Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo
Abstract
1. Introduction
2. Results
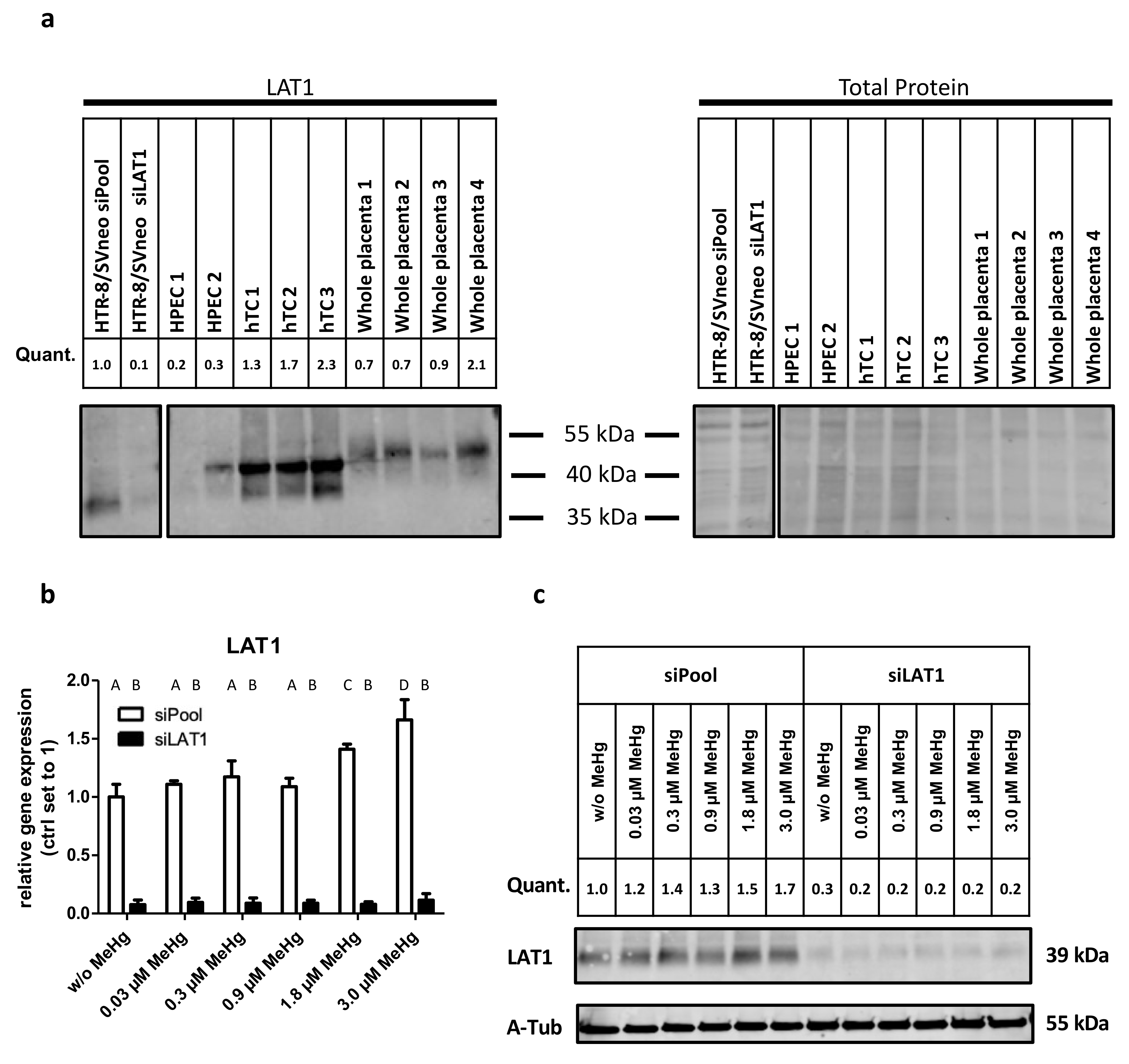
2.1. LAT1 Protein Expression in the Placenta and Verification of siRNA Mediated Gene Knockdown of LAT1 in HTR-8/SVneo Cells
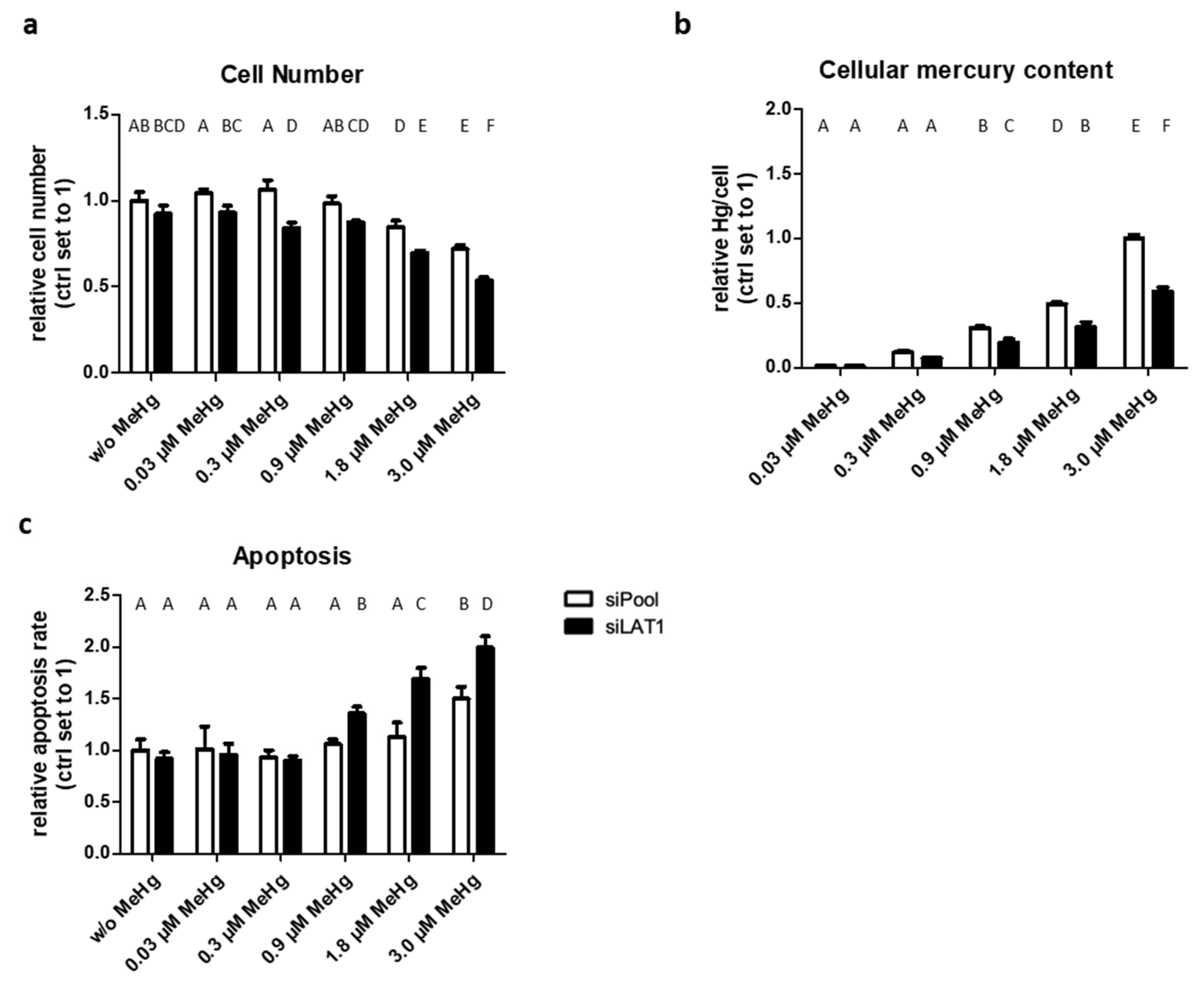
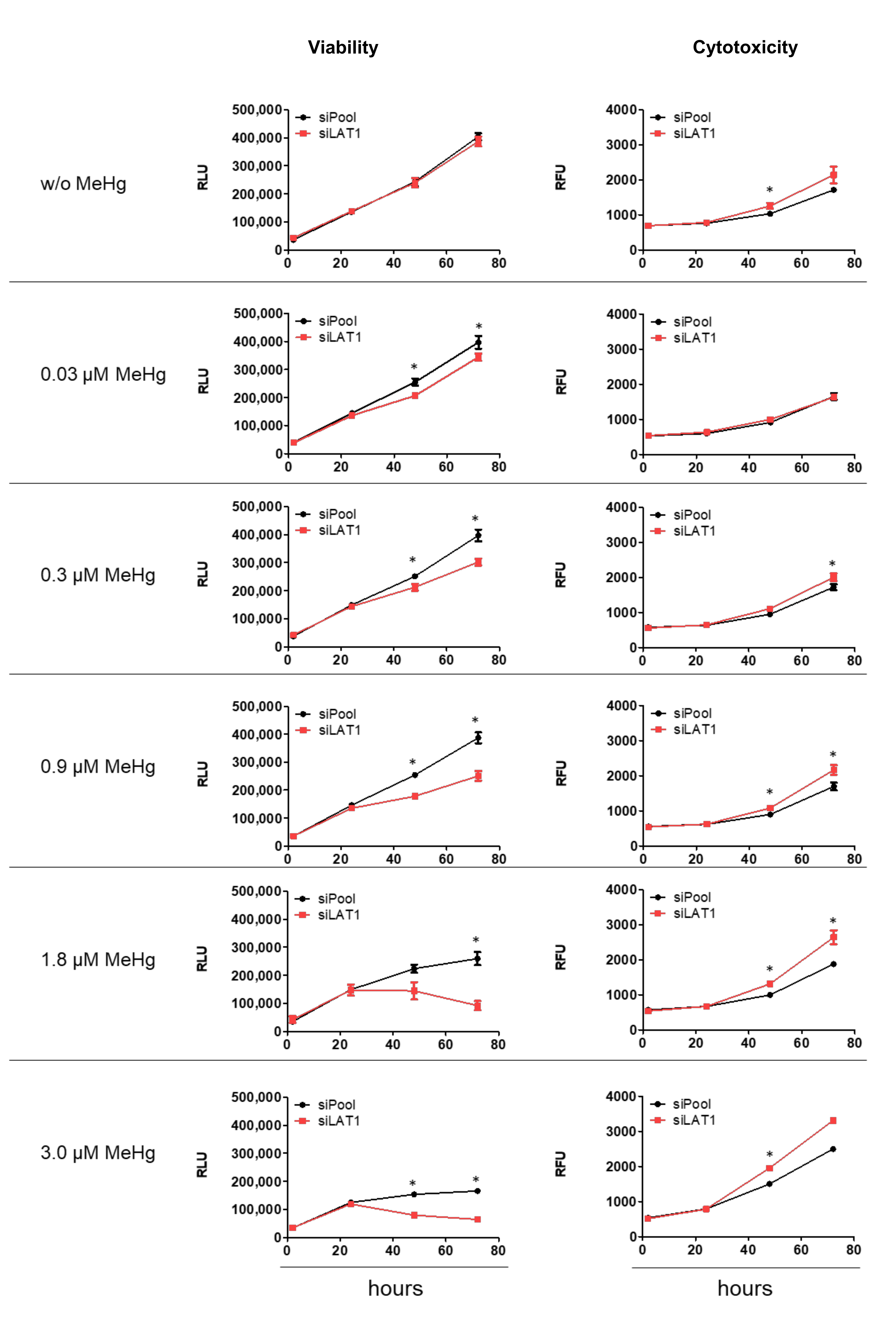
2.2. LAT1 Downregulation of MeHg-Treated HTR-8/SVneo Cells Decreases Cellular Hg Content and Cell Viability but Increases Cytotoxicity and Apoptosis
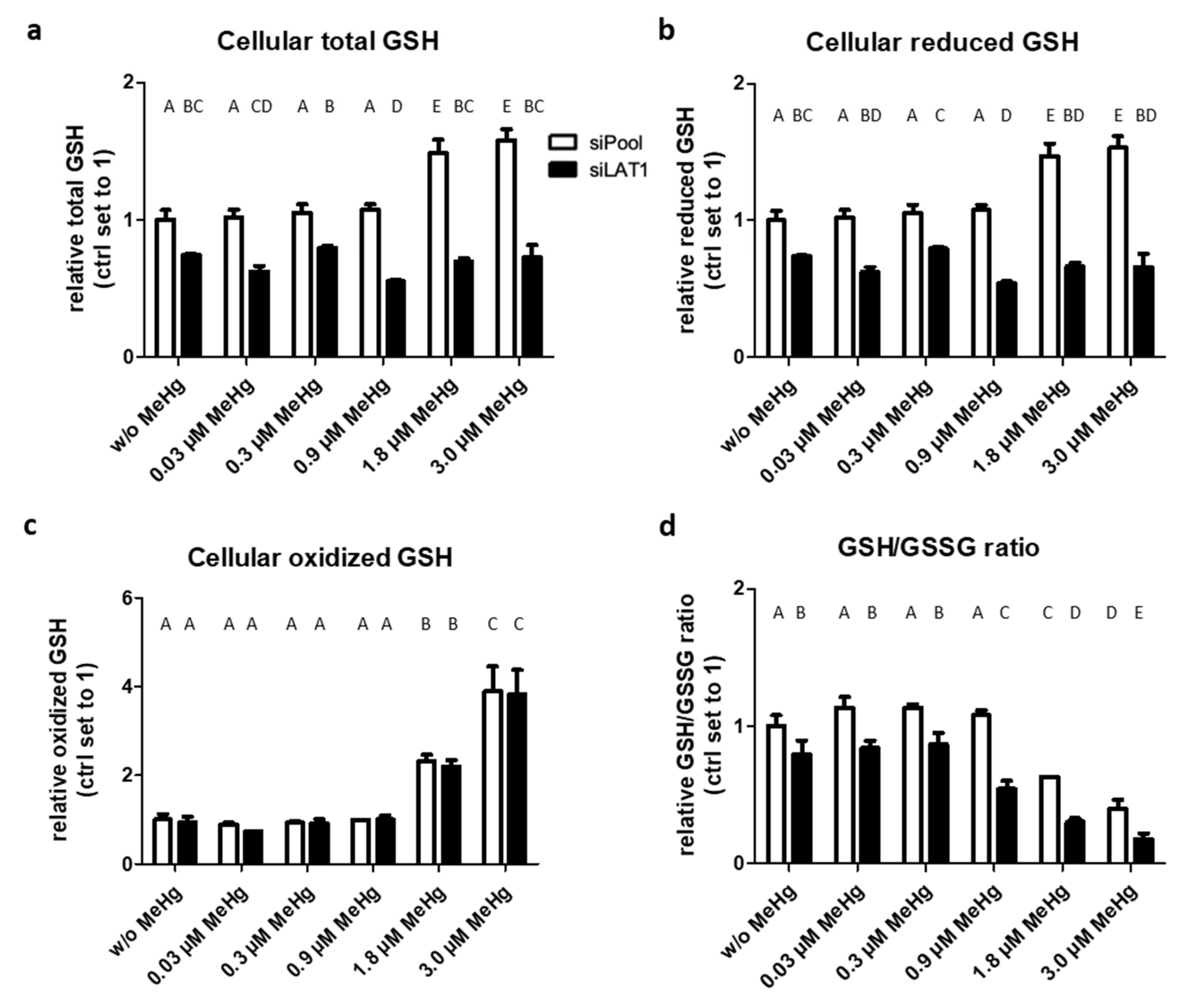
2.3. LAT1 Knockdown Decreases GSH Levels and Promotes Oxidative Stress
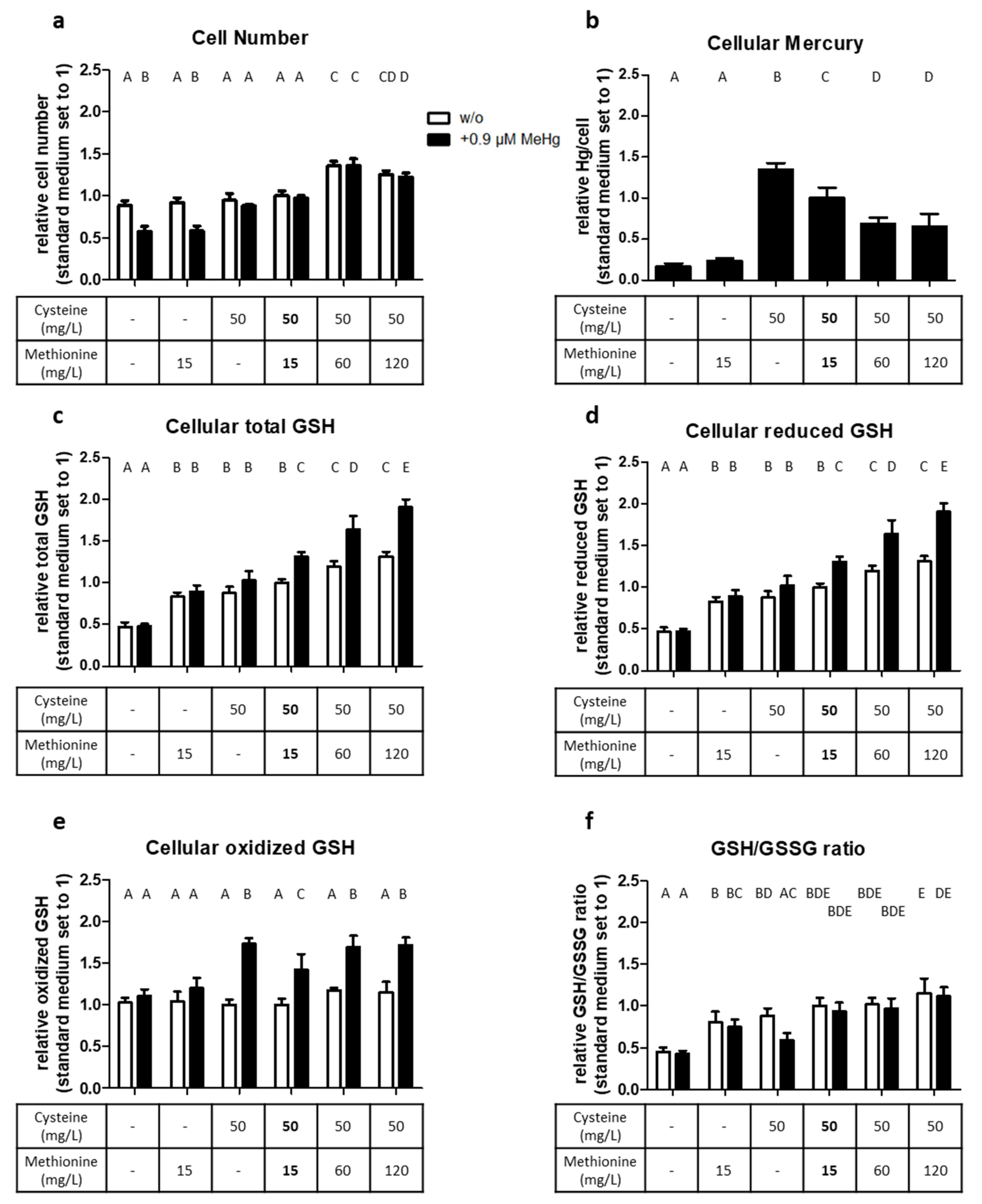
2.4. Cysteine and Methionine Availability Affect the Oxidative Stress Response
3. Discussion
3.1. LAT1 Is Required for MeHg Uptake but also for Protection from the Metal’s Toxicity
3.2. Cysteine and Methionine Are Required for GSH Synthesis
4. Material and Methods
4.1. Cell Culture
4.2. Methylmercury (MeHg) Dosages
4.3. siRNA Mediated Knockdown
4.4. Analysis of Total Hg
4.5. Cytotoxicity and Cell Viability Assays
4.6. Apoptosis Assay
4.7. GSH/GSSG Assay
4.8. RNA Isolation, cDNA Synthesis and Quantitative PCR
4.9. Immunoblotting
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grillo, M.A.; Lanza, A.; Colombatto, S. Transport of amino acids through the placenta and their role. Amino Acids 2008, 34, 517–523. [Google Scholar] [CrossRef]
- Kanai, Y.; Endou, H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J. Toxicol. Sci. 2003, 28, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Jiang, H.; Syversen, T.; Rocha, J.B.; Farina, M.; Aschner, M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J. Neurochem. 2008, 107, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Straka, E.; Ellinger, I.; Balthasar, C.; Scheinast, M.; Schatz, J.; Szattler, T.; Bleichert, S.; Saleh, L.; Knofler, M.; Zeisler, H.; et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology 2016, 340, 34–42. [Google Scholar] [CrossRef]
- Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a neurotoxicant by molecular mimicry: The methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem. J. 2002, 367 Pt 1, 239–246. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef]
- Sheehan, M.C.; Burke, T.A.; Navas-Acien, A.; Breysse, P.N.; McGready, J.; Fox, M.A. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: A systematic review. Bull. World Health Organ. 2014, 92, 254–269F. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, Y.M.; Lee, K.E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strahle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef]
- Guzzi, G.; La Porta, C.A. Molecular mechanisms triggered by mercury. Toxicology 2008, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Grandjean, P.; Herz, K.T. Methylmercury and brain development: Imprecision and underestimation of developmental neurotoxicity in humans. Mt. Sinai J. Med. 2011, 78, 107–118. [Google Scholar] [CrossRef]
- Grandjean, P.; Herz, K.T. Trace elements as paradigms of developmental neurotoxicants: Lead, methylmercury and arsenic. J. Trace Elem. Med. Biol. 2015, 31, 130–134. [Google Scholar] [CrossRef]
- Gupta, R.C.; Milatovic, D.; Lall, R.; Srivastava, A. Mercury. In Veterinary Toxicology; Academic Press: Washington, DC, USA, 2018; pp. 455–462. [Google Scholar]
- Gundacker, C.; Neesen, J.; Straka, E.; Ellinger, I.; Dolznig, H.; Hengstschlager, M. Genetics of the human placenta: Implications for toxicokinetics. Arch. Toxicol. 2016, 90, 2563–2581. [Google Scholar] [CrossRef]
- Huppertz, B. The anatomy of the normal placenta. J. Clin. Pathol. 2008, 61, 1296–1302. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Vasallo, M.D.; Aragones, N.; Pollan, M.; Lopez-Abente, G.; Perez-Gomez, B. Mercury, cadmium, and lead levels in human placenta: A systematic review. Environ. Health Perspect. 2012, 120, 1369–1377. [Google Scholar] [CrossRef]
- Balthasar, C.; Stangl, H.; Widhalm, R.; Granitzer, S.; Hengstschlager, M.; Gundacker, C. Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter. Int. J. Mol. Sci. 2017, 18, 1730. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129285. [Google Scholar] [CrossRef]
- Granitzer, S.; Ellinger, I.; Khan, R.; Gelles, K.; Widhalm, R.; Hengstschlager, M.; Zeisler, H.; Desoye, G.; Tupova, L.; Ceckova, M.; et al. In vitro function and in situ localization of Multidrug Resistance-associated Protein (MRP)1 (ABCC1) suggest a protective role against methyl mercury-induced oxidative stress in the human placenta. Arch. Toxicol. 2020, 94, 3799–3817. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, A.R.; Jarmer, S.; Harbison, R.D. Heavy Metal Inhibition of Carnitine Acetyltransferase Activity in Human Placental Syncytiottophoblast: Possible Site of Action of HgC12, CHSHgCI, and CdClz. Teratog. Carcinog. Mutagenes. 1986, 6, 351–360. [Google Scholar] [CrossRef]
- Tucker, E.K.; Nowak, R.A. Methylmercury alters proliferation, migration, and antioxidant capacity in human HTR8/SV-neo trophoblast cells. Reprod. Toxicol. 2018, 78, 60–68. [Google Scholar] [CrossRef]
- Daher, B.; Vucetic, M.; Pouyssegur, J. Cysteine Depletion, a Key Action to Challenge Cancer Cells to Ferroptotic Cell Death. Front. Oncol. 2020, 10, 723. [Google Scholar] [CrossRef]
- Lin, J.; Raoof, D.A.; Thomas, D.G.; Greenson, J.K.; Giordano, T.J.; Robinson, G.S.; Bourner, M.J.; Bauer, C.T.; Orringer, M.B.; Beer, D.G. L-type amino acid transporter-1 overexpression and melphalan sensitivity in Barrett’s adenocarcinoma. Neoplasia 2004, 6, 74–84. [Google Scholar] [CrossRef]
- Ihara, H.; Kasamatsu, S.; Kitamura, A.; Nishimura, A.; Tsutsuki, H.; Ida, T.; Ishizaki, K.; Toyama, T.; Yoshida, E.; Abdul Hamid, H.; et al. Exposure to Electrophiles Impairs Reactive Persulfide-Dependent Redox Signaling in Neuronal Cells. Chem. Res. Toxicol. 2017, 30, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tsui, M.T.; Lee, E.; Fowler, J.; Jia, Z. Uptake, efflux, and toxicity of inorganic and methyl mercury in the endothelial cells (EA.hy926). Sci. Rep. 2020, 10, 9023. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lopez, M.E.; Costa-Malaquias, A.; Oliveira, E.H.; Miranda, M.S.; Arrifano, G.P.; Souza-Monteiro, J.R.; Sagica, F.E.; Fontes-Junior, E.A.; Maia, C.S.; Macchi, B.M.; et al. Is Low Non-Lethal Concentration of Methylmercury Really Safe? A Report on Genotoxicity with Delayed Cell Proliferation. PLoS ONE 2016, 11, e0162822. [Google Scholar] [CrossRef] [PubMed]
- Pieper, I.; Wehe, C.A.; Bornhorst, J.; Ebert, F.; Leffers, L.; Holtkamp, M.; Hoseler, P.; Weber, T.; Mangerich, A.; Burkle, A.; et al. Mechanisms of Hg species induced toxicity in cultured human astrocytes: Genotoxicity and DNA-damage response. Metallomics 2014, 6, 662–671. [Google Scholar] [CrossRef]
- Ou, L.; Chen, L.; Chen, C.; Yang, T.; Wang, H.; Tong, Y.; Hu, D.; Zhang, W.; Long, W.; Wang, X. Associations of methylmercury and inorganic mercury between human cord blood and maternal blood: A meta-analysis and its application. Environ. Pollut. 2014, 191, 25–30. [Google Scholar] [CrossRef]
- Ask, K.; Akesson, A.; Berglund, M.; Vahter, M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ. Health Perspect. 2002, 110, 523–526. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta BBA Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Yothaisong, S.; Namwat, N.; Yongvanit, P.; Khuntikeo, N.; Puapairoj, A.; Jutabha, P.; Anzai, N.; Tassaneeyakul, W.; Tangsucharit, P.; Loilome, W. Increase in L-type amino acid transporter 1 expression during cholangiocarcinogenesis caused by liver fluke infection and its prognostic significance. Parasitol. Int. 2017, 66, 471–478. [Google Scholar] [CrossRef]
- Usuki, F.; Fujimura, M.; Yamashita, A. Endoplasmic reticulum stress preconditioning modifies intracellular mercury content by upregulating membrane transporters. Sci. Rep. 2017, 7, 12390. [Google Scholar] [CrossRef]
- Poncet, N.; Mitchell, F.E.; Ibrahim, A.F.; McGuire, V.A.; English, G.; Arthur, J.S.; Shi, Y.B.; Taylor, P.M. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS ONE 2014, 9, e89547. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Huttunen, J.; Montaser, A.; Huttunen, K.M. Hemocompatible LAT1-inhibitor can induce apoptosis in cancer cells without affecting brain amino acid homeostasis. Apoptosis 2020, 25, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Sakamoto, S.; Ando, K.; Maimaiti, M.; Takeshita, N.; Okunushi, K.; Reien, Y.; Imamura, Y.; Sazuka, T.; Nakamura, K.; et al. Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma. Sci. Rep. 2019, 9, 16776. [Google Scholar] [CrossRef] [PubMed]
- Hansson, S.R.; Naav, A.; Erlandsson, L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front. Physiol. 2014, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Dhingra, R.; Arya, D.S.; Kalaivani, M.; Bhatla, N.; Kumar, R. Role of oxidative stress markers and antioxidants in the placenta of preeclamptic patients. J. Obstet. Gynaecol. Res. 2010, 36, 1189–1194. [Google Scholar] [CrossRef]
- Aiko, Y.; Askew, D.J.; Aramaki, S.; Myoga, M.; Tomonaga, C.; Hachisuga, T.; Suga, R.; Kawamoto, T.; Tsuji, M.; Shibata, E. Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC Pregnancy Childbirth 2014, 14, 181. [Google Scholar] [CrossRef]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef]
- Cosco, J.; Scalise, M.; Colas, C.; Galluccio, M.; Martini, R.; Rovella, F.; Mazza, T.; Ecker, G.F.; Indiveri, C. ATP modulates SLC7A5 (LAT1) synergistically with cholesterol. Sci. Rep. 2020, 10, 16738. [Google Scholar] [CrossRef]
- Prasad, P.D.; Wang, H.; Huang, W.; Kekuda, R.; Rajan, D.P.; Leibach, F.H.; Ganapathy, V. Human LAT1, a subunit of system L amino acid transporter: Molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999, 255, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, W.; Tan, D.; Liang, H.; Zhang, Q.; Tian, N.; Cao, K.; Tan, Y.; Ma, J.; Han, R. Positive regulation of placentation by L-amino acid transporter-1 (lat1) in pregnant mice. Int. J. Clin. Exp. Pathol. 2017, 10, 9551–9558. [Google Scholar]
- Ohgaki, R.; Ohmori, T.; Hara, S.; Nakagomi, S.; Kanai-Azuma, M.; Kaneda-Nakashima, K.; Okuda, S.; Nagamori, S.; Kanai, Y. Essential Roles of L-Type Amino Acid Transporter 1 in Syncytiotrophoblast Development by Presenting Fusogenic 4F2hc. Mol. Cell. Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.C.; Thomas, R.L.; Pavisic, J.; James, S.J.; Ulrich, C.M.; Nijhout, H.F. A mathematical model of glutathione metabolism. Theor. Biol. Med. Model. 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Chen, H.W.; Sheen, L.Y.; Lii, C.K. Methionine and cysteine affect glutathione level, glutathione-related enzyme activities and the expression of glutathione S-transferase isozymes in rat hepatocytes. J. Nutr. 1997, 127, 2135–2141. [Google Scholar] [CrossRef]
- Chrostowski, M.K.; McGonnigal, B.G.; Stabila, J.P.; Padbury, J.F. Role of the L-amino acid transporter-1 (LAT-1) in mouse trophoblast cell invasion. Placenta 2010, 31, 528–534. [Google Scholar] [CrossRef]
- Roos, D.H.; Puntel, R.L.; Farina, M.; Aschner, M.; Bohrer, D.; Rocha, J.B.; de Vargas Barbosa, N.B. Modulation of methylmercury uptake by methionine: Prevention of mitochondrial dysfunction in rat liver slices by a mimicry mechanism. Toxicol. Appl. Pharmacol. 2011, 252, 28–35. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Yasutake, A.; Adachi, T.; Hirayama, K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch. Toxicol. 1996, 70, 310–314. [Google Scholar] [CrossRef]
- Lang, I.; Pabst, M.A.; Hiden, U.; Blaschitz, A.; Dohr, G.; Hahn, T.; Desoye, G. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur. J. Cell Biol. 2003, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Scheinast, M.; Damjanovic, L.; Fuchs, C.; Rosner, M.; Hengstschlager, M. Proliferation potential of human amniotic fluid stem cells differently responds to mercury and lead exposure. Amino Acids 2012, 43, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.; Siegel, N.; Fuchs, C.; Slabina, N.; Dolznig, H.; Hengstschlager, M. Efficient siRNA-mediated prolonged gene silencing in human amniotic fluid stem cells. Nat. Protoc. 2010, 5, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redox potential of GSH/GSSG couple: Assay and biological significance. Methods Enzymol. 2002, 348, 93–112. [Google Scholar]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
| Cell Type | Species | MeHg (µM) | Exposure Time | Cell Number Reduction | Reference |
|---|---|---|---|---|---|
| HTR-8/SVneo | human | 3.0 | 72 h | ~40% | [21] |
| HTR-8/SVneo | human | 4.6 | 72 h | ~50% | [23] |
| HTR-8/SVneo | human | 7.8 | 24 h | ~30% | [23] |
| BeWo cells | human | 3.0 | 24 h | ~30% | [4] |
| Cerebellar granule neurons | rat | 1.9 | 24 h | ~50% | [26] |
| SH-SY5Y | human | 2.8 | 24 h | ~50% | [26] |
| Endothelial cells | human | 4.1 | 24 h | ~50% | [27] |
| Glioma cells | rat | 6.1 | 24 h | ~40% | [28] |
| P12 cells | rat | 14.3 | 24 h | ~40% | [28] |
| Astrocytes | human | 15.0 | 24 h | ~30% | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granitzer, S.; Widhalm, R.; Forsthuber, M.; Ellinger, I.; Desoye, G.; Hengstschläger, M.; Zeisler, H.; Salzer, H.; Gundacker, C. Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo. Int. J. Mol. Sci. 2021, 22, 1707. https://doi.org/10.3390/ijms22041707
Granitzer S, Widhalm R, Forsthuber M, Ellinger I, Desoye G, Hengstschläger M, Zeisler H, Salzer H, Gundacker C. Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo. International Journal of Molecular Sciences. 2021; 22(4):1707. https://doi.org/10.3390/ijms22041707
Chicago/Turabian StyleGranitzer, Sebastian, Raimund Widhalm, Martin Forsthuber, Isabella Ellinger, Gernot Desoye, Markus Hengstschläger, Harald Zeisler, Hans Salzer, and Claudia Gundacker. 2021. "Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo" International Journal of Molecular Sciences 22, no. 4: 1707. https://doi.org/10.3390/ijms22041707
APA StyleGranitzer, S., Widhalm, R., Forsthuber, M., Ellinger, I., Desoye, G., Hengstschläger, M., Zeisler, H., Salzer, H., & Gundacker, C. (2021). Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo. International Journal of Molecular Sciences, 22(4), 1707. https://doi.org/10.3390/ijms22041707








