Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age
Abstract
1. Introduction
2. Results
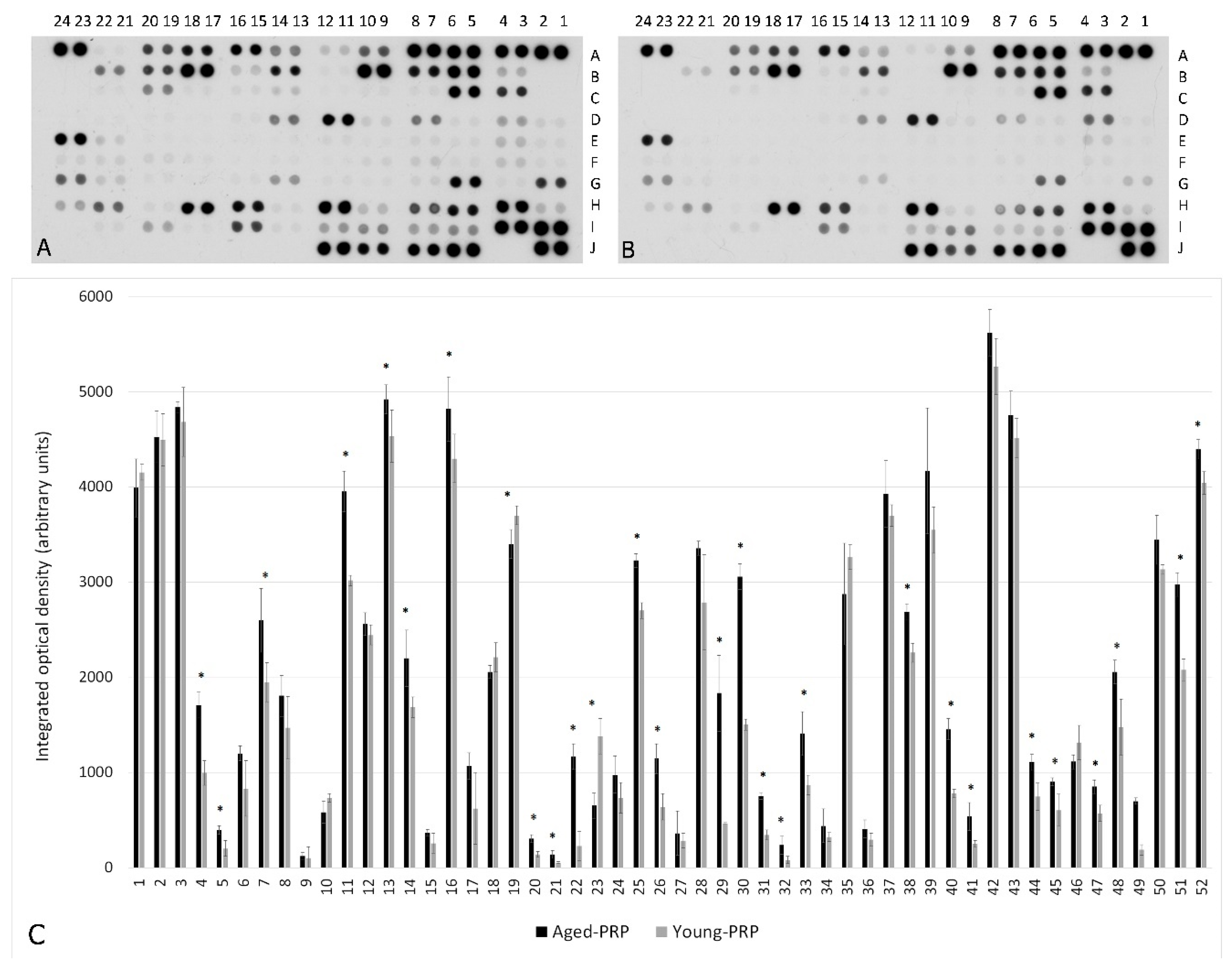
2.1. PRP Characterization: Growth Factors and Cytokine Measurement
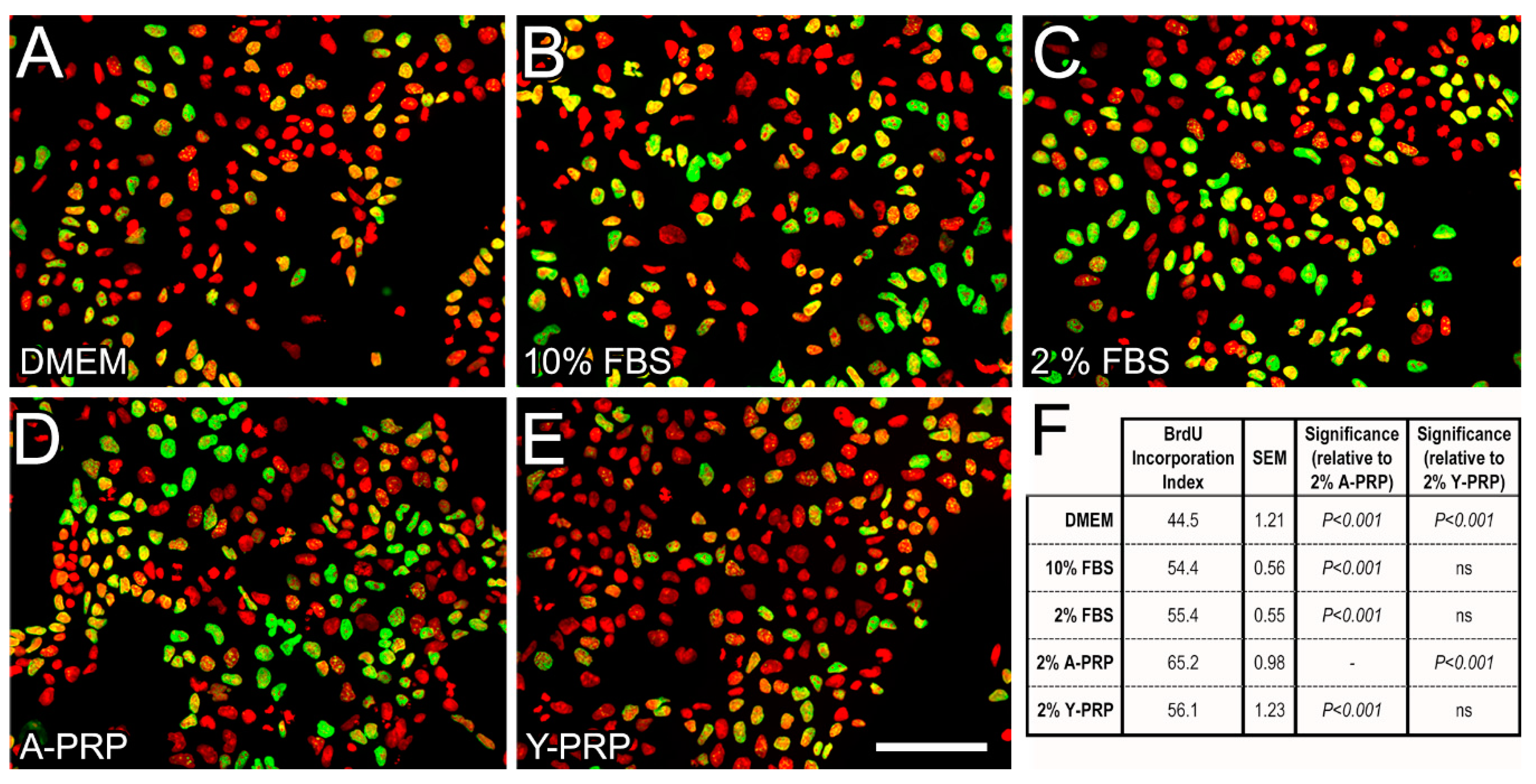
2.2. Effect of PRP on BrdU Incorporation in NT2 Progenitors
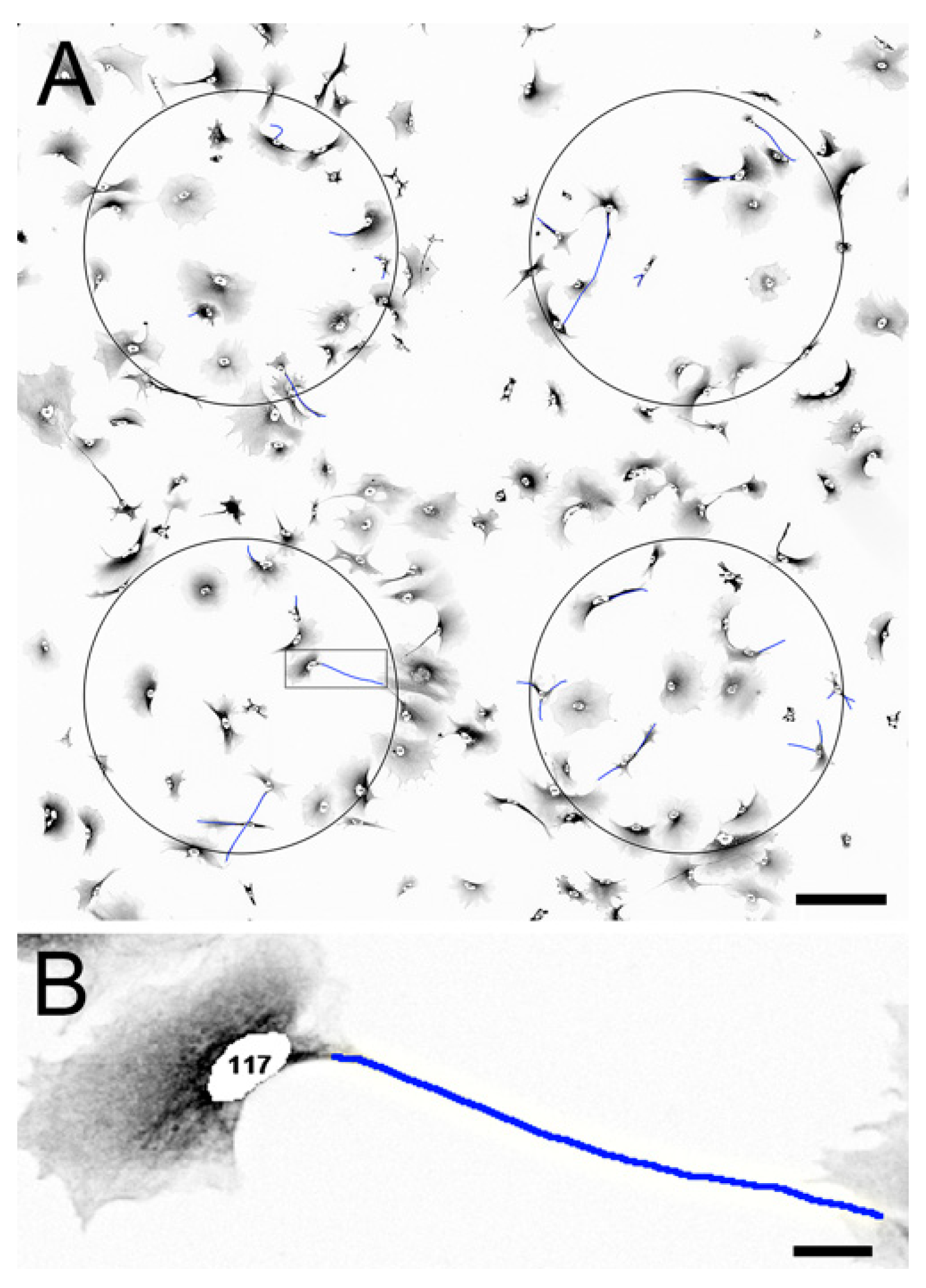
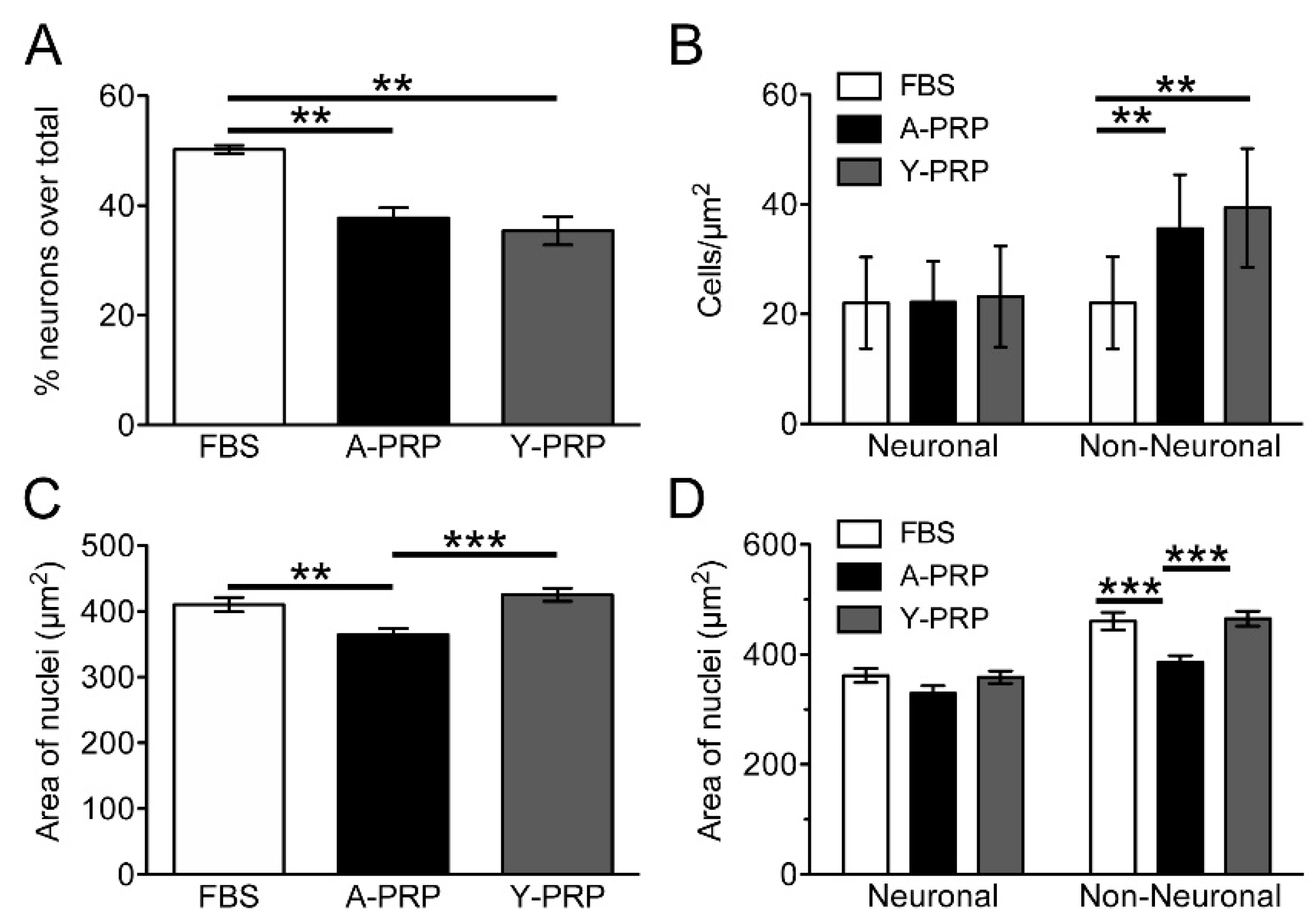
2.3. Effect of PRP on Cell Survival and Morphology of AraC-Differentiated NT2 Cells
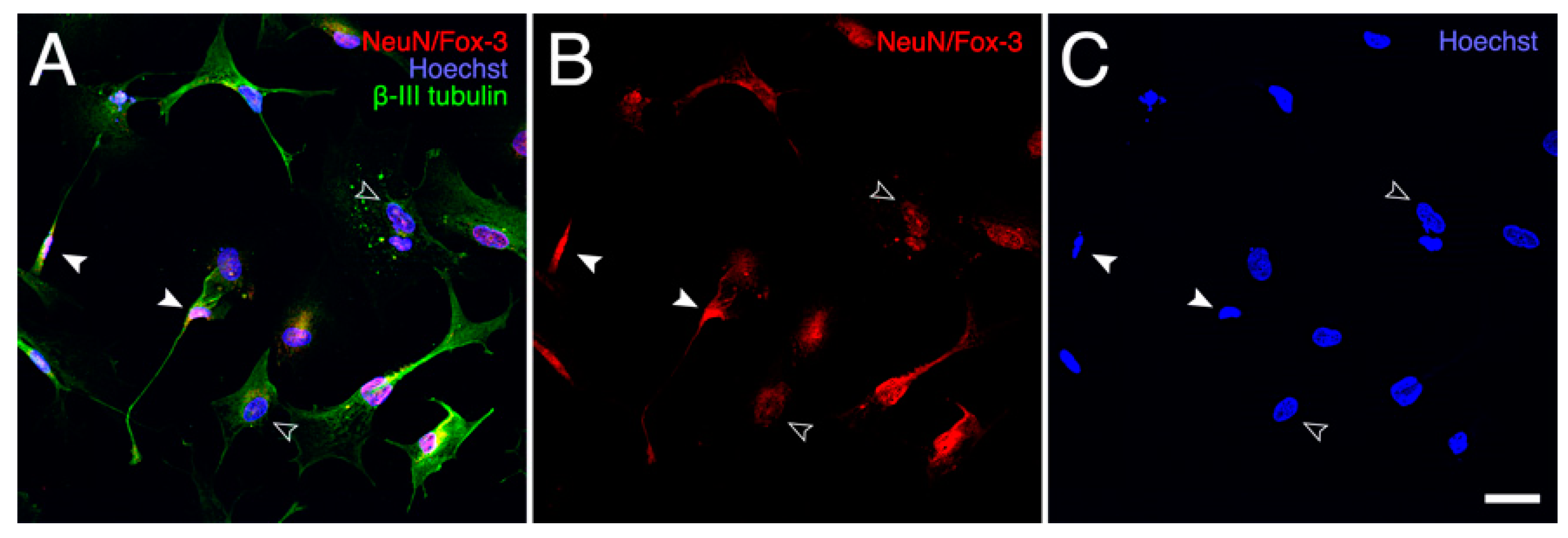
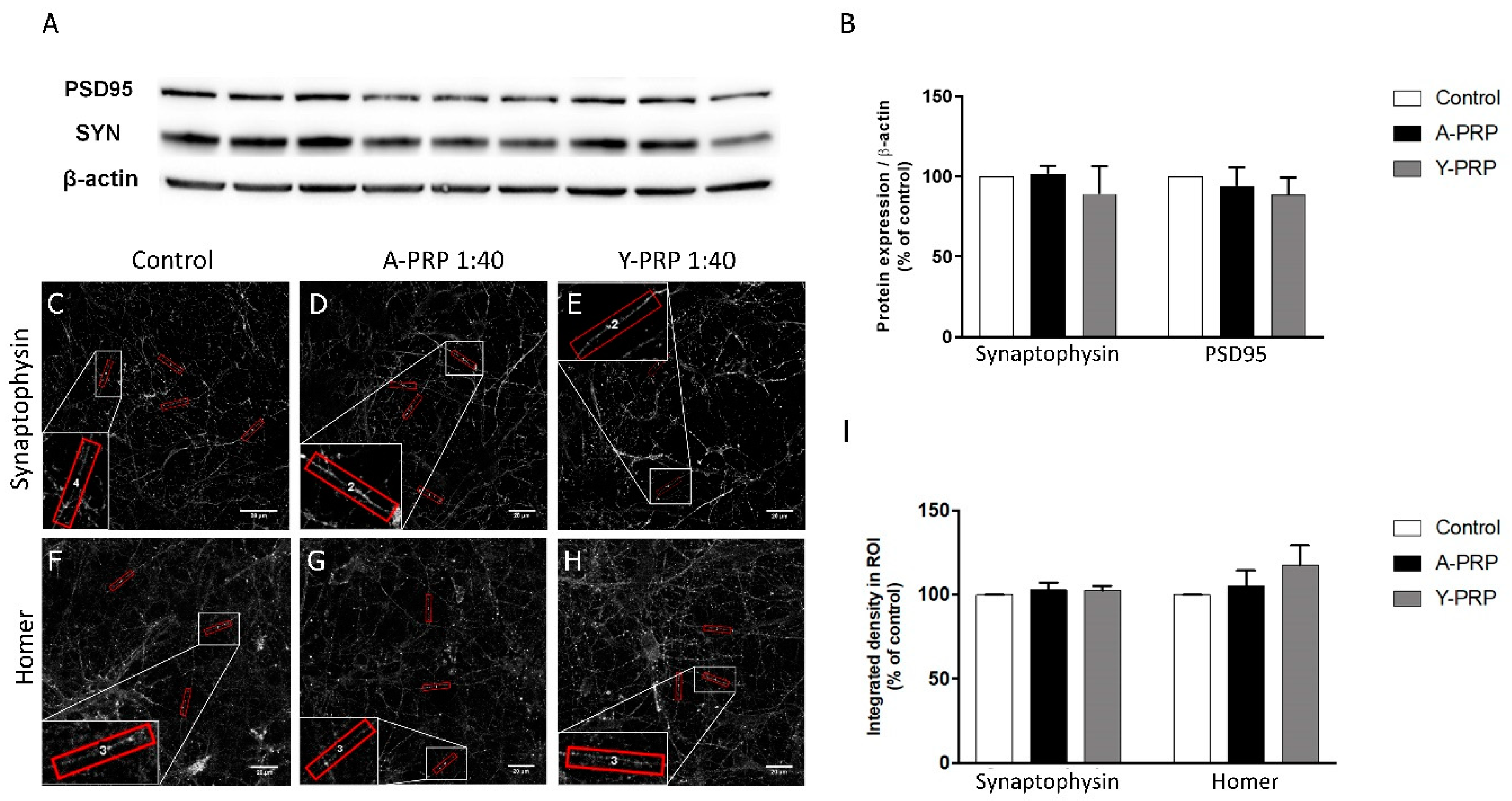
2.4. Effect of PRP on Toxicity and Quantification of Pre- and Postsynaptic Markers
2.5. Effect of PRP on Microglia Inflammation
3. Discussion
4. Materials and Methods
4.1. Platelet-Rich Plasma Preparation and Characterization
4.2. In Vitro Assays
4.2.1. Culture and Neuronal Differentiation of NT2 Cells
4.2.2. PRP Treatment, Imaging, and Analysis of BrdU Incorporation in NT2 Progenitor Cells
4.2.3. PRP Treatment, Imaging, and Analysis of AraC-Differentiated NT2 Cells
4.2.4. Neuronal Primary Cultures
4.2.5. Toxicity Assay
4.2.6. Western Blot for Synaptic Markers In Vitro
4.2.7. Immunofluorescence for Synaptic Markers In Vitro
4.2.8. Synaptic Marker Intensity and Puncta Analysis
4.2.9. Microglia Culture and Microglia Activation Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AraC | Pyrimidine nucleoside analogue cytosine β-D-arabinofuranoside |
| BrdU | 5-bromo-2′-deoxyuridine |
| CNS | Central nervous system |
| FBS | Fetal bovine serum |
| LPS | Lipopolysaccharide |
| NT-2 | Human teratocarcinoma NTERA2-D1 cells |
| PRP | Platelet-rich plasma |
| CCL-11 | C-C motif chemokine 11 |
| GDF-11 | Growth differentiation factor |
| G-CSF | Granulocyte colony-stimulating factor |
| HGF | Hepatocyte growth factor |
| IGF | Insulin-like growth factor |
| PDGF | Platelet-derived growth factor |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor |
Appendix A
Appendix A.1. BrdU Immunofluorescence Labeling
Appendix A.2. Immunofluorescence in NT2 Cells
Appendix A.3. Double Immunofluorescence Staining of AraC-Differentiated NT2 Cells
Appendix A.4. Imaging and Analysis of AraC-Differentiated NT2 Cells
References
- Le, A.D.K.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Platelet-rich plasma. Clin. Sports Med. 2019, 38, 17–44. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Delgado, D.; Sanchez, P.; Prado, R.; Orive, G.; Padilla, S. Platelet-rich plasma, a source of autologous growth factors and biomimetic scaffold for peripheral nerve regeneration. Expert Opin. Biol. Ther. 2017, 17, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-X.; Fan, Z.-H.; Zhao, J.-G.; Zhang, P. The Application of platelet-rich plasma may be a novel treatment for central nervous system diseases. Med. Hypotheses 2009, 73, 1038–1040. [Google Scholar] [CrossRef]
- Anitua, E.; Pascual, C.; Pérez-Gonzalez, R.; Antequera, D.; Padilla, S.; Orive, G.; Carro, E. Intranasal delivery of plasma and platelet growth factors using prgf-endoret system enhances neurogenesis in a mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e73118. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Pascual, C.; Pérez-Gonzalez, R.; Orive, G.; Carro, E. Intranasal PRGF-endoret enhances neuronal survival and attenuates NF-ΚB-dependent inflammation process in a mouse model of Parkinson’s disease. J. Control. Release 2015, 203, 170–180. [Google Scholar] [CrossRef]
- Kuffler, D.P. An assessment of current techniques for inducing axon regeneration and neurological recovery following peripheral nerve trauma. Prog. Neurobiol. 2014, 116, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stolle, M.; Schulze, J.; Roemer, A.; Lenarz, T.; Durisin, M.; Warnecke, A. Human Plasma rich in growth factors improves survival and neurite outgrowth of spiral ganglion neurons in vitro. Tissue Eng. Part A 2018, 24, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhu, Q.; Liu, X.; Huang, X.; He, C.; Jiang, L.; Quan, D.; Zhou, X.; Zhu, Z. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of schwann cells in vitro. J. Tissue Eng. Regen. Med. 2016, 10, 428–436. [Google Scholar] [CrossRef]
- Larsson, A.; Carlsson, L.; Gordh, T.; Lind, A.-L.; Thulin, M.; Kamali-Moghaddam, M. The effects of age and gender on plasma levels of 63 cytokines. J. Immunol. Methods 2015, 425, 58–61. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C.; Migliore, E.; Grandi, F.C.; Koltsov, J.; Lingampalli, N.; Cisar, C.; Indelli, P.F.; Sebastiano, V.; Robinson, W.H.; Bhutani, N.; et al. Platelet-rich plasma (PRP) from older males with knee osteoarthritis depresses chondrocyte metabolism and upregulates inflammation. J. Orthop. Res. 2019, 37, 1760–1770. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert. Opin. Biol. Ther. 2020, 1–14. [Google Scholar] [CrossRef]
- Sala, C.; Piëch, V.; Wilson, N.R.; Passafaro, M.; Liu, G.; Sheng, M. Regulation of dendritic spine morphology and synaptic function by shank and homer. Neuron 2001, 31, 115–130. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Yin, J. Platelet-rich plasma: A promising product for treatment of peripheral nerve regeneration after nerve injury. Int. J. Neurosci. 2011, 121, 176–180. [Google Scholar] [CrossRef]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; Garate, A.; Sánchez, P.; Bilbao, A.M.; García Del Caño, G.; Salles, J.; Sánchez, M. Biological and structural effects after intraosseous infiltrations of age-dependent platelet-rich plasma: An in vivo study. J. Orthop. Res. 2020, 38, 1931–1941. [Google Scholar] [CrossRef]
- Kizil, C.; Kyritsis, N.; Brand, M. Effects of Inflammation on stem cells: Together they strive? EMBO Rep. 2015, 16, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Covacu, R.; Brundin, L. Effects of neuroinflammation on neural stem cells. Neuroscientist 2017, 23, 27–39. [Google Scholar] [CrossRef]
- Whitney, N.P.; Eidem, T.M.; Peng, H.; Huang, Y.; Zheng, J.C. Inflammation mediates varying effects in neurogenesis: Relevance to the pathogenesis of brain injury and neurodegenerative disorders. J. Neurochem. 2009, 108, 1343–1359. [Google Scholar] [CrossRef]
- Geller, H.M.; Cheng, K.Y.; Goldsmith, N.K.; Romero, A.A.; Zhang, A.L.; Morris, E.J.; Grandison, L. Oxidative stress mediates neuronal DNA damage and apoptosis in response to cytosine arabinoside. J. Neurochem. 2001, 78, 265–275. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Chen, W.; Song, J.; Zhao, X.; Ouyang, Y.; Yuan, W.; Fan, C. Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Z.; Liu, D.; Zhao, P.; Liang, S.; Xu, K. Autologous platelet-rich plasma promotes neurogenic differentiation of human adipose-derived stem cells in vitro. Int. J. Neurosci. 2013, 123, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, L.; Guan, C.; Yang, X.; Hu, X.; Zhang, X.; Zhang, W. Effects of Platelet-Rich Plasma on Proliferation, Viability, and Odontogenic Differentiation of Neural Crest Stem-Like Cells Derived from Human Dental Apical Papilla. Available online: https://www.hindawi.com/journals/bmri/2020/4671989/ (accessed on 22 October 2020).
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet Rich Plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef]
- Mo, W.; Wu, J.; Qiu, Q.; Zhang, F.; Luo, H.; Xu, N.; Zhu, W.; Liang, M. Platelet-rich plasma inhibits osteoblast apoptosis and actin cytoskeleton disruption induced by gingipains through upregulating integrin Β. Cell Biol. Int. 2020. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Yu, T.-Y.; Chang, G.-J.; Lin, L.-P.; Lin, M.-S.; Pang, J.-H.S. Platelet-rich plasma releasate promotes regeneration and decreases inflammation and apoptosis of injured skeletal muscle. Am. J. Sports Med. 2018, 46, 1980–1986. [Google Scholar] [CrossRef]
- McMahon, S.S.; Dockery, P.; McDermott, K.W. Estimation of nuclear volume as an indicator of maturation of glial precursor cells in the developing rat spinal cord: A stereological approach. J. Anat. 2003, 203, 339–344. [Google Scholar] [CrossRef]
- Grosch, M.; Ittermann, S.; Rusha, E.; Greisle, T.; Ori, C.; Truong, D.-J.J.; O’Neill, A.C.; Pertek, A.; Westmeyer, G.G.; Drukker, M. Nucleus size and DNA accessibility are linked to the regulation of paraspeckle formation in cellular differentiation. BMC Biol. 2020, 18, 42. [Google Scholar] [CrossRef]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- Augustin, H.; McGourty, K.; Steinert, J.R.; Cochemé, H.M.; Adcott, J.; Cabecinha, M.; Vincent, A.; Halff, E.F.; Kittler, J.T.; Boucrot, E.; et al. Myostatin-like proteins regulate synaptic function and neuronal morphology. Development 2017, 144, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lei, X.X.; Xuan, L.; Tang, J.B.; Cheng, B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets 2019, 30, 773–792. [Google Scholar] [CrossRef]
- Sominsky, L.; De Luca, S.; Spencer, S.J. Microglia: Key players in neurodevelopment and neuronal plasticity. Int. J. Biochem. Cell Biol. 2018, 94, 56–60. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-F.; Sung, C.-S.; Wen, Z.-H.; Chen, C.-H.; Feng, C.-W.; Hung, H.-C.; Yang, S.-N.; Tsui, K.-H.; Chen, W.-F. Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Bluthé, R.-M.; Kelley, K.W.; Dantzer, R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav. Immun. 2006, 20, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Ruiz, C.R.; Lebson, L.; Selenica, M.-L.B.; Rizer, J.; Hunt, J.B.; Rojiani, R.; Reid, P.; Kammath, S.; Nash, K.; et al. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol. Aging 2013, 34, 1610–1620. [Google Scholar] [CrossRef]
- Noh, M.Y.; Lim, S.M.; Oh, K.-W.; Cho, K.-A.; Park, J.; Kim, K.-S.; Lee, S.-J.; Kwon, M.-S.; Kim, S.H. Mesenchymal stem cells modulate the functional properties of microglia via TGF-β secretion. Stem. Cells Transl. Med. 2016, 5, 1538–1549. [Google Scholar] [CrossRef]
- Anitua, E.; Prado, R.; Sánchez, M.; Orive, G. Platelet-rich plasma: Preparation and formulation. Oper. Tech. Orthop. 2012, 22, 25–32. [Google Scholar] [CrossRef]
- Sánchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Aduriz Recalde, E.; Garcia Gutierrez, A.; Albillos, J.; Gárate, R.; Aguirre, J.J.; Padilla, S.; et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 2012, 28, 1070–1078. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Detection of mycoplasma contamination in cell cultures. Curr. Protoc. Mol. Biol. 2014, 106, 28.4.1–28.4.14. [Google Scholar] [CrossRef]
- Alberdi, E.; Sánchez-Gómez, M.V.; Cavaliere, F.; Pérez-Samartín, A.; Zugaza, J.L.; Trullas, R.; Domercq, M.; Matute, C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 2010, 47, 264–272. [Google Scholar] [CrossRef]
- Almeida, C.G.; Tampellini, D.; Takahashi, R.H.; Greengard, P.; Lin, M.T.; Snyder, E.M.; Gouras, G.K. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005, 20, 187–198. [Google Scholar] [CrossRef]
- Domercq, M.; Sánchez-Gómez, M.V.; Sherwin, C.; Etxebarria, E.; Fern, R.; Matute, C. System Xc- and Glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J. Immunol. 2007, 178, 6549–6556. [Google Scholar] [CrossRef]




| n | Mean ± S.D. (Years) | Age Range (Years) | |
|---|---|---|---|
| Aged | 12 | 73.71 ± 5.00 | (65–85) |
| Young | 8 | 22.13 ± 1.81 | (20–25) |
| Aged PRP | Young PRP | |
|---|---|---|
| 1. PRP Preparation | ||
| Initial blood volume | 81 mL per subject (9 mL per tube) | 81 mL per subject (9 mL per tube) |
| Anticoagulant | Sodium citrate 3.8% (wt/V) | Sodium citrate 3.8% (wt/V) |
| System | Close | Close |
| Centrifugation | Yes | Yes |
| number | 1 | 1 |
| Speed | 580 g—8 min | 580 g—8 min |
| Final PRP volume | 18 mL per subject | 18 mL per subject |
| 2. PRP Characteristics | ||
| PRP Type | 12-00-11 | 12-00-11 |
| MPV | 10.4 ± 0.5 fL | 9.6 ± 0.5 fL |
| Red Blood Cells | <0.01 × 106/µL | <0.01 × 106/µL |
| White Blood Cells | <0.05 × 106/µL | <0.05 × 106/µL |
| Neutrophils | --- | --- |
| Lymphocytes | --- | --- |
| Monocytes | --- | --- |
| Eosinophils | --- | --- |
| Basophils | --- | --- |
| Activation | CaCl2 (10% wt/vol) | CaCl2 (10% wt/vol) |
| 3. Application Characteristics | ||
| Dose | 2% | 2% |
| Direct/Indirect | Direct | Direct |
| Cell line | CNS cells | CNS cells |
| 4. Other Remarkable PRP and Study Features | ||
| The product added to the cell cultures was the platelet lysate obtained after activation of PRP with calcium chloride (10%) | ||
| Growth Factors and Cytokines | Aged PRP Mean ± S.D. (pg/mL) | Young PRP Mean ± S.D. (pg/mL) | p |
|---|---|---|---|
| CCL-11 | 72.51 ± 19.00 | 46.75 ± 13.56 | 0.022 * |
| GDF-11 | 0 | 169.04 ± 6.25 | <0.001 *** |
| G-CSF | 209.91 ± 88.92 | 143.82 ± 69.10 | 0.181 |
| HGF | 1101.45 ± 44.09 | 699.33 ± 5.66 | 0.001 ** |
| IGF | 53,036.72 ± 9254.97 | 106,320.62 ± 10,470.11 | <0.001 *** |
| PDGF | 2834.75 ± 427.59 | 3908.59 ± 705.04 | 0.016 * |
| TGF-β | 34,732.53 ± 3657.98 | 42,332.98 ± 4958.67 | 0.025 * |
| TNF-α | 17.55 ± 0.98 | 18.10 ± 1.75 | 0.600 |
| Number | Coordinate | Analyte | Aged PRP Group (Mean ± S.D.) (IOD) | Young PRP Group (Mean ± S.D.) (IOD) | p Value | Number | Coordinate | Analyte | Aged PRP Group (Mean ± S.D.) (IOD) | Young PRP Group (Mean ± S.D.) (IOD) | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A3, A4 | Adiponectin | 3989.26 ± 304.47 | 4154.84 ± 80.55 | 0.333 | 27 | E3, E4 | IL-5 | 364.37 ± 230.30 | 286.47 ± 77.85 | 0.545 |
| 2 | A5, A6 | Apolipoprotein A-I | 4524.69 ± 269.84 | 4493.79 ± 272.99 | 0.877 | 28 | E23, E24 | IL-18 Bpa | 3355.77 ± 75.21 | 2785.41 ± 499.27 | 0.064 |
| 3 | A7, A8 | Angiogenin | 4835.93 ± 61.23 | 4682.65 ± 364.25 | 0.438 | 29 | G1, G2 | Leptin | 1832.24 ± 397.51 | 468.69 ± 3.88 | <0.001 * |
| 4 | A9, A10 | Angiopoietin-1 | 1705.54 ± 134.90 | 995.56 ± 128.65 | <0.001 * | 30 | G5, G6 | Lipocalin-2 | 3055.21 ± 136.35 | 1501.53 ± 60.29 | <0.001 * |
| 5 | A11, A12 | Angiopoietin-2 | 397.67 ± 46.55 | 203.83 ± 81.25 | 0.006 * | 31 | G13, G14 | MIF | 752.34 ± 36.06 | 344.14 ± 52.74 | <0.001 * |
| 6 | A13, A14 | BAFF | 1199.89 ± 75.92 | 833.94 ± 289.97 | 0.050 | 32 | G21, G22 | MIP-3β | 239.42 ± 93.85 | 81.19 ± 37.61 | 0.020 * |
| 7 | A17, A18 | C5/C5a | 2596.91 ± 336.86 | 1945.71 ± 203.49 | 0.016 * | 33 | G23, G24 | MMP-9 | 1409.28 ± 229.17 | 871.01 ± 102.66 | 0.005 * |
| 8 | A19, A20 | CD14 | 1803.53 ± 217.21 | 1471.18 ± 329.11 | 0.142 | 34 | H1, H2 | Myeloperoxidase | 439.91 ± 177.34 | 322.67 ± 45.61 | 0.247 |
| 9 | A21, A22 | CD30 | 124.93 ± 37.07 | 101.39 ± 118.70 | 0.718 | 35 | H3, H4 | Osteopontin | 2872.29 ± 534.43 | 3260.3 ± 129.74 | 0.088 |
| 10 | B3, B4 | CD40 ligand | 585.14 ± 117.44 | 735.12 ± 44.84 | 0.054 | 36 | H9, H10 | Pentraxin 3 | 407.46 ± 93.16 | 293.89 ± 69.21 | 0.098 |
| 11 | B5, B6 | Chitinase 3-like 1 | 3953.46 ± 211.37 | 3015.32 ± 54.49 | <0.001 * | 37 | H11, H12 | PF4 | 3927.03 ± 350.75 | 3697.05 ± 114.58 | 0.263 |
| 12 | B7, B8 | Complement Factor D | 2556.74 ± 118.44 | 2444.56 ± 105.74 | 0.207 | 38 | H15, H16 | RANTES | 2685.81 ± 85.30 | 2257.70 ± 98.68 | 0.001 * |
| 13 | B9, B10 | C-Reactive Protein | 4920.45 ± 152.84 | 4531.85 ± 273.50 | 0.047 * | 39 | H17, H18 | RBP-4 | 4169.65 ± 662.79 | 3547.42 ± 242.99 | 0.128 |
| 14 | B11, B12 | Cripto-1 | 2199.56 ± 296.10 | 1683.65 ± 108.46 | 0.016 * | 40 | H21, H22 | Resistin | 1452.82 ± 110.10 | 781.19 ± 43.31 | <0.001 * |
| 15 | B15, B16 | Dkk-1 | 369.85 ± 32.40 | 259.23 ± 104.68 | 0.090 | 41 | H23, H24 | SDF-1α | 540.59 ± 146.77 | 252.61 ± 35.56 | 0.008 * |
| 16 | B17, B18 | DPPIV | 4820.59 ± 337.84 | 4297.65 ± 253.27 | 0.047 * | 42 | I1, I2 | Serpin E1 | 5621.19 ± 241.19 | 5263.41 ± 297.57 | 0.110 |
| 17 | B21, B22 | Emmprin | 1067.68 ± 137.51 | 622.89 ± 376.77 | 0.068 | 43 | I3, I4 | SHBG | 4755.72 ± 249.73 | 4513.15 ± 206.17 | 0.184 |
| 18 | C3, C4 | ENA-78 | 2057.37 ± 66.20 | 2209.68 ± 150.08 | 0.112 | 44 | I5, I6 | ST2 | 1107.68 ± 81.92 | 746.5 ± 144.46 | 0.004 * |
| 19 | C5, C6 | Endoglin | 3398.28 ± 152.91 | 3700.11 ± 98.83 | 0.016 * | 45 | I7, I8 | TARC | 905.01 ± 42.82 | 603.39 ± 169.32 | 0.013 * |
| 20 | C13, C14 | FGF-19 | 305.75 ± 42.74 | 142.15 ± 29.98 | <0.001 * | 46 | I9, I10 | TFF3 | 1112.75 ± 73.10 | 1314.63 ± 179.57 | 0.082 |
| 21 | C15, C16 | Flt-3 Ligand | 139.46 ± 42.29 | 54.35 ± 8.87 | 0.007 * | 47 | I11, I12 | TfR | 852.16 ± 73.98 | 571.47 ± 85.82 | 0.002 * |
| 22 | C19, C20 | GDF-15 | 1166.25 ± 133.84 | 230.15 ± 153.84 | <0.001 * | 48 | I15, I16 | Thrombospondin1 | 2055.38 ± 123.63 | 1473.03 ± 297.34 | 0.025 * |
| 23 | D3, D4 | Growth Hormone | 652.69 ± 130.68 | 1380.33 ± 187.64 | <0.001 * | 49 | I19, I20 | uPAR | 700.47 ± 33.23 | 189.58 ± 56.00 | 0.001 * |
| 24 | D7, D8 | ICAM-1 | 978.39 ± 193.86 | 733.12 ± 160.05 | 0.098 | 50 | J7, J8 | CD31 | 3444.59 ± 256.88 | 3132.6 ± 49.38 | 0.054 |
| 25 | D11, D12 | IGFBP-2 | 3227.19 ± 71.56 | 2700.56 ± 82.07 | <0.001 * | 51 | J9, J10 | TIM-3 | 2973.56 ± 122.94 | 2075.5 ± 114.34 | <0.001 * |
| 26 | D13, D14 | IGFBP-3 | 1146.28 ± 155.31 | 640.54 ± 138.50 | 0.002 * | 52 | J11, J12 | VCAM-1 | 4394.29 ± 100.97 | 4041.8 ± 116.81 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, D.; Bilbao, A.M.; Beitia, M.; Garate, A.; Sánchez, P.; González-Burguera, I.; Isasti, A.; López De Jesús, M.; Zuazo-Ibarra, J.; Montilla, A.; et al. Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age. Int. J. Mol. Sci. 2021, 22, 1725. https://doi.org/10.3390/ijms22041725
Delgado D, Bilbao AM, Beitia M, Garate A, Sánchez P, González-Burguera I, Isasti A, López De Jesús M, Zuazo-Ibarra J, Montilla A, et al. Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age. International Journal of Molecular Sciences. 2021; 22(4):1725. https://doi.org/10.3390/ijms22041725
Chicago/Turabian StyleDelgado, Diego, Ane Miren Bilbao, Maider Beitia, Ane Garate, Pello Sánchez, Imanol González-Burguera, Amaia Isasti, Maider López De Jesús, Jone Zuazo-Ibarra, Alejandro Montilla, and et al. 2021. "Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age" International Journal of Molecular Sciences 22, no. 4: 1725. https://doi.org/10.3390/ijms22041725
APA StyleDelgado, D., Bilbao, A. M., Beitia, M., Garate, A., Sánchez, P., González-Burguera, I., Isasti, A., López De Jesús, M., Zuazo-Ibarra, J., Montilla, A., Domercq, M., Capetillo-Zarate, E., García del Caño, G., Sallés, J., Matute, C., & Sánchez, M. (2021). Effects of Platelet-Rich Plasma on Cellular Populations of the Central Nervous System: The Influence of Donor Age. International Journal of Molecular Sciences, 22(4), 1725. https://doi.org/10.3390/ijms22041725







