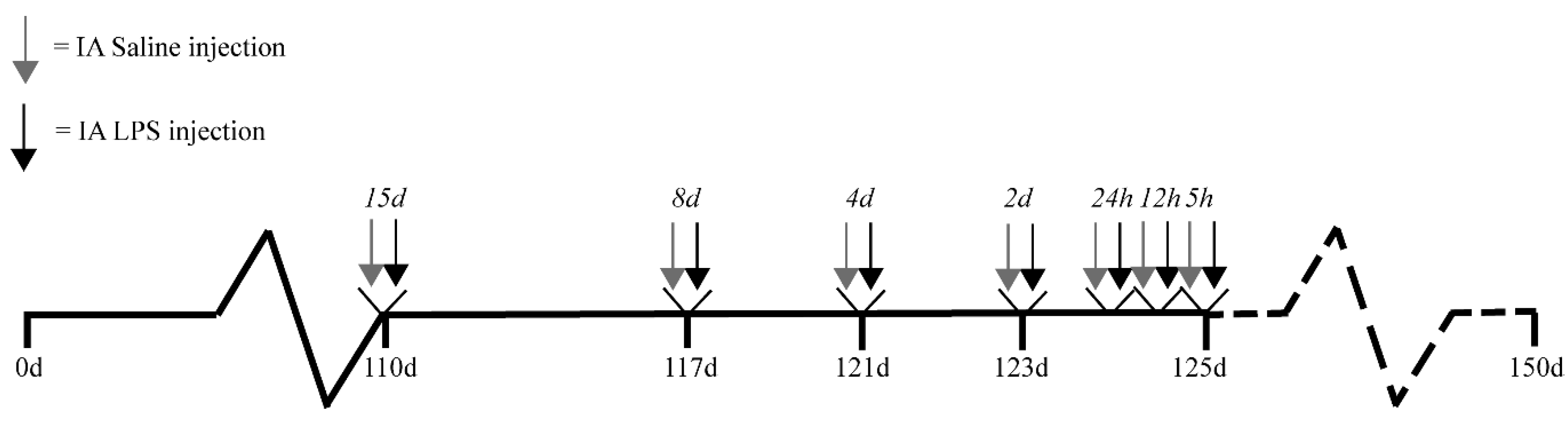
4.1. Experimental Design
4.2. Antibodies
4.3. Alcian Blue/Periodic Acid–Schiff (AB/PAS)
4.4. Immunohistochemistry
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Chaiyasit, N.; Yoon, B.H.; Kim, Y.M. Acute chorioamnionitis and funisitis: Definition, pathologic features, and clinical significance. Am. J. Obs. Gynecol. 2015, 213, S29–S52. [Google Scholar] [CrossRef] [PubMed]
- Gussenhoven, R.; Westerlaken, R.J.J.; Ophelders, D.; Jobe, A.H.; Kemp, M.W.; Kallapur, S.G.; Zimmermann, L.J.; Sangild, P.T.; Pankratova, S.; Gressens, P.; et al. Chorioamnionitis, neuroinflammation, and injury: Timing is key in the preterm ovine fetus. J. Neuroinflammation 2018, 15, 113. [Google Scholar] [CrossRef]
- Gotsch, F.; Romero, R.; Kusanovic, J.P.; Mazaki-Tovi, S.; Pineles, B.L.; Erez, O.; Espinoza, J.; Hassan, S.S. The fetal inflammatory response syndrome. Clin. Obs. Gynecol. 2007, 50, 652–683. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Thymann, T.; Goericke-Pesch, S.K.; Ren, S.; Wei, W.; Skovgaard, K.; Damborg, P.; Brunse, A.; van Gorp, C.; Kramer, B.W.; et al. Prenatal Intra-Amniotic Endotoxin Induces Fetal Gut and Lung Immune Responses and Postnatal Systemic Inflammation in Preterm Pigs. Am. J. Pathol. 2018, 188, 2629–2643. [Google Scholar] [CrossRef]
- Nikiforou, M.; Vanderlocht, J.; Chougnet, C.A.; Jellema, R.K.; Ophelders, D.R.; Joosten, M.; Kloosterboer, N.; Senden-Gijsbers, B.L.; Germeraad, W.T.; Kramer, B.W.; et al. Prophylactic Interleukin-2 Treatment Prevents Fetal Gut Inflammation and Injury in an Ovine Model of Chorioamnionitis. Inflamm. Bowel Dis. 2015, 21, 2026–2038. [Google Scholar] [CrossRef]
- Wolfs, T.G.; Buurman, W.A.; Zoer, B.; Moonen, R.M.; Derikx, J.P.; Thuijls, G.; Villamor, E.; Gantert, M.; Garnier, Y.; Zimmermann, L.J.; et al. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS ONE 2009, 4, e5837. [Google Scholar] [CrossRef]
- Elgin, T.G.; Fricke, E.M.; Gong, H.; Reese, J.; Mills, D.A.; Kalantera, K.M.; Underwood, M.A.; McElroy, S.J. Fetal exposure to maternal inflammation interrupts murine intestinal development and increases susceptibility to neonatal intestinal injury. Dis. Models Mech. 2019, 12, dmm040808. [Google Scholar] [CrossRef] [PubMed]
- van Gorp, C.; de Lange, I.H.; Spiller, O.B.; Dewez, F.; Cillero Pastor, B.; Heeren, R.M.A.; Kessels, L.; Kloosterboer, N.; van Gemert, W.G.; Beeton, M.L.; et al. Protection of the Ovine Fetal Gut against Ureaplasma-Induced Chorioamnionitis: A Potential Role for Plant Sterols. Nutrients 2019, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Heymans, C.; de Lange, I.H.; Hütten, M.C.; Lenaerts, K.; de Ruijter, N.J.E.; Kessels, L.; Rademakers, G.; Melotte, V.; Boesmans, W.; Saito, M.; et al. Chronic Intra-Uterine Ureaplasma parvum Infection Induces Injury of the Enteric Nervous System in Ovine Fetuses. Front. Immunol. 2020, 11, 189. [Google Scholar] [CrossRef]
- Been, J.V.; Lievense, S.; Zimmermann, L.J.; Kramer, B.W.; Wolfs, T.G. Chorioamnionitis as a risk factor for necrotizing enterocolitis: A systematic review and meta-analysis. J. Pediatr. 2013, 162, 236–242.e2. [Google Scholar] [CrossRef] [PubMed]
- Gantert, M.; Been, J.V.; Gavilanes, A.W.; Garnier, Y.; Zimmermann, L.J.; Kramer, B.W. Chorioamnionitis: A multiorgan disease of the fetus? J. Perinatol. 2010, 30, S21–S30. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.J.; Eaton, S.; Pierro, A. Royal Australasia of Surgeons Guest Lecture. Necrotizing enterocolitis: Prevention, treatment, and outcome. J. Pediatr. Surg. 2013, 48, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Sjovall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergstrom, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schutte, A.; van der Post, S.; Svensson, F.; Rodriguez-Pineiro, A.M.; Nystrom, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Schaart, M.W.; de Bruijn, A.C.; Bouwman, D.M.; de Krijger, R.R.; van Goudoever, J.B.; Tibboel, D.; Renes, I.B. Epithelial functions of the residual bowel after surgery for necrotising enterocolitis in human infants. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 31–41. [Google Scholar] [CrossRef]
- McElroy, S.J.; Prince, L.S.; Weitkamp, J.H.; Reese, J.; Slaughter, J.C.; Polk, D.B. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: A potential role in neonatal necrotizing enterocolitis. Am. J. Physiol. Gastrointest Liver Physiol. 2011, 301, G656–G666. [Google Scholar] [CrossRef]
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Sato, T.; Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef]
- Lu, P.; Struijs, M.-C.; Mei, J.; Witte-Bouma, J.; Korteland-van Male, A.M.; de Bruijn, A.C.J.M.; van Goudoever, J.B.; Renes, I.B. Endoplasmic reticulum stress, unfolded protein response and altered T cell differentiation in necrotizing enterocolitis. PLoS ONE 2013, 8, e78491. [Google Scholar] [CrossRef]
- Afrazi, A.; Branca, M.F.; Sodhi, C.P.; Good, M.; Yamaguchi, Y.; Egan, C.E.; Lu, P.; Jia, H.; Shaffiey, S.; Lin, J.; et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J. Biol. Chem. 2014, 289, 9584–9599. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Eri, R.D.; Das, I.; Lourie, R.; Florin, T.H. ER stress and the unfolded protein response in intestinal inflammation. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G820–G832. [Google Scholar] [CrossRef]
- Li, B.; Zani, A.; Lee, C.; Zani-Ruttenstock, E.; Zhang, Z.; Li, X.; Ip, W.; Gonska, T.; Pierro, A. Endoplasmic reticulum stress is involved in the colonic epithelium damage induced by maternal separation. J. Pediatr. Surg. 2016, 51, 1001–1004. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Günther, C.; Neumann, H.; Neurath, M.F.; Becker, C. Apoptosis, necrosis and necroptosis: Cell death regulation in the intestinal epithelium. Gut 2013, 62, 1062–1071. [Google Scholar] [CrossRef]
- Heymans, C.; de Lange, I.H.; Lenaerts, K.; Kessels, L.; Hadfoune, M.; Rademakers, G.; Melotte, V.; Boesmans, W.; Kramer, B.W.; Jobe, A.H.; et al. Chorioamnionitis induces enteric nervous system injury: Effects of timing and inflammation in the ovine fetus. Mol. Med. 2020, 26, 82. [Google Scholar] [CrossRef]
- Gregorieff, A.; Stange, D.E.; Kujala, P.; Begthel, H.; van den Born, M.; Korving, J.; Peters, P.J.; Clevers, H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 2009, 137, 1333–1345. [Google Scholar] [CrossRef]
- Chen, G.; Sun, L.; Kato, T.; Okuda, K.; Martino, M.B.; Abzhanova, A.; Lin, J.M.; Gilmore, R.C.; Batson, B.D.; O’Neal, Y.K.; et al. IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J. Clin. Investig. 2019, 129, 4433–4450. [Google Scholar] [CrossRef]
- Negroni, A.; Cucchiara, S.; Stronati, L. Apoptosis, Necrosis, and Necroptosis in the Gut and Intestinal Homeostasis. Mediators Inflamm. 2015, 2015, 250762. [Google Scholar] [CrossRef]
- Asada, R.; Saito, A.; Kawasaki, N.; Kanemoto, S.; Iwamoto, H.; Oki, M.; Miyagi, H.; Izumi, S.; Imaizumi, K. The endoplasmic reticulum stress transducer OASIS is involved in the terminal differentiation of goblet cells in the large intestine. J. Biol. Chem. 2012, 287, 8144–8153. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, J.; van Lidth de Jeude, J.F.; Koo, B.-K.; Rosekrans, S.L.; Wielenga, M.C.B.; van de Wetering, M.; Ferrante, M.; Lee, A.S.; Onderwater, J.J.M.; Paton, J.C.; et al. ER Stress Causes Rapid Loss of Intestinal Epithelial Stemness through Activation of the Unfolded Protein Response. Cell Rep. 2013, 3, 1128–1139. [Google Scholar] [CrossRef]
- Clevers, H.C.; Bevins, C.L. Paneth cells: Maestros of the small intestinal crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Bose, C.L.; Laughon, M.M.; Allred, E.N.; O’Shea, T.M.; Van Marter, L.J.; Ehrenkranz, R.A.; Fichorova, R.N.; Leviton, A. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine 2013, 61, 315–322. [Google Scholar] [CrossRef]
- Machado, J.R.; Soave, D.F.; da Silva, M.V.; de Menezes, L.B.; Etchebehere, R.M.; Monteiro, M.L.; dos Reis, M.A.; Correa, R.R.; Celes, M.R. Neonatal sepsis and inflammatory mediators. Mediators Inflamm. 2014, 2014, 269681. [Google Scholar] [CrossRef]
- Dvorak, B.; Khailova, L.; Clark, J.A.; Hosseini, D.M.; Arganbright, K.M.; Reynolds, C.A.; Halpern, M.D. Comparison of epidermal growth factor and heparin-binding epidermal growth factor-like growth factor for prevention of experimental necrotizing enterocolitis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 11–18. [Google Scholar] [CrossRef]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef]
- Tian, F.; Liu, G.R.; Li, N.; Yuan, G. Insulin-like growth factor I reduces the occurrence of necrotizing enterocolitis by reducing inflammatory response and protecting intestinal mucosal barrier in neonatal rats model. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4711–4719. [Google Scholar]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef]
- Miyake, H.; Lee, C.; Chusilp, S.; Bhalla, M.; Li, B.; Pitino, M.; Seo, S.; O’Connor, D.L.; Pierro, A. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int. 2020, 36, 155–163. [Google Scholar] [CrossRef]
- Wu, R.Y.; Li, B.; Koike, Y.; Maattanen, P.; Miyake, H.; Cadete, M.; Johnson-Henry, K.C.; Botts, S.R.; Lee, C.; Abrahamsson, T.R.; et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol. Nutr. Food Res. 2019, 63, e1800658. [Google Scholar] [CrossRef]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D.; et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, E.; Wolfs, T.G.; Collins, J.J.; Jellema, R.K.; Newnham, J.P.; Kemp, M.W.; Kallapur, S.G.; Jobe, A.H.; Kramer, B.W. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod. Sci. 2013, 20, 946–956. [Google Scholar] [CrossRef]
- Newnham, J.P.; Kallapur, S.G.; Kramer, B.W.; Moss, T.J.; Nitsos, I.; Ikegami, M.; Jobe, A.H. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am. J. Obstet. Gynecol. 2003, 189, 1458–1466. [Google Scholar] [CrossRef]
- Yamabayashi, S. Periodic acid-Schiff-alcian blue: A method for the differential staining of glycoproteins. Histochem. J. 1987, 19, 565–571. [Google Scholar] [CrossRef]
- Hurd, E.A.; Holmén, J.M.; Hansson, G.C.; Domino, S.E. Gastrointestinal mucins of Fut2-null mice lack terminal fucosylation without affecting colonization by Candida albicans. Glycobiology 2005, 15, 1002–1007. [Google Scholar] [CrossRef]
- Chen, G.; Korfhagen, T.R.; Xu, Y.; Kitzmiller, J.; Wert, S.E.; Maeda, Y.; Gregorieff, A.; Clevers, H.; Whitsett, J.A. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Investig. 2009, 119, 2914–2924. [Google Scholar] [CrossRef]
- Noah, T.K.; Kazanjian, A.; Whitsett, J.; Shroyer, N.F. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp. Cell Res. 2010, 316, 452–465. [Google Scholar] [CrossRef]
- Willems, M.G.; Ophelders, D.R.; Nikiforou, M.; Jellema, R.K.; Butz, A.; Delhaas, T.; Kramer, B.W.; Wolfs, T.G. Systemic interleukin-2 administration improves lung function and modulates chorioamnionitis-induced pulmonary inflammation in the ovine fetus. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L1–L7. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gorp, C.; de Lange, I.H.; Massy, K.R.I.; Kessels, L.; Jobe, A.H.; Cleutjens, J.P.M.; Kemp, M.W.; Saito, M.; Usada, H.; Newnham, J.; et al. Intestinal Goblet Cell Loss during Chorioamnionitis in Fetal Lambs: Mechanistic Insights and Postnatal Implications. Int. J. Mol. Sci. 2021, 22, 1946. https://doi.org/10.3390/ijms22041946
van Gorp C, de Lange IH, Massy KRI, Kessels L, Jobe AH, Cleutjens JPM, Kemp MW, Saito M, Usada H, Newnham J, et al. Intestinal Goblet Cell Loss during Chorioamnionitis in Fetal Lambs: Mechanistic Insights and Postnatal Implications. International Journal of Molecular Sciences. 2021; 22(4):1946. https://doi.org/10.3390/ijms22041946
Chicago/Turabian Stylevan Gorp, Charlotte, Ilse H. de Lange, Kimberly R. I. Massy, Lilian Kessels, Alan H. Jobe, Jack P. M. Cleutjens, Matthew W. Kemp, Masatoshi Saito, Haruo Usada, John Newnham, and et al. 2021. "Intestinal Goblet Cell Loss during Chorioamnionitis in Fetal Lambs: Mechanistic Insights and Postnatal Implications" International Journal of Molecular Sciences 22, no. 4: 1946. https://doi.org/10.3390/ijms22041946
APA Stylevan Gorp, C., de Lange, I. H., Massy, K. R. I., Kessels, L., Jobe, A. H., Cleutjens, J. P. M., Kemp, M. W., Saito, M., Usada, H., Newnham, J., Hütten, M., Kramer, B. W., Zimmermann, L. J., & Wolfs, T. G. A. M. (2021). Intestinal Goblet Cell Loss during Chorioamnionitis in Fetal Lambs: Mechanistic Insights and Postnatal Implications. International Journal of Molecular Sciences, 22(4), 1946. https://doi.org/10.3390/ijms22041946






