Early Enriched Environment Prevents Epigenetic p11 Gene Changes Induced by Adulthood Stress in Mice
Abstract
1. Introduction
2. Results
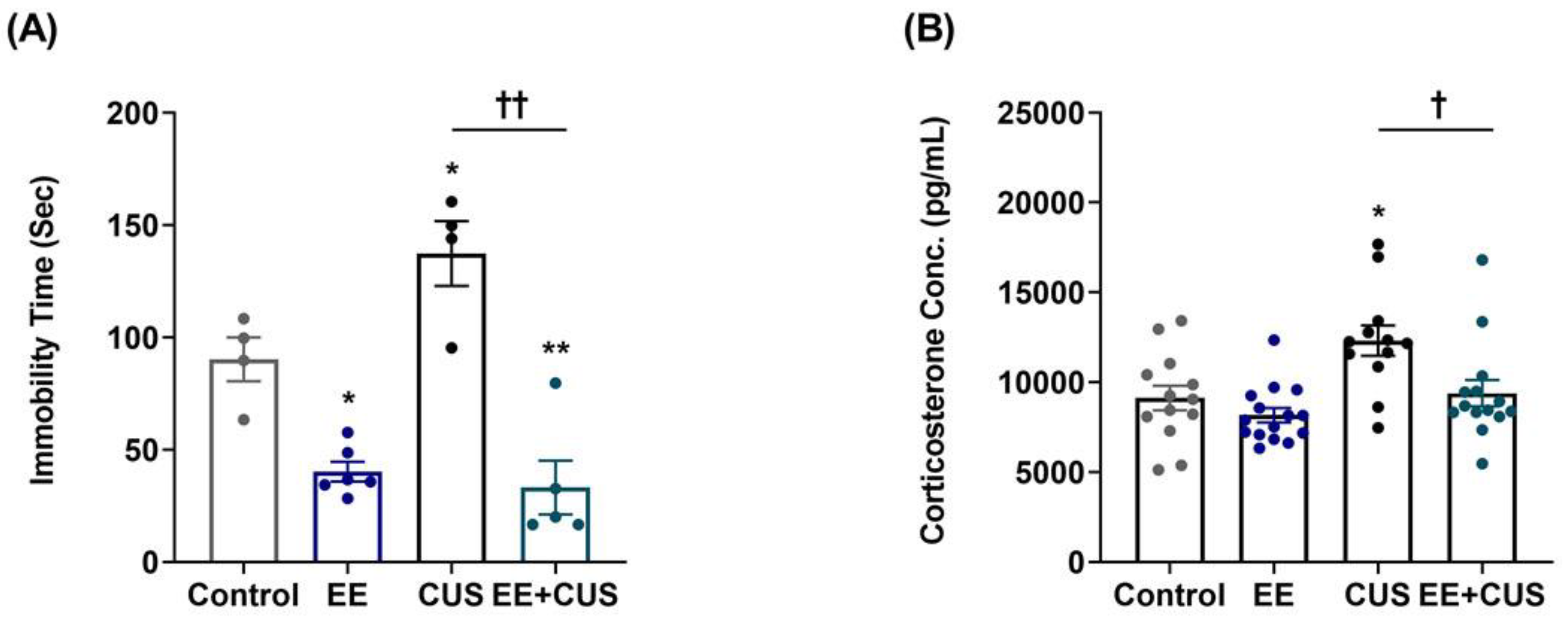
2.1. Effects of EE and CUS on Depression-Like Behavior and Plasma Corticosterone Levels
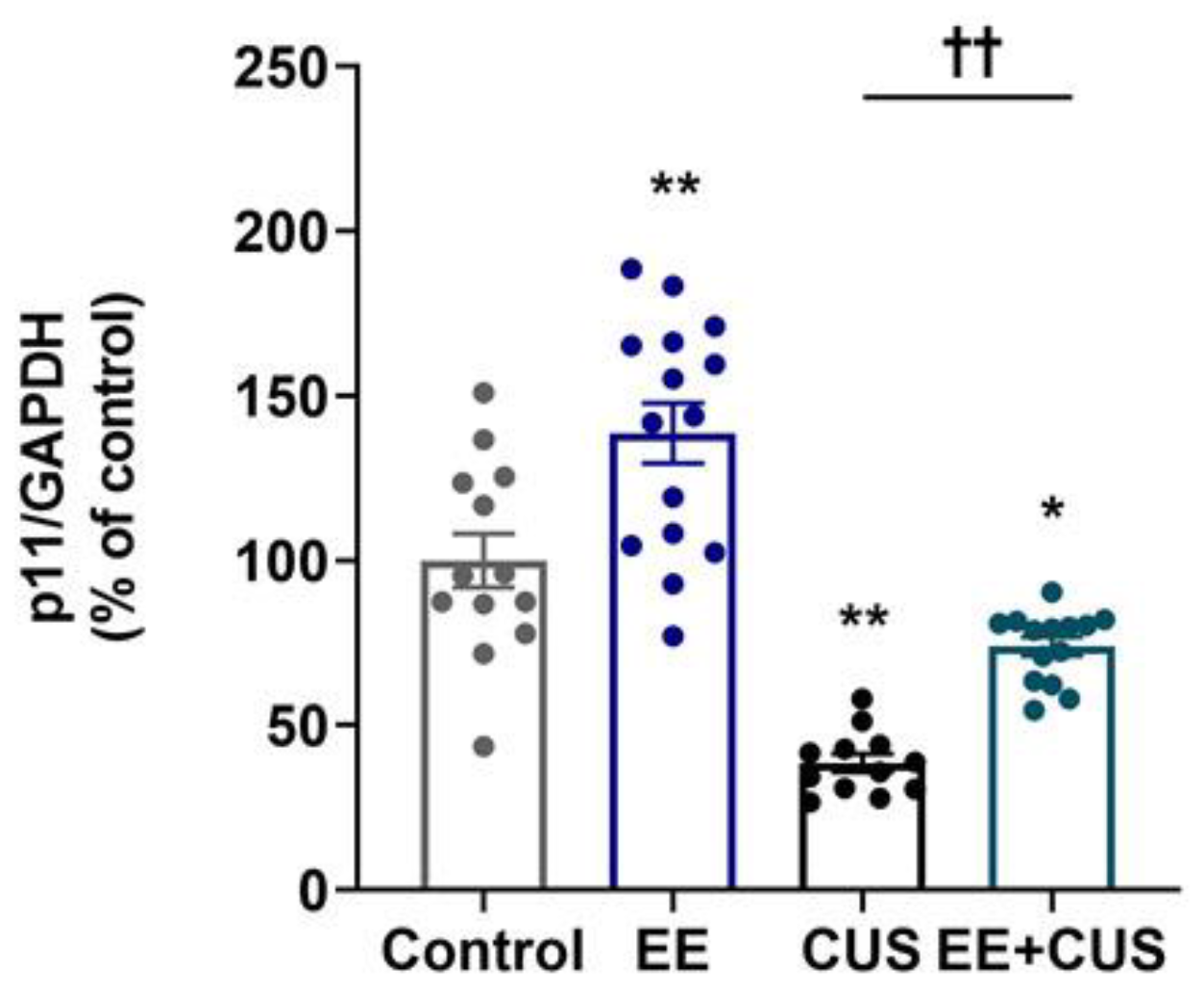
2.2. Effects of EE and CUS on Hippocampal p11 mRNA Levels
2.3. Effects of EE and CUS on the Epigenetic State of the p11 Gene
3. Discussion
4. Materials and Methods
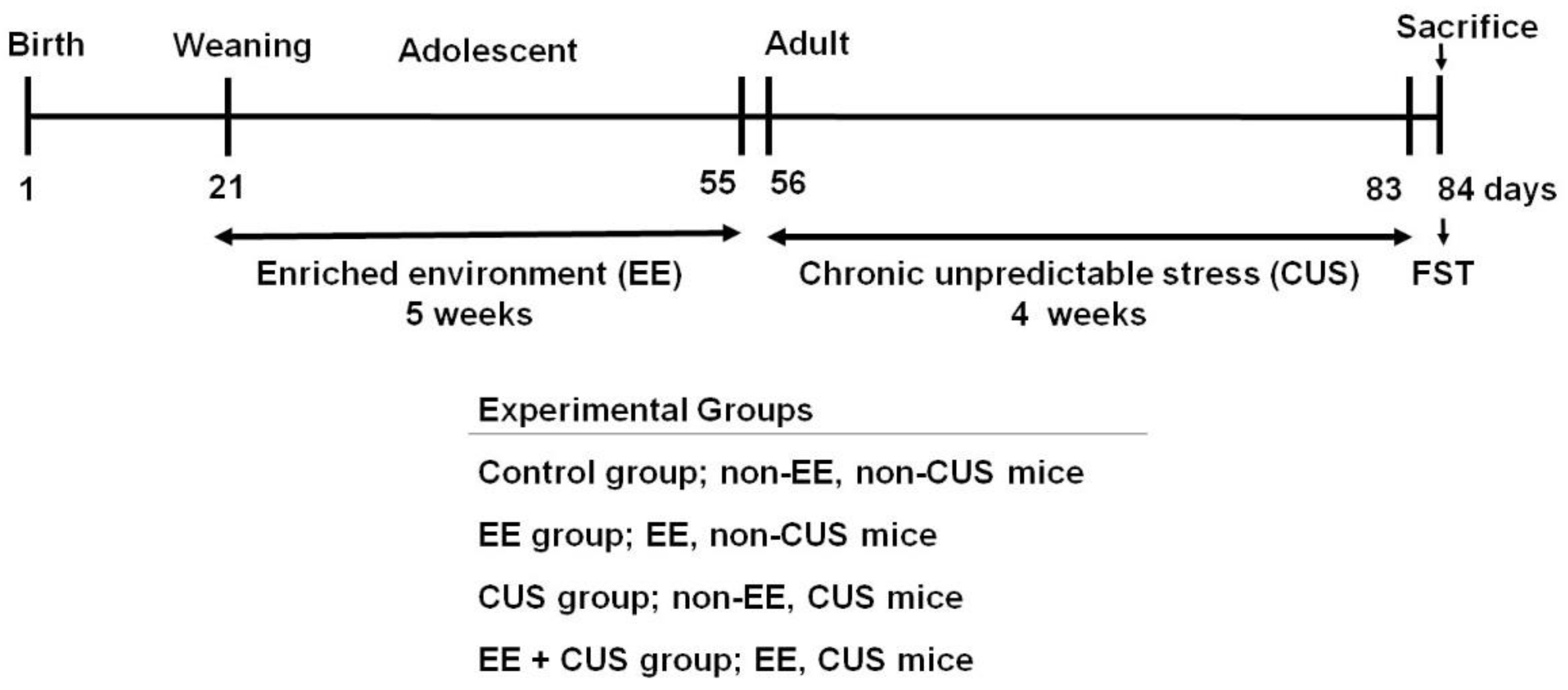
4.1. Animals and Groups
4.2. Enriched Environment (EE)
4.3. Chronic Unpredictable Stress (CUS)
4.4. Forced Swimming Test (FST)
4.5. Plasma Corticosterone Measurements
4.6. Measurement of mRNA Levels by qRT-PCR
4.7. Chromatin Immunoprecipitation (ChIP) Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcH3 | Acetylation of histone H3 |
| ANOVA | Analysis of variance |
| BDNF | Brain-derived neurotropic factor |
| cDNA | Complementary deoxyribonucleic acid |
| ChIP | Chromatin immunoprecipitation |
| Ct | Cycle threshold |
| CUS | Chronic unpredictable stress |
| DNA | Deoxyribonucleic acid |
| EE | Enriched environment |
| EZH2 | Enhancer of zeste homolog 2 |
| FST | Forced swimming test |
| G9a | Euchromatic histone-lysine N-methyltransferase 2 (EHMT2) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| H3 | Histone H3 |
| H3K4 | Histone H3 lysine 4 |
| H3K4me3 | Trimethylation of H3K4 |
| H3K9 | Histone H3 lysine 9 |
| H3K9me3 | Trimethylation of H3K9 |
| H3K20 | Histone H3 lysine 20 |
| H3K27 | Histone H3 lysine 27 |
| H3K27me3 | Trimethylation of H3K27 |
| H3K36 | Histone H3 lysine 36 |
| H4 | Histone H4 |
| HATs | Histone acetyltransferases |
| HDAC5 | Histone deacetylase 5 |
| HDACs | Histone deacetylases |
| i.p. | Intraperitoneal |
| IP | Immunoprecipitation |
| K | Lysine |
| mRNA | Messenger RNA |
| PND | Postnatal day |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RNA | Ribonucleic acid |
| RPL30 | Ribosomal protein L30 |
| SEM | Standard error of the mean |
| Set7/9 | SET domain-containing lysine N-methyltransferase 7 (SETD7) |
References
- Bonanno, G.A. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004, 59, 20–28. [Google Scholar] [CrossRef]
- Southwick, S.; Charney, D.S. The science of resilience: Implications for the prevention and treatment of depression. Science 2012, 338, 79–82. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Patchev, A.V.; Wu, Y.; Micale, V.; Bockmühl, Y.; Fischer, D.; Holsboer, F.; Wotjak, C.T.; Almeida, O.F.; Spengler, D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009, 12, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.E.; Turecki, G. DNA methylation and childhood maltreatment: From animal models to human studies. Neuroscience 2014, 264, 142–156. [Google Scholar] [CrossRef]
- Lutz, P.E.; Almeida, D.; Flori, L.M.; Turecki, G. Childhood maltreatment and stress-related psychopathology: The epigenetic memory hypothesis. Curr. Pharm. Des. 2015, 21, 1413–1417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Borrelli, E.; Nestler, E.J.; Allis, C.D.; Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 2008, 60, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Howe, L.J. Histone acetylation: Truth of consequences? Biochem. Cell Biol. 2009, 87, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Vogelauer, M.; Wu, J.; Suka, N.; Grunstein, M. Global histone acetylation and deacetylation in yeast. Nature 2000, 408, 495–498. [Google Scholar] [CrossRef]
- de Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef]
- Izzo, A.; Schneider, R. Chatting histone modifications in mammals. Brief Funct. Genom. 2010, 9, 429–443. [Google Scholar] [CrossRef]
- Svenningsson, P.; Kim, Y.; Warner-Schmidt, J.; Oh, Y.S.; Greengard, P. p11 and its role in depression and therapeutic responses to antidepressants. Nat. Rev. Neurosci. 2013, 14, 673–680. [Google Scholar] [CrossRef]
- Svenningsson, P.; Chergui, K.; Rachleff, I.; Flajolet, M.; Zhang, X.; El-Yacoubi, M.; Vaugeois, J.M.; Nomikos, G.G.; Greengard, P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 2006, 311, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Flajolet, M.; Maller, A.; Chen, E.Y.; Qi, H.; Svenningsson, P.; Greengard, P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J. Neurosci. 2009, 29, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Chen, E.Y.; Zhang, X.; Marshall, J.J.; Morozov, A.; Svenningsson, P.; Greengard, P. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol. Psychiatry 2010, 68, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, L.; Braschi, C.; Spolidoro, M.; Begenisic, T.; Sale, A.; Maffei, L. Nurturing brain plasticity: Impact of environmental enrichment. Cell Death Differ. 2010, 17, 1092–1103. [Google Scholar] [CrossRef]
- Sale, A.; Berardi, N.; Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Kempermann, G.; Gage, F.H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Baldini, S.; Restani, L.; Baroncelli, L.; Coltelli, M.; Franco, R.; Cenni, M.C.; Maffei, L.; Berardi, N. Enriched early life experiences reduce adult anxiety-like behavior in rats: A role for insulin-like growth factor 1. J. Neurosci. 2013, 33, 11715–11723. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Henriksson, B.G.; Söderström, S.; Ebendal, T.; Olsson, T.; Seckl, J.R. Environmental influences on the central nervous system and their implications for the aging rat. Behav. Brain Res. 1993, 57, 183–191. [Google Scholar] [CrossRef]
- Olsson, T.; Mohammed, A.H.; Donaldson, L.F.; Henriksson, B.G.; Seckl, J.R. Glucocorticoid receptor and NGFI-A gene expression are induced in the hippocampus after environmental enrichment in adult rats. Brain Res. Mol. Brain Res. 1994, 23, 349–353. [Google Scholar] [CrossRef]
- Yang, J.; Hou, C.; Ma, N.; Liu, J.; Zhang, Y.; Zhou, J.; Xu, L.; Li, L. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol. Learn Mem. 2007, 87, 257–263. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. Lond. B 2012, 367, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Tomporowski, P.D. Exercise and Cognition. Pediatr. Exerc. Sci. 2016, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Hollis, F.; Isgor, C.; Kabbaj, M. The consequences of adolescent chronic unpredictable stress exposure on brain and behavior. Neuroscience 2013, 249, 232–241. [Google Scholar] [CrossRef]
- Monteiro, S.; Roque, S.; de Sá-Calçada, D.; Sousa, N.; Correia-Neves, M.; Cerqueira, J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry 2015, 6, 6. [Google Scholar] [CrossRef]
- Seo, J.S.; Wei, J.; Qin, L.; Kim, Y.; Yan, Z.; Greengard, P. Cellular and molecular basis for stress-induced depression. Mol. Psychiatry 2017, 22, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Deussing, J.M. Animal models of depression. Drug Discov. Today Dis. Models 2006, 3, 375–383. [Google Scholar] [CrossRef]
- Liu, D.; Qiu, H.M.; Fei, H.Z.; Hu, X.Y.; Xia, H.J.; Wang, L.J.; Qin, L.J.; Jiang, X.H.; Zhou, Q.X. Histone acetylation and expression of mono-aminergic transmitters synthetases involved in CUS-induced depressive rats. Exp. Biol. Med. 2014, 239, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Erburu, M.; Muñoz-Cobo, I.; Domínguez-Andrés, J.; Beltran, E.; Suzuki, T.; Mai, A.; Valente, S.; Puerta, E.; Tordera, R.M. Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur. Neuropsychopharmacol. 2015, 25, 2036–2048. [Google Scholar] [CrossRef] [PubMed]
- Ferland, C.L.; Schrader, L.A. Regulation of histone acetylation in the hippocampus of chronically stressed rats: A potential role of sirtuins. Neuroscience 2011, 174, 104–114. [Google Scholar] [CrossRef]
- Gallinari, P.; Di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519–525. [Google Scholar] [CrossRef]
- Seo, M.K.; Ly, N.N.; Lee, C.H.; Cho, H.Y.; Choi, C.M.; Nhu, L.H.; Lee, J.G.; Lee, B.J.; Kim, G.M.; Yoon, B.J.; et al. Early life stress increases stress vulnerability through BDNF gene epigenetic changes in the rat hippocampus. Neuropharmacology 2016, 105, 388–397. [Google Scholar] [CrossRef]
- Iga, J.; Ueno, S.; Yamauchi, K.; Numata, S.; Kinouchi, S.; Tayoshi-Shibuya, S.; Song, H.; Ohmori, T. Altered HDAC5 and CREB mRNA expressions in the peripheral leukocytes of major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 628–632. [Google Scholar] [CrossRef]
- Renthal, W.; Maze, I.; Krishnan, V.; Covington, H.E., III; Xiao, G.; Kumar, A.; Russo, S.J.; Graham, A.; Tsankova, N.; Kippin, T.E.; et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron 2007, 56, 517–529. [Google Scholar] [CrossRef]
- Hunter, R.G.; McCarthy, K.J.; Milne, T.A.; Pfaff, D.W.; McEwen, B.S. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc. Natl. Acad. Sci. USA 2009, 106, 20912–20917. [Google Scholar] [CrossRef]
- Cui, M.; Yang, Y.; Yang, J.; Zhang, J.; Han, H.; Ma, W.; Li, H.; Mao, R.; Xu, L.; Hao, W.; et al. Enriched environment experience overcomes the memory deficits and depressive-like behavior induced by early life stress. Neurosci. Lett. 2006, 404, 208–212. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Ortiz-López, L.; Gómez-Sánchez, A.; Oikawa-Sala, J.; Estrada-Camarena, E.M.; Ramírez-Rodríguez, G.B. The neurogenic effects of an enriched environment and its protection against the behavioral consequences of chronic mild stress persistent after enrichment cessation in six-month-old female Balb/C mice. Behav. Brain Res. 2016, 301, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Morley-Fletcher, S.; Rea, M.; Maccari, S.; Laviola, G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur. J. Neurosci. 2003, 18, 3367–3374. [Google Scholar] [CrossRef]
- Llorens-Martín, M.V.; Rueda, N.; Martínez-Cué, C.; Torres-Alemán, I.; Flórez, J.; Trejo, J.L. Both increases in immature dentate neuron number and decreases of immobility time in the forced swim test occurred in parallel after environmental enrichment of mice. Neuroscience 2007, 147, 631–638. [Google Scholar] [CrossRef][Green Version]
- Veena, J.; Srikumar, B.N.; Mahati, K.; Bhagya, V.; Raju, T.R.; Shankaranarayana Rao, B.S. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J. Neurosci. Res. 2009, 87, 831–843. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Tejeda, G.S.; Trejo, J.L. Antidepressant and proneurogenic influence of environmental enrichment in mice: Protective effects vs. recovery. Neuropsychopharmacology 2011, 36, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Caldji, C.; Tannenbaum, B.; Sharma, S.; Francis, D.; Plotsky, P.M.; Meaney, M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of behavioral fearfulness in adulthood in the rat. Proc. Natl. Acad. Sci. USA 1998, 95, 5335–5340. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.D.; Diorio, J.; Liu, D.; Meaney, M.J. Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science 1999, 286, 1155–1158. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Zahir, S.; Schmold, N.; Ilnytskyy, S.; Kovalchuk, O.; Gibb, R. Parental enrichment and offspring development: Modifications to brain, behavior and the epigenome. Behav. Brain Res. 2012, 228, 294–298. [Google Scholar] [CrossRef]
- Irier, H.; Street, R.C.; Dave, R.; Lin, L.; Cai, C.; Davis, T.H.; Yao, B.; Cheng, Y.; Jin, P. Environmental Enrichment Modulates 5-hydroxymethylcytosine Dynamics in Hippocampus. Genomics 2014, 104, 376–382. [Google Scholar] [CrossRef]
- Kuzumaki, N.; Ikegami, D.; Tamura, R.; Hareyama, N.; Imai, S.; Narita, M.; Torigoe, K.; Niikura, K.; Takeshima, H.; Ando, T.; et al. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus 2011, 21, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Brenes, J.C.; Padilla, M.; Fornaguera, J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav. Brain Res. 2009, 197, 125–137. [Google Scholar] [CrossRef]
- Jha, S.; Dong, B.; Sakata, K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl. Psychiatry 2011, 1, e40. [Google Scholar] [CrossRef] [PubMed]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural Transm. 2019, 126, 383–1408. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Qu, C.; Xu, L.; Sun, H.; Zhang, J. The enriched environment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive impairment by activating the SIRT1/miR-134 signaling pathway in hippocampus. J. Affect Disord. 2019, 248, 81–90. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar]


| Primer Sequence (5′-3′) | ||
|---|---|---|
| qRT-PCR for mRNA | ||
| p11 * | Forward | TGCTCATGGAAAGGGAGTTC |
| Reverse | CCCCGCCACTATTGATAGAA | |
| HDAC5 # | Forward | CCATTGGAGATGTGGAATAC |
| Reverse | CAGTGGAGACAGATGTCCTT | |
| GAPDH † | Forward | AACAGCAACTCCCATTCTTC |
| Reverse | TGGTCCAGGGTTTCTTACTC | |
| qRT-PCR for histone modification | ||
| p11 promoter § | Forward | CGTTCCTCCTGCTTATCTAG |
| Reverse | GCTCTTAGTATTTCAGGGCA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.K.; Choi, A.J.; Seog, D.-H.; Lee, J.G.; Park, S.W. Early Enriched Environment Prevents Epigenetic p11 Gene Changes Induced by Adulthood Stress in Mice. Int. J. Mol. Sci. 2021, 22, 1928. https://doi.org/10.3390/ijms22041928
Seo MK, Choi AJ, Seog D-H, Lee JG, Park SW. Early Enriched Environment Prevents Epigenetic p11 Gene Changes Induced by Adulthood Stress in Mice. International Journal of Molecular Sciences. 2021; 22(4):1928. https://doi.org/10.3390/ijms22041928
Chicago/Turabian StyleSeo, Mi Kyoung, Ah Jeong Choi, Dae-Hyun Seog, Jung Goo Lee, and Sung Woo Park. 2021. "Early Enriched Environment Prevents Epigenetic p11 Gene Changes Induced by Adulthood Stress in Mice" International Journal of Molecular Sciences 22, no. 4: 1928. https://doi.org/10.3390/ijms22041928
APA StyleSeo, M. K., Choi, A. J., Seog, D.-H., Lee, J. G., & Park, S. W. (2021). Early Enriched Environment Prevents Epigenetic p11 Gene Changes Induced by Adulthood Stress in Mice. International Journal of Molecular Sciences, 22(4), 1928. https://doi.org/10.3390/ijms22041928






