Low-Temperature Argon Plasma Regulates Skin Moisturizing and Melanogenesis-Regulating Markers through Yes-Associated Protein
Abstract
1. Introduction
2. Results
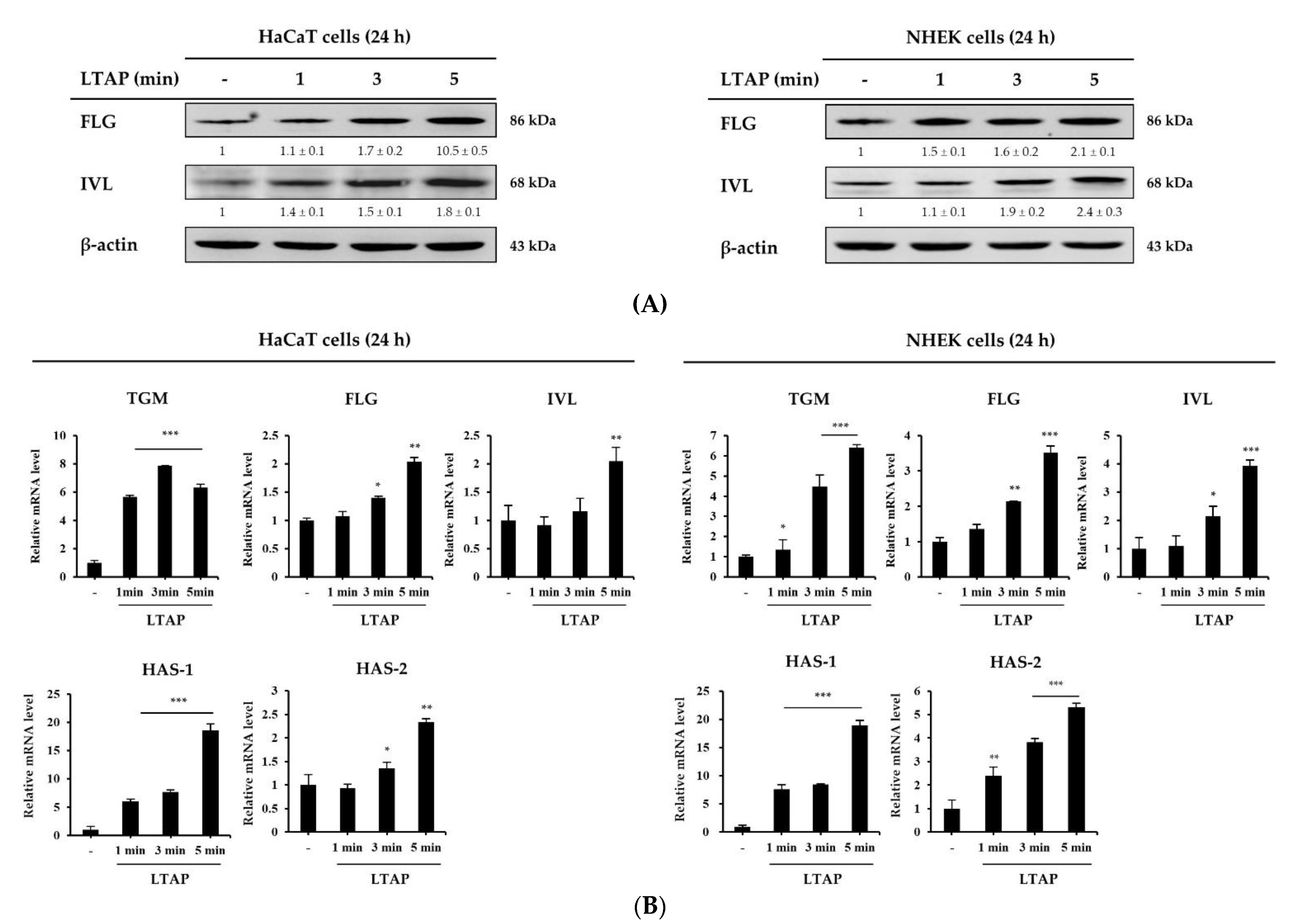
2.1. LTAP Induces Skin Barrier and Moisturizing Factors
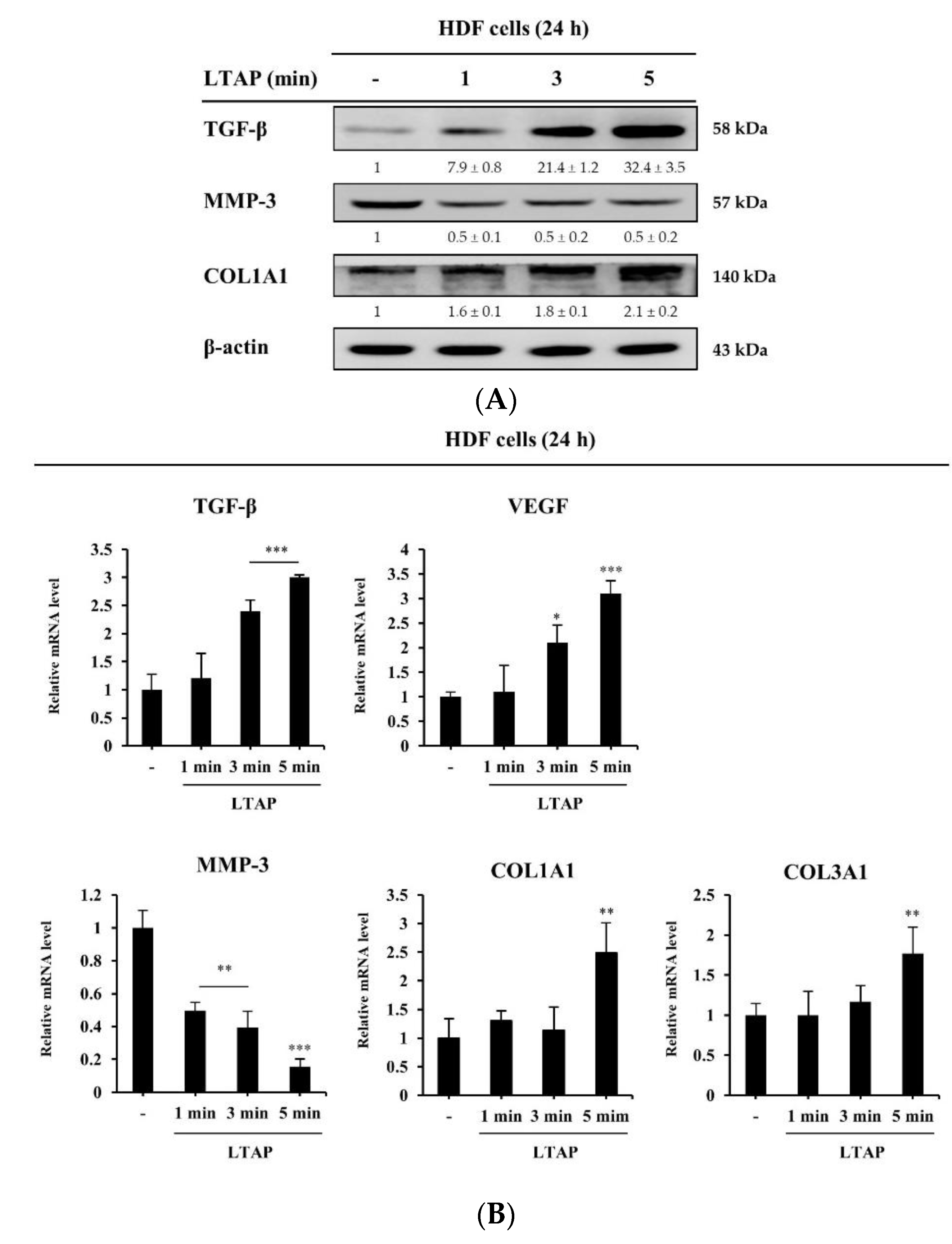
2.2. Effects of LTAP on the Skin Proliferation and Aging-Related Gene
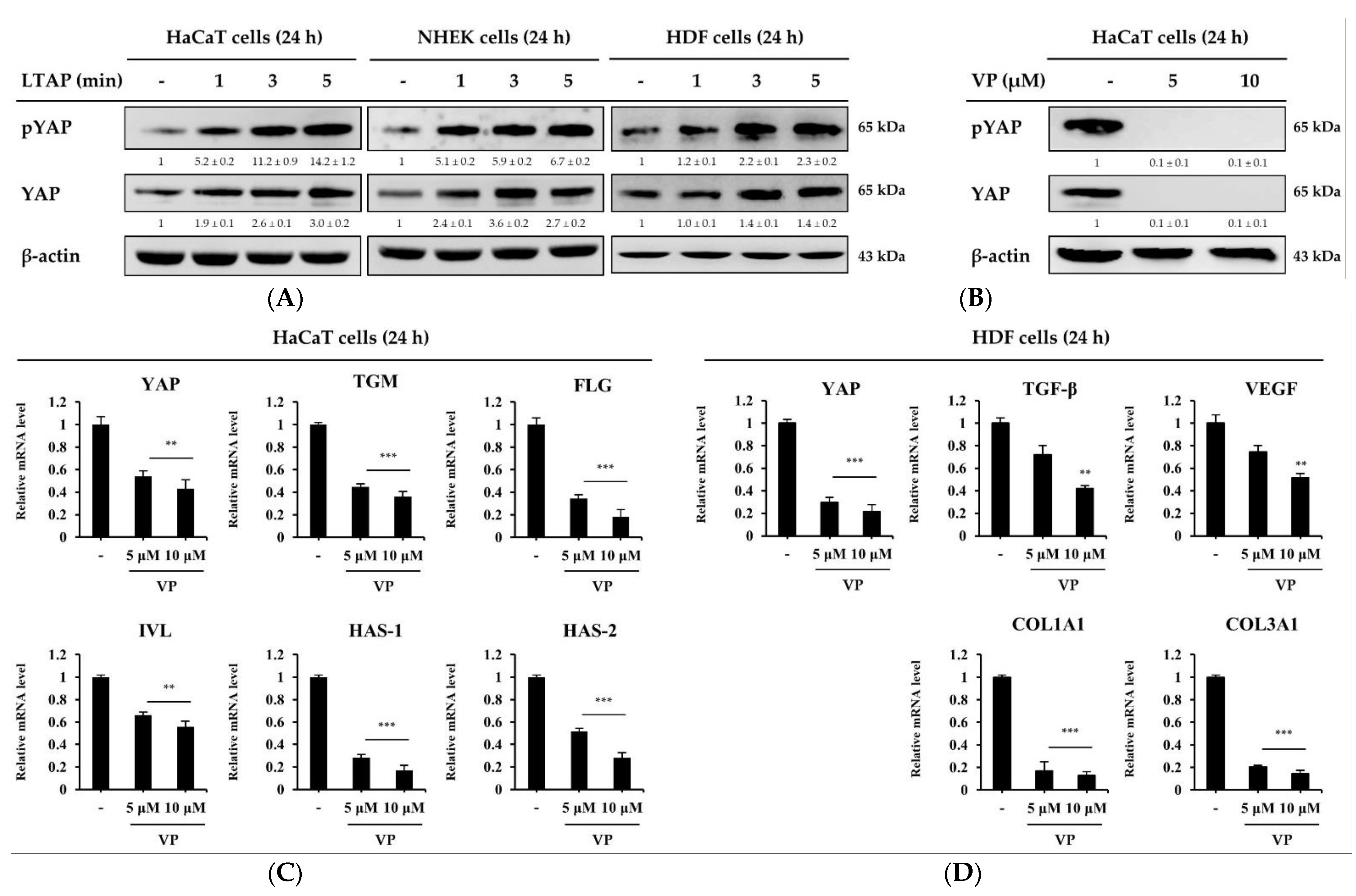
2.3. The Skin Moisturizing and Aging Factors Are Mediated by YAP
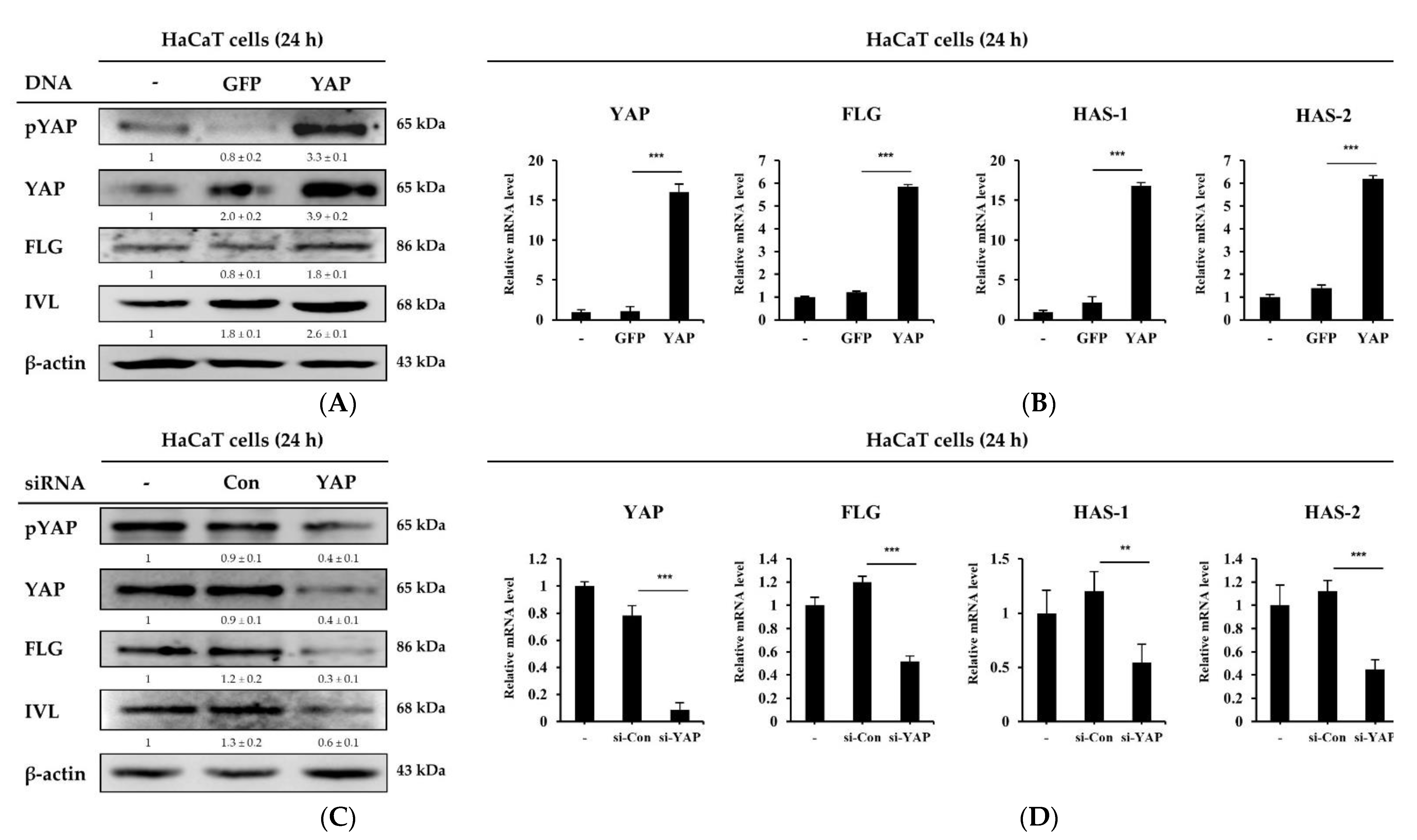
2.4. YAP Overexpression or Knockdown in HaCaT Cells Regulates Skin Barrier and Moisturizing Factors
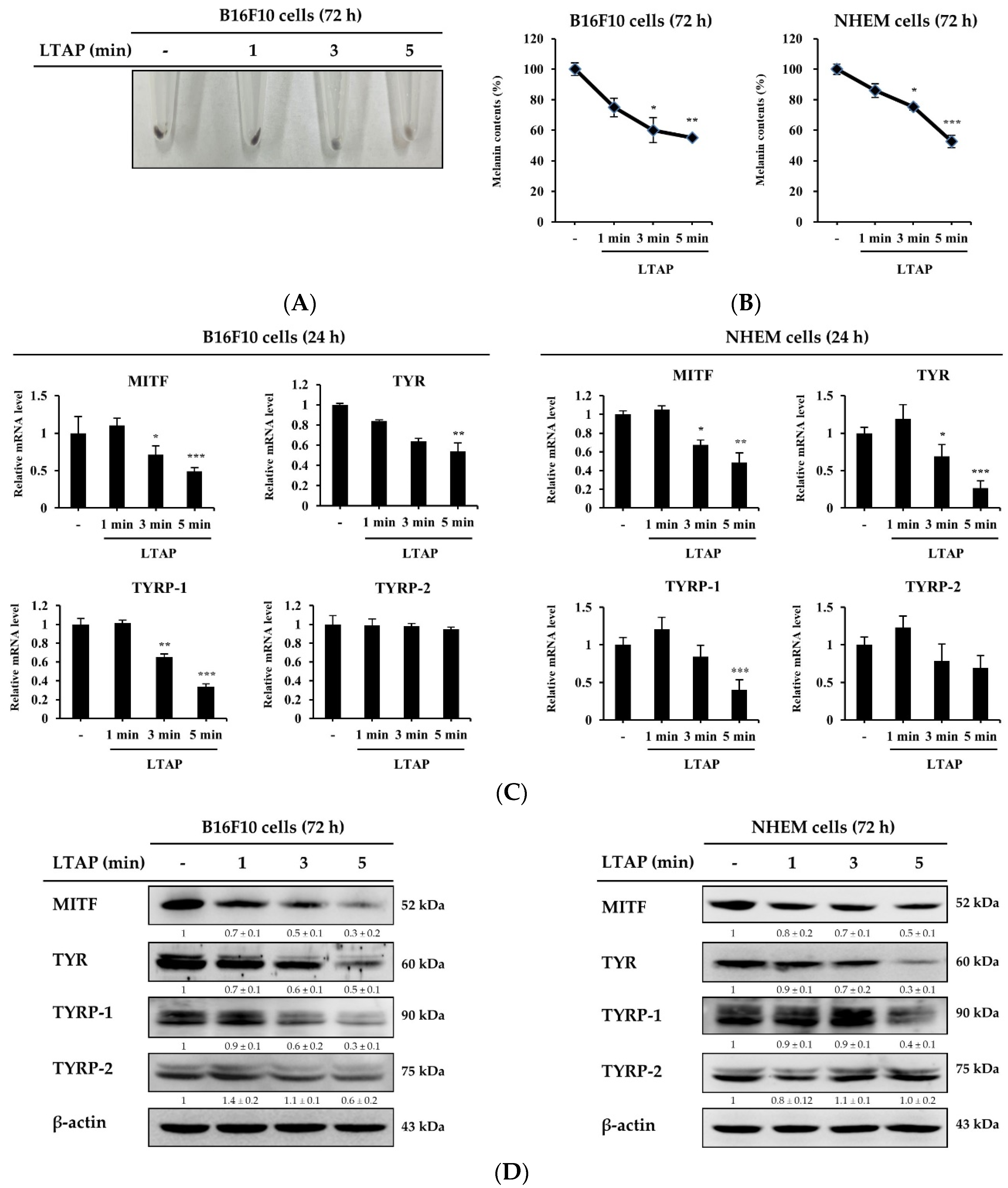
2.5. LTAP Inhibits Melanin Production in B16F10 Cells and NHEMs
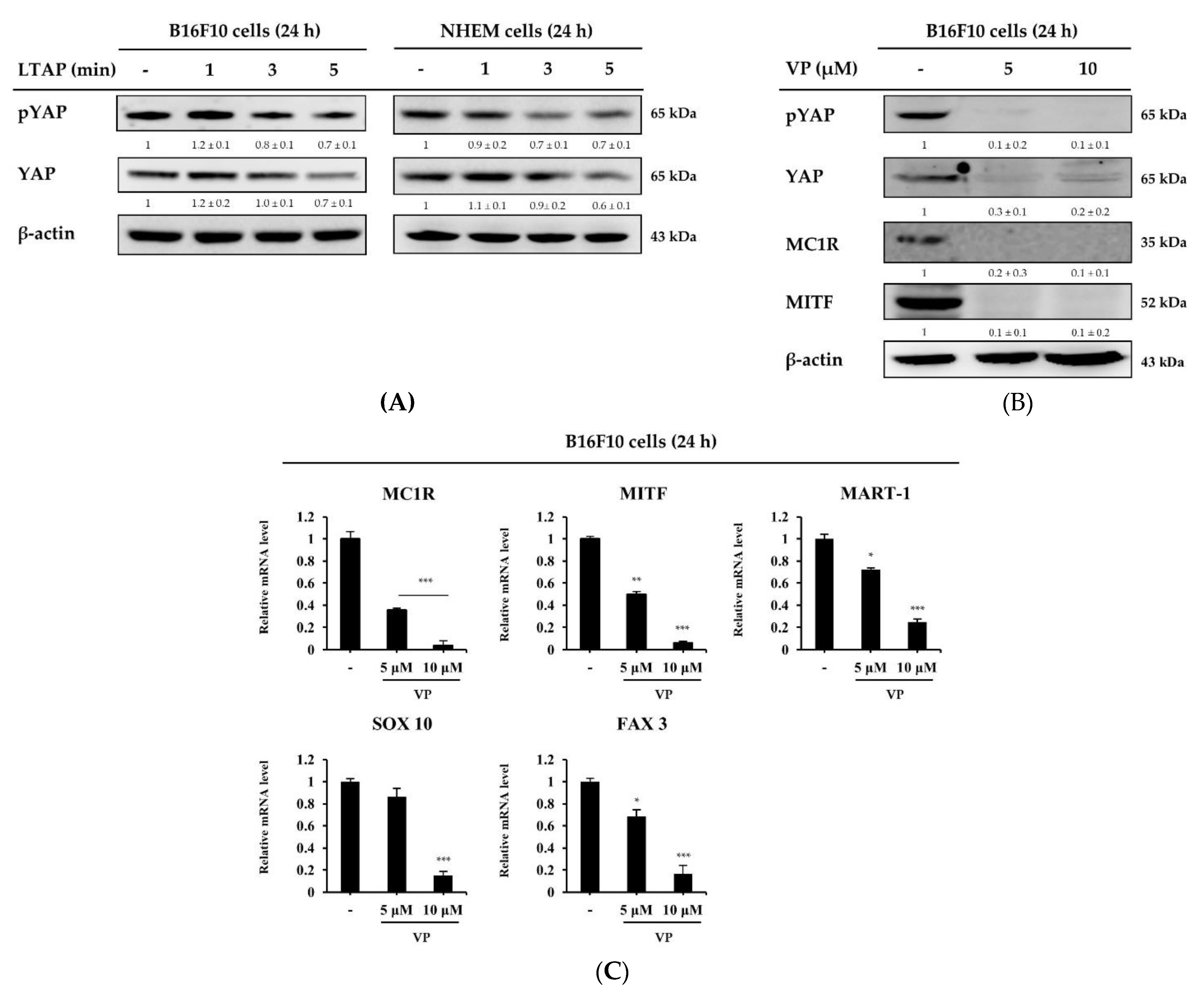
2.6. LTAP Inhibits Receptors and Genes Involved in Melanin Production and Melanoma Proliferation
2.7. Melanogenesis Related Genes Are Regulated by Verteporine on YAP Inhibitors
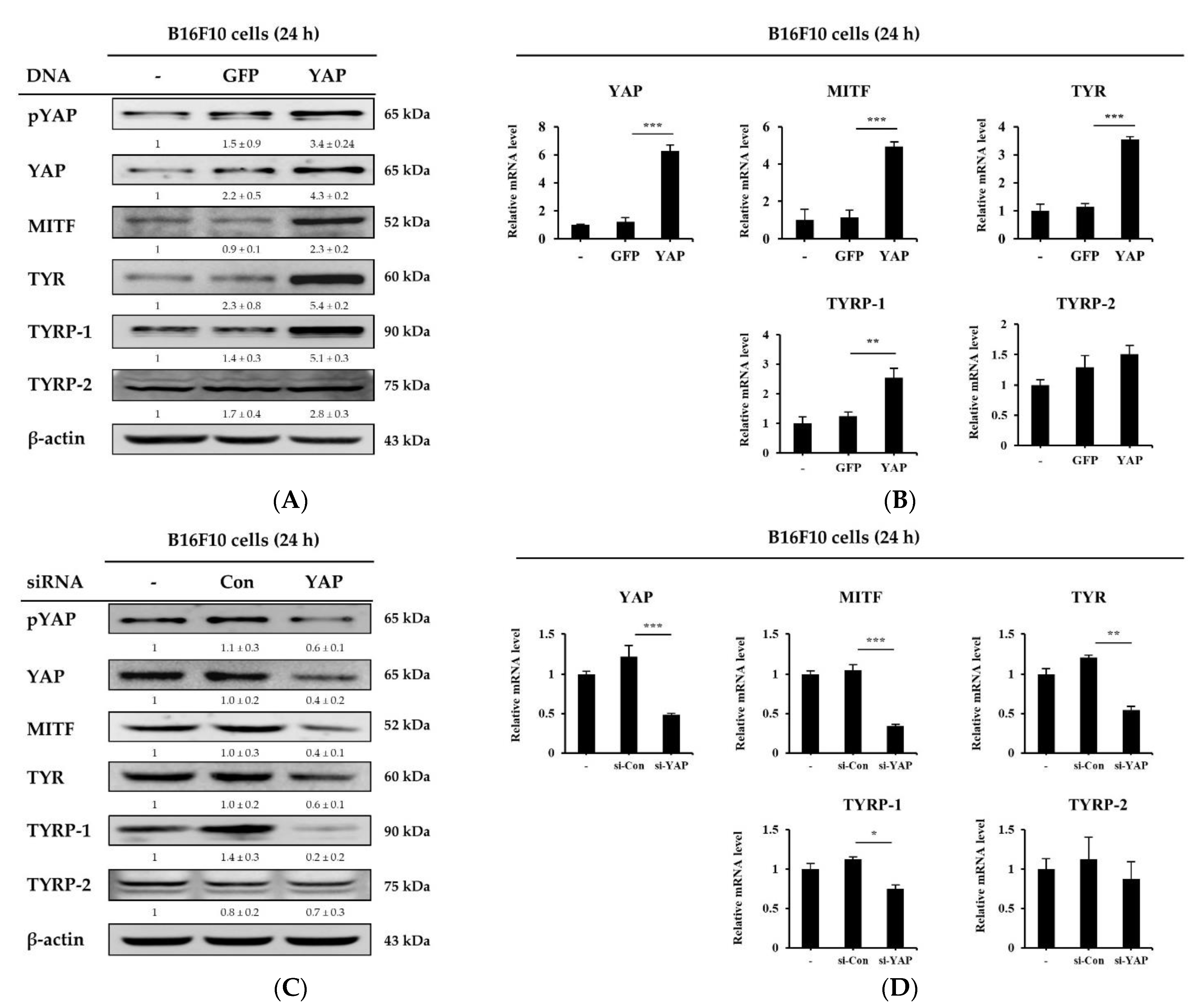
2.8. Overexpression and Knockdown of YAP Regulates Melanogenesis-Related Molecule Expression in B16F10 Cells
3. Discussion
4. Materials and Methods
4.1. LTAP Device
4.2. Cell Culture and LTAP Exposure
4.3. VP Treatment, siRNA, and Plasmid DNA Transfection
4.4. Measurement of Melanin Content
4.5. cDNA synThesis and Quantitative Real-Time PCR (qRT-PCR)
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTAP | Low-temperature argon plasma |
| YAP | Yes-associated protein |
| NMF | Natural moisturizing factor |
| TGM | Transglutaminase |
| FLG | Filaggrin |
| IVL | Involucrin |
| HA | Hyaluronic acid |
| HAS | Hyaluronic acid synthase |
| VEGF | Vascular endothelial growth factor |
| TGF-β | Transforming growth factor-β |
| COL1A1/COL3A1 | Collagen, type I/III, alpha 1 |
| MMP-3 | Matrix metallopeptidase-3 |
| VP | Verteporfin |
| siRNA | Small interfering RNA |
| MITF | Microphthalmia-associated transcription factor |
| TYR | Tyrosinase |
| TYRP-1/2 | Tyrosinase-related protein-1/2 |
| EDN | Endothelin |
| WNT | Wingless-type protein |
| MC1R | Melanocortin 1 receptor |
| α-MSH | Alpha-melanocyte stimulating hormone |
| PKA | Protein kinase A |
| cAMP | Cyclic adenosine monophosphate |
| MAPK | Mitogen-activated protein kinase |
| CREB | cAMP response element-binding protein |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun-N-terminal kinase |
| SOX10 | SRY (sex determining region Y)-box 10 |
| PAX3 | Paired box gene 3 |
| MART-1 | Protein melan-A |
References
- Jenkins, G. Molecular Mechanisms of Skin Ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhong, A.; Friedrich, E.E.; Jia, S.; Xie, P.; Galiano, R.D.; Mustoe, T.A.; Hong, S.J. S100A12 Induced in the Epidermis by Reduced Hydration Activates Dermal Fibroblasts and Causes Dermal Fibrosis. J. Investig. Dermatol. 2017, 137, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Harding, C.R. Moisturization and Skin Barrier Function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef]
- Kim, H.; Lim, Y.J.; Park, J.H.; Cho, Y. Dietary Silk Protein, Sericin, Improves Epidermal Hydration with Increased Levels of Filaggrins and Free Amino Acids in NC/Nga Mice. Br. J. Nutr. 2012, 108, 1726–1735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Candi, E.; Schmidt, R.; Melino, G. The Cornified Envelope: A Model of Cell Death in the Skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Tammi, R.; Ripellino, J.A.; Margolis, R.U.; Maibach, H.I.; Tammi, M. Hyaluronate accumulation in human epidermis treated with retinoic acid in skin organ culture. J. Investig. Dermatol. 1989, 92, 326–332. [Google Scholar] [CrossRef]
- Libert, A.; Ghanem, G.; Arnould, R.; Lejeune, F.J. Use of an Alpha-Melanocyte-Stimulating Hormone Analogue to Improve Alpha-Melanocyte-Stimulating Hormone Receptor Binding Assay in Human Melanoma. Pigment. Cell Res. 1989, 2, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Setlow, R.; Berwick, M.; Polsky, D.; Marghoob, A.A.; Kopf, A.W.; Bart, R.S. Ultraviolet A and Melanoma: A Review. J. Am. Acad. Dermatol. 2001, 44, 837–846. [Google Scholar] [CrossRef]
- Michaela, B.; Vincent, J. Modifying skin pigmentation—Approaches through intrinsic biochemistry and exogenous agents. Bone 2008, 5, 189–199. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, W. The Emerging Role of the Hippo Pathway in Cell Contact Inhibition, Organ Size Control, and Cancer Development in Mammals. Cancer Cell 2008, 13, 188–192. [Google Scholar] [CrossRef]
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo Pathway in Organ Size Control, Tissue Regeneration and Stem Cell Self-Renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Kim, C.-H. New Conversing Technology; Plasma Medicine. Korean J. Otorhinolaryngol. Neck Surg. 2010, 53, 593. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, H.W.; Lee, J.K.; Hong, J.W.; Kim, G.C. Low-Temperature Atmospheric Plasma Increases the Expression of Anti-Aging Genes of Skin Cells without Causing Cellular Damages. Arch. Dermatol. Res. 2013, 305, 133–140. [Google Scholar] [CrossRef]
- Choi, J.H.; Song, Y.S.; Lee, H.J.; Kim, G.C.; Hong, J.W. The Topical Application of Low-Temperature Argon Plasma Enhances the Anti-Inflammatory Effect of Jaun-Ointment on DNCB-Induced NC/Nga Mice. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shon, C.H.; Kim, G.C.; Lee, H.J. Research on the Apoptotic Death of Melanoma by the irradiation of Micro Plasma. Natl. Digit. Sci. Lib. 2017, 220–221. [Google Scholar]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of Skin Aging and Photoaging. Dermatol. Nurs. 2008, 20, 177–183. [Google Scholar]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; Estevão, B.M.; Gianotti, E.; Marchese, L.; Renò, F. Verteporfin Based Silica Nanoparticle for in Vitro Selective Inhibition of Human Highly Invasive Melanoma Cell Proliferation. J. Photochem. Photobiol. B Biol. 2017, 167, 1–6. [Google Scholar] [CrossRef]
- Kim, H.Y.; Sah, S.K.; Choi, S.S.; Kim, T.Y. Inhibitory Effects of Extracellular Superoxide Dismutase on Ultraviolet B-Induced Melanogenesis in Murine Skin and Melanocytes. Life Sci. 2018, 210, 201–208. [Google Scholar] [CrossRef]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Swope, V.B.; Abdel-Malek, Z.A. MC1R: Front and Center in the Bright Side of Dark Eumelanin and DNA Repair. Int. J. Mol. Sci. 2018, 19, 2667. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.; Heidl, M.; Imfeld, D.; Meeus, L.; Schuetz, R.; Campiche, R. Discovery of a Highly Selective Mc1r Agonists Pentapeptide to Be Used as a Skin Pigmentation Enhancer and with Potential Anti-Aging Properties. Int. J. Mol. Sci. 2019, 20, 6143. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, Z.; Swope, V.B.; Suzuki, I.; Akcali, C.; Harriger, M.D.; Boyce, S.T.; Urabe, K.; Hearing, V.J. Mitogenic and Melanogenic Stimulation of Normal Human Melanocytes by Melanotropic Peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 1789–1793. [Google Scholar] [CrossRef]
- Fetsch, P.A.; Cormier, J.; Hijazi, Y.M. Immunocytochemical Detection of MART-1 in Fresh and Paraffin-Embedded Malignant Melanomas. J. Immunother. 1997, 20, 60–64. [Google Scholar] [CrossRef]
- Watanabe, A.; Takeda, K.; Ploplis, B.; Tachibana, M. Epistatic Relationship between Waardenburg Syndrome Genes MITF and PAX3. Nat. Genet. 1998, 18, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Bondurand, N.; Pingault, V.; Goerich, D.E.; Lemort, N.; Sock, E.; Le Caignec, C.; Wegner, M.; Goossens, M. Interaction among SOX10, PAX3 and MITF, Three Genes Altered in Waardenburg Syndrome. Hum. Mol. Genet. 2000, 9, 1907–1917. [Google Scholar] [CrossRef]
- Choi, J.H.; Nam, S.H.; Song, Y.S.; Lee, H.W.; Lee, H.J.; Song, K.; Hong, J.W.; Kim, G.C. Treatment with Low-Temperature Atmospheric Pressure Plasma Enhances Cutaneous Delivery of Epidermal Growth Factor by Regulating E-Cadherin-Mediated Cell Junctions. Arch. Dermatol. Res. 2014, 306, 635–643. [Google Scholar] [CrossRef]
- Nakai, K.; Yoneda, K.; Hosokawa, Y.; Moriue, T.; Presland, R.B.; Fallon, P.G.; Kabashima, K.; Kosaka, H.; Kubota, Y. Reduced Expression of Epidermal Growth Factor Receptor, E-Cadherin, and Occludin in the Skin of Flaky Tail Mice Is Due to Filaggrin and Loricrin Deficiencies. Am. J. Pathol. 2012, 181, 969–977. [Google Scholar] [CrossRef]
- Hines, M.D.; Jin, H.C.; Wheelock, M.J.; Jensen, P.J. Inhibition of Cadherin Function Differentially Affects Markers of Terminal Differentiation in Cultured Human Keratinocytes. J. Cell Sci. 1999, 112, 4569–4579. [Google Scholar]
- Sanchez, I.M.; Aplin, A.E. Hippo—Hungry, hungry for melanoma invasion. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Sciences, M.; Wales, N.S. The Role of Yes-Associated Protein (YAP) in Skin Regeneration. Bassem Akladios. Ph.D. Thesis, Philosophy School of Medical Sciences, Faculty of Medicine, Sydney, NSW, Australia, 2017. [Google Scholar]
- Sambandam, S.A.T.; Kasetti, R.B.; Xue, L.; Dean, D.C.; Lu, Q.; Li, Q. 14-3-3Σ Regulates Keratinocyte Proliferation and Differentiation by Modulating Yap1 Cellular Localization. J. Investig. Dermatol. 2015, 135, 1621–1628. [Google Scholar] [CrossRef]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Thompson, B.J. YAP and TAZ in Epithelial Stem Cells: A Sensor for Cell Polarity, Mechanical Forces and Tissue Damage. BioEssays 2016, 38, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 Acts Downstream of α-Catenin to Control Epidermal Proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef]
- Zhang, H.; Pasolli, H.A.; Fuchs, E. Yes-Associated Protein (YAP) Transcriptional Coactivator Functions in Balancing Growth and Differentiation in Skin. Proc. Natl. Acad. Sci. USA 2011, 108, 2270–2275. [Google Scholar] [CrossRef]
- Perra, A.; Kowalik, M.A.; Ghiso, E.; Ledda-Columbano, G.M.; Di Tommaso, L.; Angioni, M.M.; Raschioni, C.; Testore, E.; Roncalli, M.; Giordano, S.; et al. YAP Activation Is an Early Event and a Potential Therapeutic Target in Liver Cancer Development. J. Hepatol. 2014, 61, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, F.; Wang, Y.; Li, T.; Xiu, P.; Zhong, J.; Sun, X.; Li, J. Verteporfin Suppresses Cell Survival, Angiogenesis and Vasculogenic Mimicry of Pancreatic Ductal Adenocarcinoma via Disrupting the YAP-TEAD Complex. Cancer Sci. 2017, 108, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.W.; Liu, Y.Z.; Pan, J.X. Verteporfin Induces Apoptosis and Eliminates Cancer Stem-like Cells in Uveal Melanoma in the Absence of Light Activation. Am. J. Cancer Res. 2016, 6, 2816–2830. [Google Scholar]
- Li, H.; Huang, Z.; Gao, M.; Huang, N.; Luo, Z.; Shen, H.; Wang, X.; Wang, T.; Hu, J.; Feng, W. Inhibition of YAP Suppresses CML Cell Proliferation and Enhances Efficacy of Imatinib in Vitro and in Vivo. J. Exp. Clin. Cancer Res. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Xiong, H.; Yu, Q.; Gong, Y.; Chen, W.; Tong, Y.; Wang, Y.; Xu, H.; Shi, Y. Yes-Associated Protein (YAP) Promotes Tumorigenesis in Melanoma Cells Through Stimulation of Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1). Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nallet-Staub, F.; Marsaud, V.; Li, L.; Gilbert, C.; Dodier, S.; Bataille, V.; Sudol, M.; Herlyn, M.; Mauviel, A. Pro-Invasive Activity of the Hippo Pathway Effectors YAP and TAZ in Cutaneous Melanoma. J. Investig. Dermatol. 2014, 134, 123–132. [Google Scholar] [CrossRef]
- Lui, J.W.; Xiao, S.; Ogomori, K.; Hammarstedt, J.E.; Little, E.C.; Lang, D. The Efficiency of Verteporfin as a Therapeutic Option in Pre-Clinical Models of Melanoma. J. Cancer 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Oh, S.W.; Park, S.H.; Lee, J.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Yu, E.; Cho, J.Y.; et al. Melanogenic Effects of Maclurin Are Mediated through the Activation of CAMP/PKA/CREB and P38 MAPK/CREB Signaling Pathways. Oxidative Med. Cell. Longev. 2019, 2019, 9827519. [Google Scholar] [CrossRef] [PubMed]
- Shon, C.H.; Kim, G.C.; Lee, H.J. The Effects of an RF Plasma and Electric Fields on the Death of G361 Melanoma Cells. Trans. Korean Inst. Electr. Eng. 2007, 56, 1972–1977. [Google Scholar]
- Alimohammadi, M.; Golpur, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold Atmospheric Plasma Is a Potent Tool to Improve Chemotherapy in Melanoma in Vitro and in Vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef]
- Wellbrock, C.; Arozarena, I. Microphthalmia-Associated Transcription Factor in Melanoma Development and MAP-Kinase Pathway Targeted Therapy. Pigment. Cell Melanoma Res. 2015, 28, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Miskolczi, Z.; Smith, M.P.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen Abundance Controls Melanoma Phenotypes through Lineage-Specific Microenvironment Sensing. Oncogene 2018, 37, 3166–3182. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Agrahari, G.; Lee, M.J.; Tak, L.-J.; Ham, W.-K.; Kim, T.-Y. Low-Temperature Argon Plasma Regulates Skin Moisturizing and Melanogenesis-Regulating Markers through Yes-Associated Protein. Int. J. Mol. Sci. 2021, 22, 1895. https://doi.org/10.3390/ijms22041895
Kim H-Y, Agrahari G, Lee MJ, Tak L-J, Ham W-K, Kim T-Y. Low-Temperature Argon Plasma Regulates Skin Moisturizing and Melanogenesis-Regulating Markers through Yes-Associated Protein. International Journal of Molecular Sciences. 2021; 22(4):1895. https://doi.org/10.3390/ijms22041895
Chicago/Turabian StyleKim, Hae-Young, Gaurav Agrahari, Min Jung Lee, Lee-Jung Tak, Won-Kook Ham, and Tae-Yoon Kim. 2021. "Low-Temperature Argon Plasma Regulates Skin Moisturizing and Melanogenesis-Regulating Markers through Yes-Associated Protein" International Journal of Molecular Sciences 22, no. 4: 1895. https://doi.org/10.3390/ijms22041895
APA StyleKim, H.-Y., Agrahari, G., Lee, M. J., Tak, L.-J., Ham, W.-K., & Kim, T.-Y. (2021). Low-Temperature Argon Plasma Regulates Skin Moisturizing and Melanogenesis-Regulating Markers through Yes-Associated Protein. International Journal of Molecular Sciences, 22(4), 1895. https://doi.org/10.3390/ijms22041895






