Dual-Crystallizable Silk Fibroin/Poly(L-lactic Acid) Biocomposite Films: Effect of Polymer Phases on Protein Structures in Protein-Polymer Blends
Abstract
1. Introduction
2. Results and Discussion
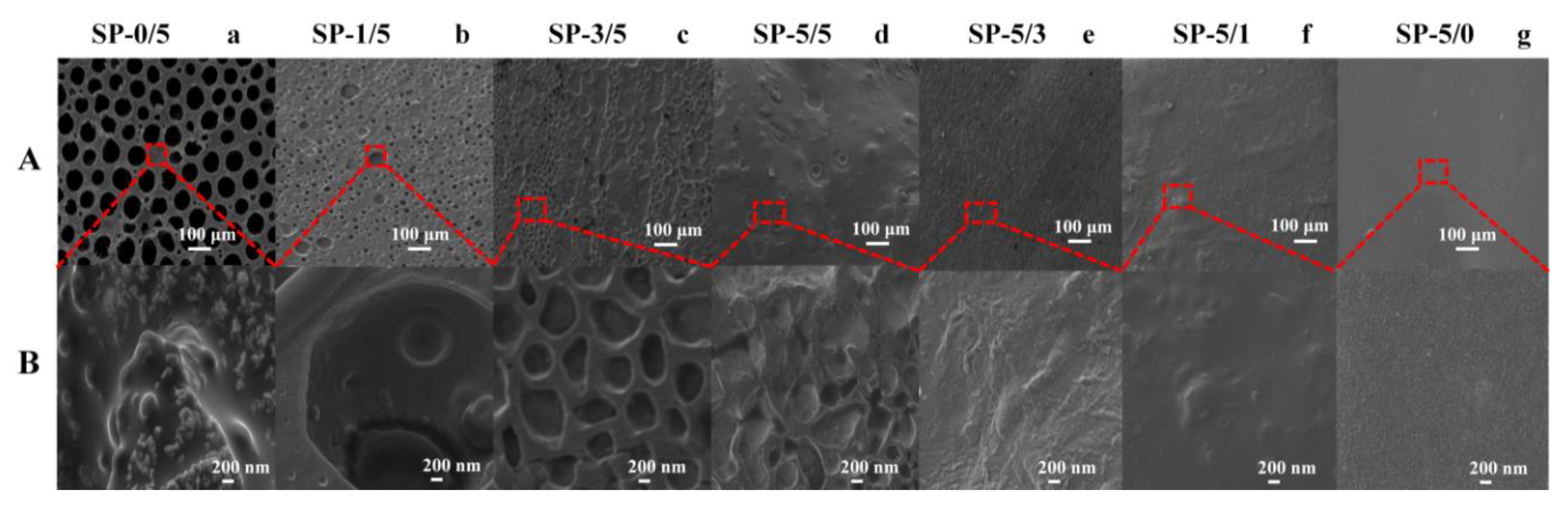
2.1. Microstructure of Blended Films
2.2. Spectroscopy Analysis
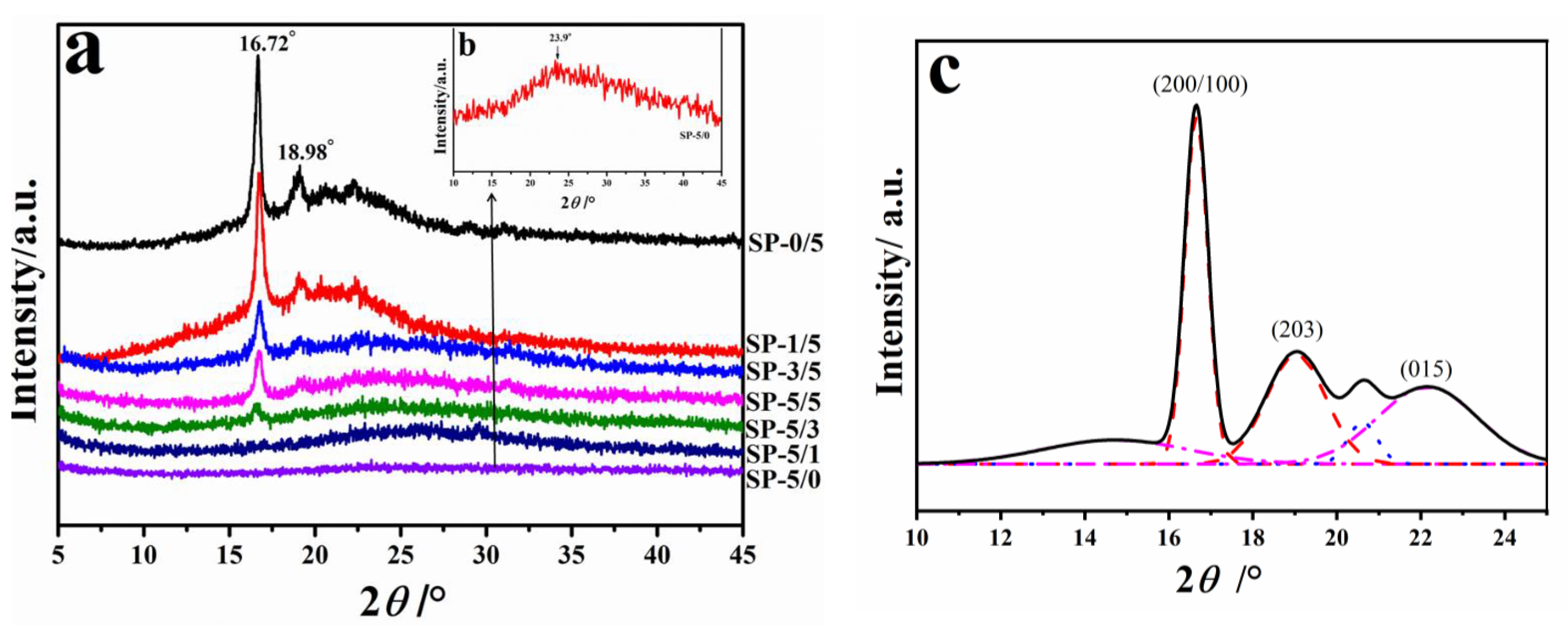
2.3. Phase Analysis
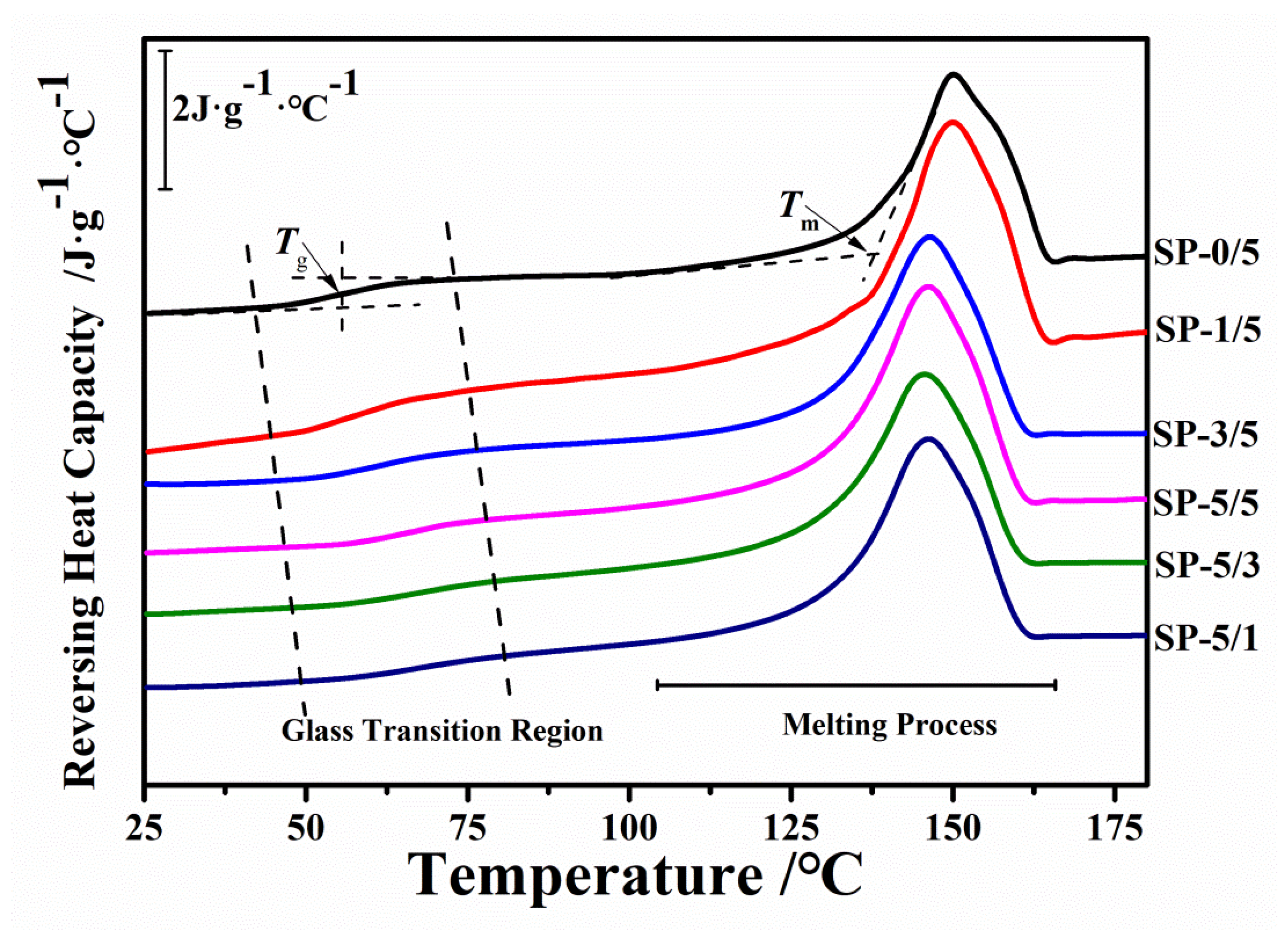
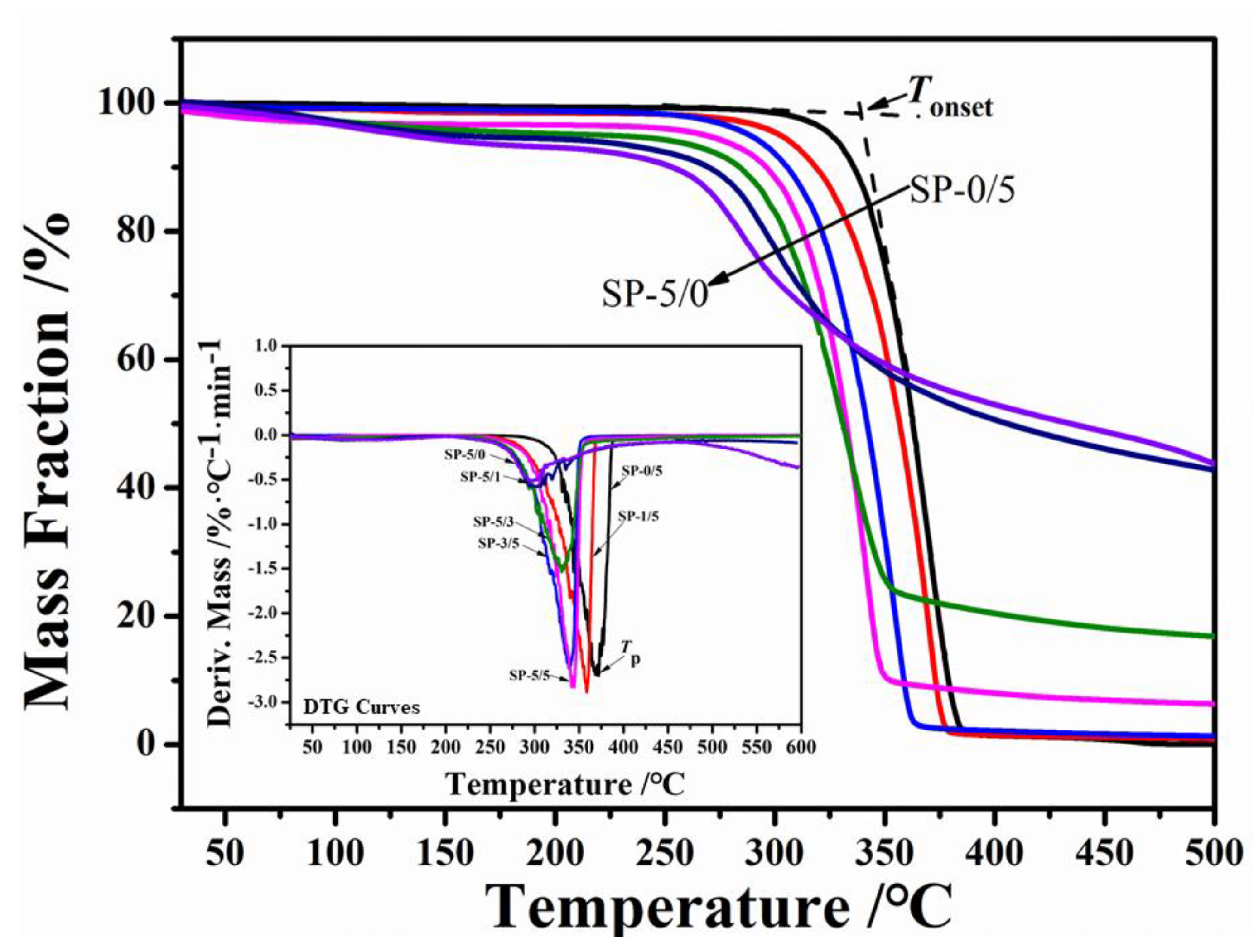
2.4. Thermal Stability
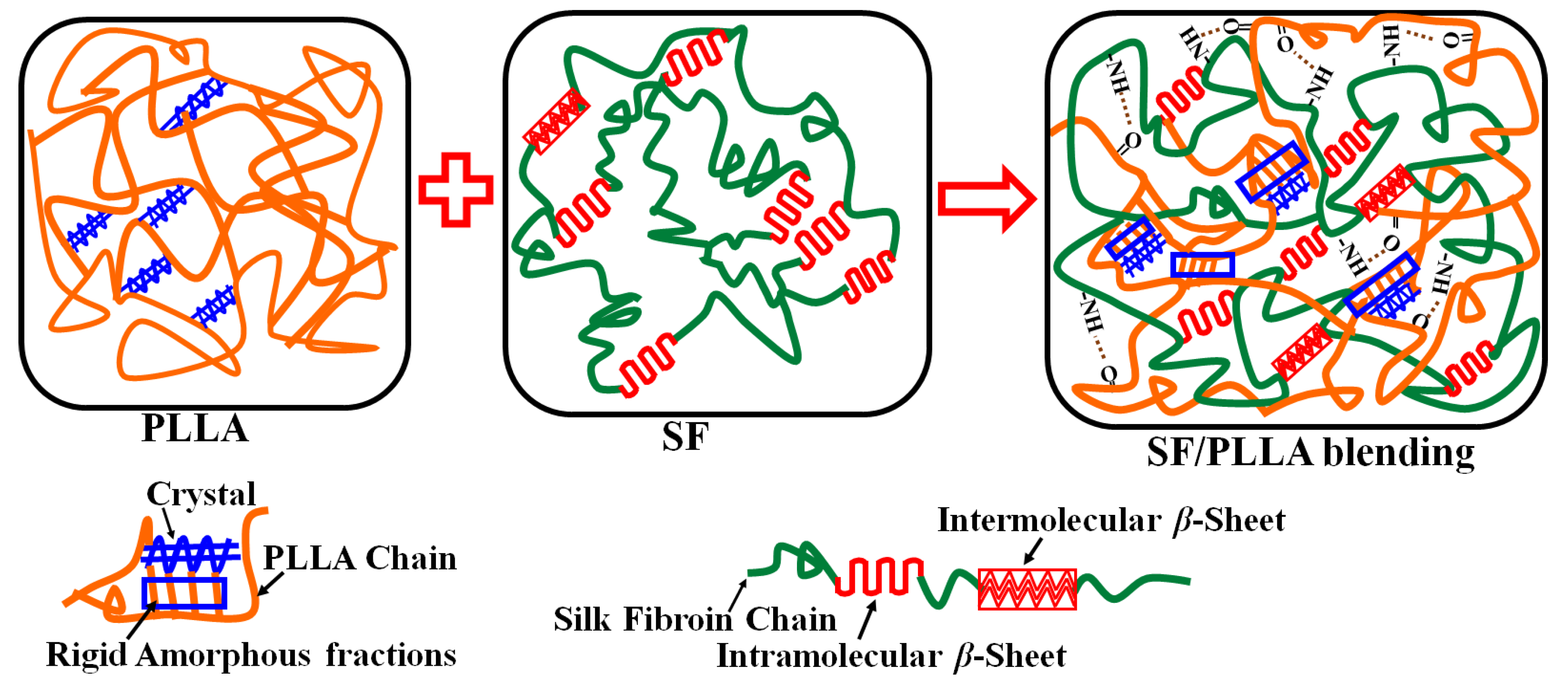
2.5. Interaction Mechanism
3. Experimental Section
3.1. Preparation of SF/PLLA Films
3.2. Experimental Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chan, A.H.P.; Filipe, E.C.; Tan, R.P.; Santos, M.; Yang, N.; Hung, J.; Feng, J.; Nazir, S.; Benn, A.J.; Ng, M.K.C.; et al. Altered processing enhances the efficacy of small-diameter silk fibroin vascular grafts. Sci. Rep. 2019, 9, 17461. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Tao, X.-M.; Hui, D. A potential material for tissue engineering: Silkworm silk/PLA biocomposite. Compos. B Eng. 2008, 39, 1026–1033. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Mitropoulos, A.N.; Marelli, B.; Ghezzi, C.E.; Applegate, M.B.; Partlow, B.P.; Kaplan, D.L.; Omenetto, F.G. Transparent, Nanostructured Silk Fibroin Hydrogels with Tunable Mechanical Properties. ACS Biomater. Sci. Eng. 2015, 1, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, N.; Murdock, C.J.; Bhattacharjee, M.; Nair, L.; Laurencin, C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020, 10, 609. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Taddei, P.; Tozzi, S.; Zuccheri, G.; Martinotti, S.; Ranzato, E.; Chiono, V.; Carmagnola, I.; Tsukada, M. Intermolecular interactions between B. mori silk fibroin and poly(l-lactic acid) in electrospun composite nanofibrous scaffolds. Mater. Sci. Eng. C 2017, 70, 777–787. [Google Scholar] [CrossRef]
- Suzuki, S.; Dawson, R.A.; Chirila, T.V.; Shadforth, A.M.A.; Hogerheyde, T.A.; Edwards, G.A.; Harkin, D.G. Treatment of Silk Fibroin with Poly(ethylene glycol) for the Enhancement of Corneal Epithelial Cell Growth. J. Funct. Biomater. 2015, 6, 345–366. [Google Scholar] [CrossRef]
- Wang, F.; Jyothirmayee Aravind, S.S.; Wu, H.; Forys, J.; Venkataraman, V.; Ramanujachary, K.; Hu, X. Tunable green graphene-silk biomaterials: Mechanism of protein-based nanocomposites. Mater. Sci. Eng. C 2017, 79, 728–739. [Google Scholar] [CrossRef]
- Aytemiz, D.; Fukuda, Y.; Higuchi, A.; Asano, A.; Nakazawa, C.; Kameda, T.; Yoshioka, T.; Nakazawa, Y. Compatibility Evaluation of Non-Woven Sheet Composite of Silk Fibroin and Polyurethane in the Wet State. Polymers 2018, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Shao, H.; Hu, X. Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Venkataraman, V.; Hu, X. Silk fibroin-poly(lactic acid) biocomposites: Effect of protein-synthetic polymer interactions and miscibility on material properties and biological responses. Mater. Sci. Eng. C 2019, 104, 109890. [Google Scholar] [CrossRef] [PubMed]
- Bindhu, B.; Renisha, R.; Roberts, L.; Varghese, T.O. Boron Nitride reinforced polylactic acid composites film for packaging: Preparation and properties. Polym. Test. 2018, 66, 172–177. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, L.; Ren, X.; Jiang, T.; Yeh, J.T. Kinetics and crystal structure of poly(lactic acid) crystallized nonisothermally: Effect of plasticizer and nucleating agent. Polym. Compos. 2010, 31, 2057–2068. [Google Scholar] [CrossRef]
- Lai, S.-M.; Hsieh, Y.-T. Preparation and Properties of Polylactic Acid (PLA)/Silica Nanocomposites. J. Macromol. Sci. Phys. 2016, 55, 211–228. [Google Scholar] [CrossRef]
- Shao, J.; Guo, Y.; Xiang, S.; Zhou, D.; Bian, X.; Sun, J.; Li, G.; Hou, H. The morphology and spherulite growth of PLA stereocomplex in linear and branched PLLA/PDLA blends: Effects of molecular weight and structure. CrystEngComm 2016, 18, 274–282. [Google Scholar] [CrossRef]
- Zhu, H.; Feng, X.; Zhang, H.; Guo, Y.; Zhang, J.; Chen, J. Structural Characteristics and Properties of Silk Fibroin/Poly(lactic acid) Blend Films. J. Biomater. Sci. Polym. Ed. 2009, 20, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wolf, N.; Rocks, E.-M.; Vuong, T.; Hu, X. Comparative studies of regenerated water-based Mori, Thai, Eri, Muga and Tussah silk fibroin films. J. Therm. Anal. Calorim. 2015, 122, 1069–1076. [Google Scholar] [CrossRef]
- Essa, S.; Rabanel, J.M.; Hildgen, P. Characterization of rhodamine loaded PEG-g-PLA nanoparticles (NPs): Effect of poly(ethylene glycol) grafting density. Int. J. Pharm. 2011, 411, 178–187. [Google Scholar] [CrossRef]
- Ying-ying, L.I.; Fang, W.; Qi-Chun, L.I.U.; Dong-Min, Z.; Xue, Z.; Qing-Yu, M.A.; Zheng-Gui, G.U. Research Progress in Silk Fibroin and Its Composite Materials. J. Mater. Eng. 2018, 46, 14–26. [Google Scholar]
- Jagdeesh, B.; Krimm, S. Vibrational Analysis of Peptides, Polypeptides, and Proteins: Characteristic amide Bands of β -Turns. Proc. Natl. Acad. Sci. USA 1979, 76, 774–777. [Google Scholar]
- Wang, F.; Yu, H.-Y.; Gu, Z.-G.; Si, L.; Liu, Q.-C.; Hu, X. Impact of calcium chloride concentration on structure and thermal property of Thai silk fibroin films. J. Therm. Anal. Calorim. 2017, 130, 851–859. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, F.; Torculas, M.; Lofland, S.; Hu, X. Formic Acid Regenerated Mori, Tussah, Eri, Thai, and Muga Silk Materials: Mechanism of Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 6361–6373. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, W.; Chen, S.; Chen, M.; Liu, Y.; Shao, Z.; Chen, X. Floxuridine-loaded silk fibroin nanospheres. RSC Adv. 2014, 4, 18171–18177. [Google Scholar] [CrossRef]
- Bai, G.-Y.; Liu, J.-L.; Wang, J.-X.; Wang, Y.-J.; Li, Y.-N.; Zhao, Y.; Yao, M.-H. Enzymatic Superactivity and Conformational Change of α-CT Induced by Cationic Gemini Surfactant. Wu Li Hua Xue Xue Bao 2017, 33, 976–983. [Google Scholar]
- Huot, A.; Lefèvre, T.; Rioux-Dubé, J.-F.; Paquet-Mercier, F.; Nault, A.-P.; Auger, M.; Pézolet, M. Effect of Mechanical Deformation on the Structure of Regenerated Bombyx mori Silk Fibroin Films as Revealed Using Raman and Infrared Spectroscopy. Appl. Spectrosc. 2015, 69, 689–698. [Google Scholar] [CrossRef]
- Ling, S.; Zhou, L.; Zhou, W.; Shao, Z.; Chen, X. Conformation transition kinetics and spinnability of regenerated silk fibroin with glycol, glycerol and polyethylene glycol. Mater. Lett. 2012, 81, 13–15. [Google Scholar] [CrossRef]
- Zhong, J.; Ma, M.; Li, W.; Zhou, J.; Yan, Z.; He, D. Self-assembly of regenerated silk fibroin from random coil nanostructures to antiparallel β-sheet nanostructures. Biopolymers 2014, 101, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zheng, X.; Yu, X.; Wang, J.; Weng, J.; Li, X.; Feng, B.; Yin, M. Hydrogen Bonding Interaction of Poly(d,l-Lactide)/hydroxyapatite Nanocomposites. Chem. Mater. 2007, 19, 247–253. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Noda, I.; Ochiai, S.; Ozaki, Y. Raman microspectroscopy study of structure, dispersibility, and crystallinity of poly(hydroxybutyrate)/poly( l-lactic acid) blends. Polymer 2006, 47, 3132–3140. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M.; Pauvert, B.; Térol, A. Vibrational analysis of poly(L-lactic acid). J. Raman Spectrosc. 1995, 26, 307–311. [Google Scholar] [CrossRef]
- Wool, R.P.; Bretzlaff, R.S.; Li, B.Y.; Wang, C.H.; Boyd, R.H. Infrared and raman spectroscopy of stressed polyethylene. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 1039–1066. [Google Scholar] [CrossRef]
- He, J.; Qin, Y.; Cui, S.; Gao, Y.; Wang, S. Structure and properties of novel electrospun tussah silk fibroin/poly(lactic acid) composite nanofibers. J. Mater. Sci. 2010, 46, 2938–2946. [Google Scholar] [CrossRef]
- Huang, S.M.; Hwang, J.J.; Liu, H.J.; Lin, L.H. Crystallization behavior of poly(L-lactic acid)/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2010, 117, 434–442. [Google Scholar] [CrossRef]
- Bruckmoser, K.; Resch, K. Effect of processing conditions on crystallization behavior and mechanical properties of poly(lactic acid) staple fibers. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Birhanu, G.; Tanha, S.; Akbari Javar, H.; Seyedjafari, E.; Zandi-Karimi, A.; Kiani Dehkordi, B. Dexamethasone loaded multi-layer poly-l-lactic acid/pluronic P123 composite electrospun nanofiber scaffolds for bone tissue engineering and drug delivery. Pharm. Dev. Technol. 2019, 24, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Avérous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Pluta, M. Morphology and properties of polylactide modified by thermal treatment, filling with layered silicates and plasticization. Polymer 2004, 45, 8239–8251. [Google Scholar] [CrossRef]
- Wu, T.-M.; Wu, C.-Y. Biodegradable poly(lactic acid)/chitosan-modified montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2006, 91, 2198–2204. [Google Scholar] [CrossRef]
- Ma, Q.; Pyda, M.; Mao, B.; Cebe, P. Relationship between the rigid amorphous phase and mesophase in electrospun fibers. Polymer 2013, 54, 2544–2554. [Google Scholar] [CrossRef]
- Schick, C.; Wurm, A.; Mohammed, A. Formation and disappearance of the rigid amorphous fraction in semicrystalline polymers revealed from frequency dependent heat capacity. Thermochim. Acta 2003, 396, 119–132. [Google Scholar] [CrossRef]
- Sheng, S.-J.; Hu, X.; Wang, F.; Ma, Q.-Y.; Gu, M.-F. Mechanical and thermal property characterization of poly- l -lactide (PLLA) scaffold developed using pressure-controllable green foaming technology. Mater. Sci. Eng. C 2015, 49, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, B. Reversible crystallization and the rigid–amorphous phase in semicrystalline macromolecules. Prog. Polym. Sci. 2003, 28, 383–450. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Iannace, S.; Maffezzoli, A.; Leo, G.; Nicolais, L. Influence of crystal and amorphous phase morphology on hydrolytic degradation of PLLA subjected to different processing conditions. Polymer 2001, 42, 3799–3807. [Google Scholar] [CrossRef]
- Pal, A.K.; Katiyar, V. Melt processing of biodegradable poly(lactic acid)/functionalized chitosan nanocomposite films: Mechanical modeling with improved oxygen barrier and thermal properties. J. Polym. Res. 2017, 24, 1–21. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, D.-X.; Xu, J.-Z.; Huang, H.-D.; Lei, J.; Li, Z.-M. Highly crystallized poly (lactic acid) under high pressure. AIP Adv. 2012, 2, 042159. [Google Scholar] [CrossRef]
- Gaur, U.; Wunderlich, B. Advanced Thermal Analysis System (ATHAS) Polymer Heat Capacity Data Bank. In Computer Applications in Applied Polymer Science; American Chemical Society: Washington, DC, USA, 1982; Volume 197, pp. 355–366. ISBN 978-084-120-733-2. [Google Scholar]
- Pritchard, E.M.; Dennis, P.B.; Omenetto, F.; Naik, R.R.; Kaplan, D.L. Physical and chemical aspects of stabilization of compounds in silk. Biopolymers 2012, 97, 479–498. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Li, Y.; Yu, H.; Gu, Z. Comparative studies of structure, thermal decomposition mechanism and thermodynamic parameters of two kinds of silk fibroin films. Sci. Sin. Chim. 2019, 49, 1014–1029. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Wang, F.; Liu, Q.-C.; Ma, Q.-Y.; Gu, Z.-G. Structure and Kinetics of Thermal Decomposition Mechanism of Novel Silk Fibroin Films. Wu Li Hua Xue Xue Bao 2017, 33, 344–355. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhou, Y.; Wu, P.; Chen, H.; Trefonas, P. Hydrogen bond mediated partially miscible poly(N-acryloyl piperidine)/poly(acrylic acid) blend with one glass transition temperature. Polymer 2018, 151, 269–278. [Google Scholar] [CrossRef]
- Shi, X. Formation mechanism of solvent-induced porous PLA microspheres. Acta Polym. Sin. 2011, 11, 866. [Google Scholar] [CrossRef]
- Jin, H.-J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Vollrath, F.; Shao, Z. Predicting the mechanical properties of spider silk as a model nanostructured polymer. Eur. Phys. J. E Soft Matter. 2005, 16, 199–206. [Google Scholar] [CrossRef] [PubMed]
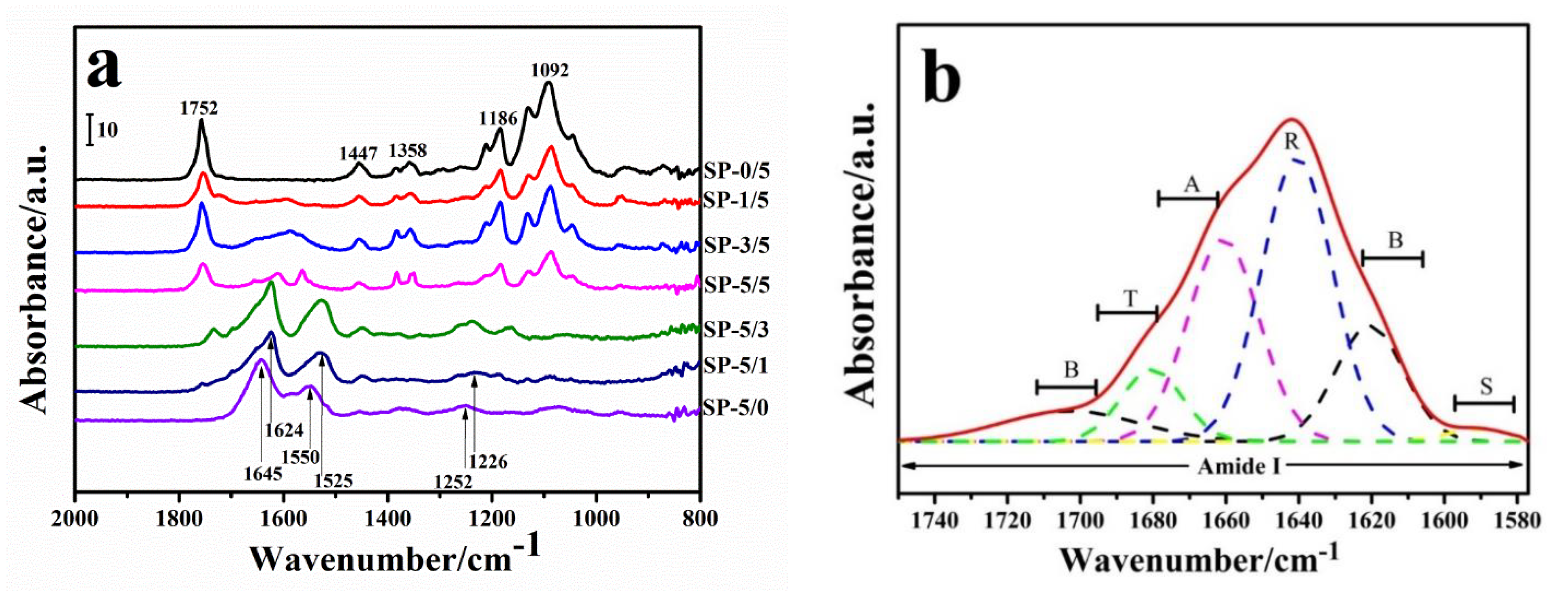

| Sample | β-Sheet (B) in Silk/ % | a-Helix & Random Coils in Silk/ % | Turns in Silk/ % | Side Chains in Silk/ % | Silk Amorphous in Sample/ % |
|---|---|---|---|---|---|
| SP-0/5 | / | / | / | / | / |
| SP-1/5 | 12.23/33.50 a | 73.89 | 12.56 | 1.32 | 11.08 |
| SP-3/5 | 13.48/42.80 a | 72.86 | 11.28 | 2.38 | 21.45 |
| SP-5/5 | 15.54/44.32 a | 71.03 | 10.79 | 2.64 | 27.84 |
| SP-5/3 | 17.03/45.03 a | 70.23 | 9.37 | 3.37 | 34.36 |
| SP-5/1 | 18.24/48.26 a | 68.99 | 9.56 | 3.21 | 43.10 |
| SP-5/0 | 23.29/58.98 a | 66.80 | 8.55 | 1.36 | 41.02 |
| Sample | SP-0/5 | SP-1/5 | SP-3/5 | SP-5/5 | SP-5/3 | SP-5/1 | SP-5/0 |
|---|---|---|---|---|---|---|---|
| SF content (%) | 0 | 16.7 | 37.5 | 50 | 62.5 | 83.3 | 100 |
| Tg (°C) | 55.81 | 58.68 | 62.31 | 65.02 | 72.03 | 74.33 | 154.32 |
| Tm (°C) | 150.29 | 150.01 | 148.93 | 147.11 | 145.57 | 144.98 | / |
| ΔHm (J·g−1) | 30.23 | 25.37 | 23.56 | 18.68 | 13.95 | 10.23 | / |
| ΔCP (J·g−1·°C−1) | 0.39 | 0.41 | 0.42 | 0.44 | 0.45 | 0.47 | / |
| XC-DSC | 0.33 | 0.27 | 0.24 | 0.19 | 0.15 | 0.11 | / |
| XMAP-DSC | 0.64 | 0.67 | 0.68 | 0.72 | 0.74 | 0.77 | / |
| XRAP-DSC | 0.03 | 0.06 | 0.08 | 0.09 | 0.11 | 0.12 | / |
| XC-XRD | 0.34 | 0.30 | 0.25 | 0.21 | 0.16 | 0.12 | / |
| XMAP-XRD | 0.62 | 0.64 | 0.68 | 0.70 | 0.73 | 0.77 | / |
| XRAP-XRD | 0.04 | 0.06 | 0.07 | 0.09 | 0.10 | 0.11 | / |
| Sample | SP-0/5 | SP-1/5 | SP-3/5 | SP-5/5 | SP-5/3 | SP-5/1 | SP-5/0 |
|---|---|---|---|---|---|---|---|
| SF content(%) | 0 | 16.7 | 37.5 | 50 | 62.5 | 83.3 | 100 |
| Tonset (°C) | 346.27 | 330.19 | 319.25 | 305.38 | 292.17 | 283.37 | 272.76 |
| Tp(°C) | 371.34 | 359.15 | 338.50 | 342.23 | 330.91 | 300.56 | 295.45 |
| ΔYw (%) | 0.42 | 1.73 | 4.48 | 4.80 | 5.52 | 6.07 | 6.99 |
| Y450 (%) | 0.58 | 1.02 | 1.89 | 7.15 | 18.10 | 46.14 | 48.77 |
| vP (wt %·°C−1) | 2.71 | 2.89 | 2.84 | 2.62 | 1.54 | 0.57 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, Y.; Gough, C.R.; Liu, Q.; Hu, X. Dual-Crystallizable Silk Fibroin/Poly(L-lactic Acid) Biocomposite Films: Effect of Polymer Phases on Protein Structures in Protein-Polymer Blends. Int. J. Mol. Sci. 2021, 22, 1871. https://doi.org/10.3390/ijms22041871
Wang F, Li Y, Gough CR, Liu Q, Hu X. Dual-Crystallizable Silk Fibroin/Poly(L-lactic Acid) Biocomposite Films: Effect of Polymer Phases on Protein Structures in Protein-Polymer Blends. International Journal of Molecular Sciences. 2021; 22(4):1871. https://doi.org/10.3390/ijms22041871
Chicago/Turabian StyleWang, Fang, Yingying Li, Christopher R. Gough, Qichun Liu, and Xiao Hu. 2021. "Dual-Crystallizable Silk Fibroin/Poly(L-lactic Acid) Biocomposite Films: Effect of Polymer Phases on Protein Structures in Protein-Polymer Blends" International Journal of Molecular Sciences 22, no. 4: 1871. https://doi.org/10.3390/ijms22041871
APA StyleWang, F., Li, Y., Gough, C. R., Liu, Q., & Hu, X. (2021). Dual-Crystallizable Silk Fibroin/Poly(L-lactic Acid) Biocomposite Films: Effect of Polymer Phases on Protein Structures in Protein-Polymer Blends. International Journal of Molecular Sciences, 22(4), 1871. https://doi.org/10.3390/ijms22041871








