HIV-1 Tat Activates Akt/mTORC1 Pathway and AICDA Expression by Downregulating Its Transcriptional Inhibitors in B Cells
Abstract
1. Introduction
2. Results
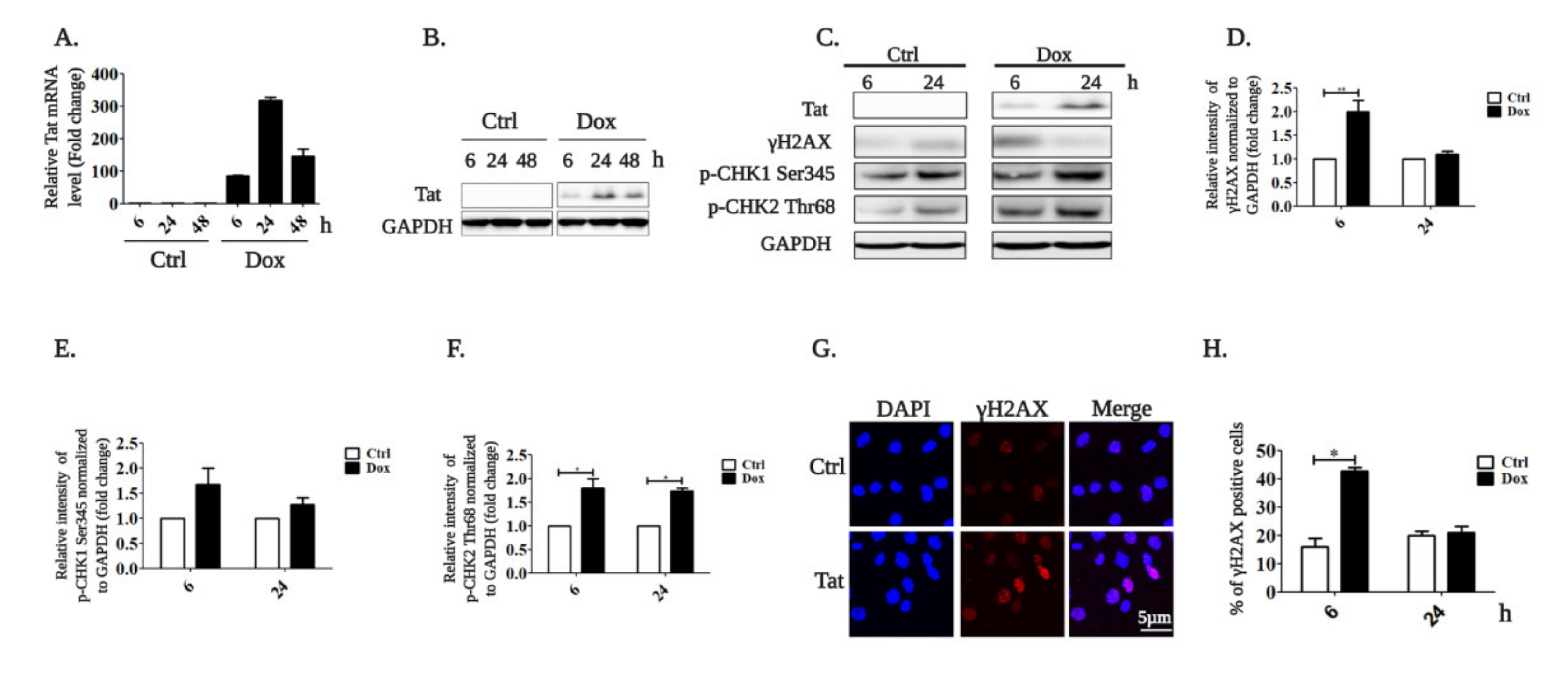
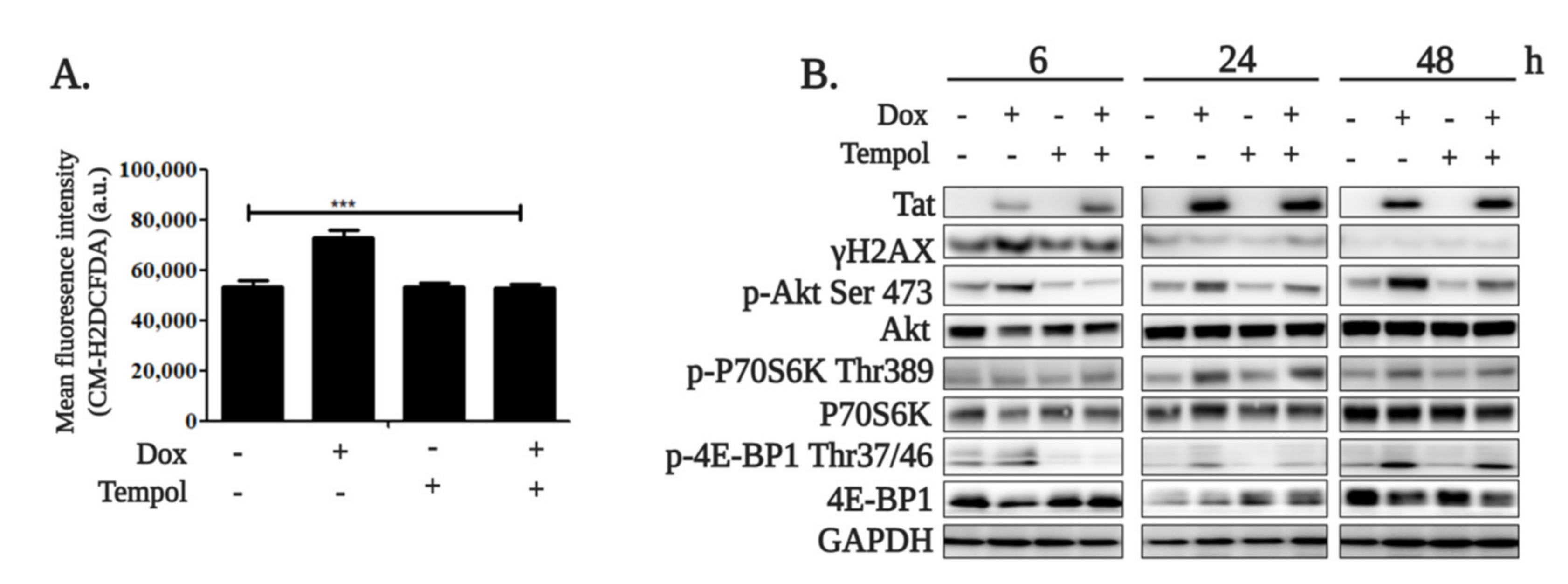
2.1. HIV-1 Tat Activates the AKT/mTORC1 Pathway in B Cells as a Response to ROS-Induced DNA Damage
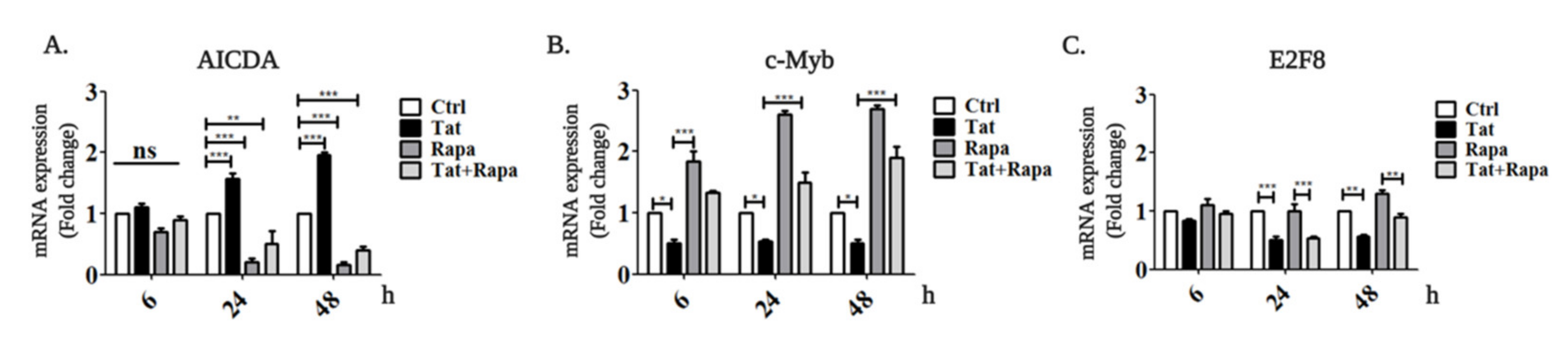
2.2. Tat Induces AICDA Expression by Downregulating c-Myb and E2F8 in an mTORC1-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Blood Samples
4.2. Treatments
4.3. Western Blotting
4.4. ROS Production Analysis
4.5. qRT-PCR
4.6. Immunofluorescence
4.7. Microscope Image Acquisition and Analysis
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayton, A.G.S.; Craig, A.; Rosen, W.C.G.; Haseltine, W.A. The Trans-Activator Gene of the Human T Cell Lymphotropic Virus Type III Is Required for Replication. Cell 1986, 44, 941–947. [Google Scholar] [CrossRef]
- Hauber, J.; Perkins, A.; Heimer, E.P.; Cullen, B.R. Trans-Activation of Human Immunodeficiency Virus Gene Expression Is Mediated by Nuclear Events. Proc. Natl. Acad. Sci. USA 1987, 84, 6364–6368. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Samaniego, F.; Nair, B.C.; Buonaguro, L.; Ensoli, B. HIV-1 Tat Protein Exits from Cells via a Leaderless Secretory Pathway and Binds to Extracellular Matrix-Associated Heparan Sulfate Proteoglycans through Its Basic Region. AIDS 1997, 11, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Hightower, G.K.; Wong, J.K.; Heaton, R.; Woods, S.; Grant, I.; Marcotte, T.D.; Ellis, R.J.; Letendre, S.L.; Collier, A.C.; et al. Genetic Features of Cerebrospinal Fluid-Derived Subtype B HIV-1 Tat. J. Neurovirol. 2012, 18, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, M.O.; Frank, R.; Ochsenbauer, C.; Stricker, K.; Dhein, J.; Walczak, H.; Debating, K.M.; Krammer, P.H. Sensitization of T Cells to CD95-Mediated Apoptosis by HIV-1 Tat and Gp120. Nature 1995, 375, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Neuveut, C.; Tiffany, H.L.; Benkirane, M.; Rich, E.A.; Murphy, P.M.; Jeang, K.-T. Selective CXCR4 Antagonism by Tat: Implications for in Vivo Expansion of Coreceptor Use by HIV-1. Proc. Natl. Acad. Sci. USA 2000, 97, 11466–11471. [Google Scholar] [CrossRef]
- Croxford, S.; Kitching, A.; Desai, S.; Kall, M.; Edelstein, M.; Skingsley, A.; Burns, F.; Copas, A.; Brown, A.E.; Sullivan, A.K.; et al. Mortality and Causes of Death in People Diagnosed with HIV in the Era of Highly Active Antiretroviral Therapy Compared with the General Population: An Analysis of a National Observational Cohort. Lancet Public Health 2017, 2, e35–e46. [Google Scholar] [CrossRef]
- Nishijima, T.; Inaba, Y.; Kawasaki, Y.; Tsukada, K.; Teruya, K.; Kikuchi, Y.; Gatanaga, H.; Oka, S. Mortality and Causes of Death in People Living with HIV in the Era of Combination Antiretroviral Therapy Compared with the General Population in Japan. AIDS 2020, 34, 913–921. [Google Scholar] [CrossRef]
- Akbay, B.; Shmakova, A.; Vassetzky, Y.; Dokudovskaya, S. Modulation of MTORC1 Signaling Pathway by HIV-1. Cells 2020, 9, 1090. [Google Scholar] [CrossRef]
- Shiels, M.S.; Engels, E.A. Evolving Epidemiology of HIV-Associated Malignancies. Curr. Opin. HIV AIDS 2017, 12, 6–11. [Google Scholar] [CrossRef]
- Shmakova, A.; Germini, D.; Vassetzky, Y. HIV-1, HAART and Cancer: A Complex Relationship. Int. J. Cancer 2020, 146, 2666–2679. [Google Scholar] [CrossRef]
- Germini, D.; Tsfasman, T.; Klibi, M.; El-Amine, R.; Pichugin, A.; Iarovaia, O.V.; Bilhou-Nabera, C.; Subra, F.; Bou Saada, Y.; Sukhanova, A.; et al. HIV Tat Induces a Prolonged MYC Relocalization next to IGH in Circulating B-Cells. Leukemia 2017, 31, 2515–2522. [Google Scholar] [CrossRef]
- Dolcetti, R.; Giagulli, C.; He, W.; Selleri, M.; Caccuri, F.; Eyzaguirre, L.M.; Mazzuca, P.; Corbellini, S.; Campilongo, F.; Marsico, S.; et al. Role of HIV-1 Matrix Protein P17 Variants in Lymphoma Pathogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, 14331–14336. [Google Scholar] [CrossRef]
- Wang, X.; Duan, Z.; Yu, G.; Fan, M.; Scharff, M.D. Human Immunodeficiency Virus Tat Protein Aids V Region Somatic Hypermutation in Human B Cells. mBio 2018, 9, e02315-17. [Google Scholar] [CrossRef]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.N.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat Protein Induces DNA Damage in Human Peripheral Blood B-Lymphocytes via Mitochondrial ROS Production. Redox Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef]
- Ágnes Márk, M.H.; Zsófia, V.; Sticz, T.B.; Nagy, N.; Csomor, J.; Berczi, L.; Varga, V.; Csóka, M.; Sebestyén, A.; Kopper, L. Characteristic MTOR Activity in Hodgkin-Lymphomas Offers a Potential Therapeutic Target in High Risk Disease—A Combined Tissue Microarray, in Vitro and in Vivo Study. BMC Cancer 2013, 13. [Google Scholar] [CrossRef]
- Browne, S.H.; Diaz-Perez, J.A.; Preziosi, M.; King, C.C.; Jones, G.A.; Jain, S.; Sun, X.; Reid, E.G.; Vandenberg, S.; Wang, H.Y. MTOR Activity in AIDS-Related Diffuse Large B-Cell Lymphoma. PLoS ONE 2017, 12, e0170771. [Google Scholar] [CrossRef] [PubMed]
- Col, J.D.; Zancai, P.; Terrin, L.; Guidoboni, M.; Ponzoni, M.; Pavan, A.; Spina, M.; Bergamin, S.; Rizzo, S.; Tirelli, U.; et al. Distinct Functional Significance of Akt and MTOR Constitutive Activation in Mantle Cell Lymphoma. Blood 2008, 111, 5142–5151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Salem, M.; Raghunath, P.N.; Marzec, M.; Liu, X.; Kasprzycka, M.; Robertson, E.; Wasik, M.A. Activation of MTORC1 Signaling Pathway in AIDS-Related Lymphomas. Am. J. Pathol. 2009, 175, 817–824. [Google Scholar] [CrossRef][Green Version]
- Sebestyén, A.; Sticz, T.B.; Márk, Á.; Hajdu, M.; Timár, B.; Nemes, K.; Nagy, N.; Váradi, Z.; Kopper, L. Activity and Complexes of MTOR in Diffuse Large B-Cell Lymphomas—A Tissue Microarray Study. Mod. Pathol. 2012, 25, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Sekihara, K.; Saitoh, K.; Han, L.; Ciurea, S.; Yamamoto, S.; Kikkawa, M.; Kazuno, S.; Taka, H.; Kaga, N.; Arai, H.; et al. Targeting Mantle Cell Lymphoma Metabolism and Survival through Simultaneous Blockade of MTOR and Nuclear Transporter Exportin-1. Oncotarget 2017, 8, 34552–34564. [Google Scholar] [CrossRef]
- Le Sage, V.; Cinti, A.; Amorim, R.; Mouland, A. Adapting the Stress Response: Viral Subversion of the MTOR Signaling Pathway. Viruses 2016, 8, 152. [Google Scholar] [CrossRef]
- Ma, Y.; Vassetzky, Y.; Dokudovskaya, S. MTORC1 Pathway in DNA Damage Response. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1293–1311. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H.; Asara, J.M.; Manning, B.D. MTORC1 Induces Purine Synthesis through Control of the Mitochondrial Tetrahydrofolate Cycle. Science 2016, 351, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Hudson, C.C.; Sekulić, A.; Williams, J.M.; Hosoi, H.; Houghton, P.J.; Lawrence, J.C.; Abraham, R.T. Phosphorylation of the Translational Repressor PHAS-I by the Mammalian Target of Rapamycin. Science 1997, 277, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.D.; Jefferson, L.S.; Kimball, S.R. Role of P70S6K1-Mediated Phosphorylation of EIF4B and PDCD4 Proteins in the Regulation of Protein Synthesis. J. Biol. Chem. 2012, 287, 42890–42899. [Google Scholar] [CrossRef] [PubMed]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a Metabolic Gene Regulatory Network Downstream of MTOR Complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, A.; Sonenberg, N. EIF4G Dramatically Enhances the Binding of EIF4E to the MRNA 5′-Cap Structure. J. Biol. Chem. 1997, 272, 21677–21680. [Google Scholar] [CrossRef]
- Linke, M.; Fritsch, S.D.; Sukhbaatar, N.; Hengstschläger, M.; Weichhart, T. MTORC1 and MTORC2 as Regulators of Cell Metabolism in Immunity. Febs. Lett. 2017, 591, 3089–3103. [Google Scholar] [CrossRef]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of Immune Responses by MTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef]
- Iwata, T.N.; Ramírez, J.A.; Tsang, M.; Park, H.; Margineantu, D.H.; Hockenbery, D.M.; Iritani, B.M. Conditional Disruption of Raptor Reveals an Essential Role for MTORC1 in B Cell Development, Survival, and Metabolism. J. Immunol. 2016, 197, 2250–2260. [Google Scholar] [CrossRef]
- Jones, D.D.; Weiss, B.M.; Allman, D.; Jones, D.D.; Gaudette, B.T.; Wilmore, J.R.; Chernova, I.; Bortnick, A.; Weiss, B.M.; Allman, D. MTOR Has Distinct Functions in Generating versus Sustaining Humoral Immunity. J. Clin. Investig. 2016, 126, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Raybuck, A.L.; Cho, S.H.; Li, J.; Rogers, M.C.; Lee, K.; Williams, C.L.; Shlomchik, M.; Thomas, J.W.; Chen, J.; Williams, J.V.; et al. B Cell–Intrinsic MTORC1 Promotes Germinal Center–Defining Transcription Factor Gene Expression, Somatic Hypermutation, and Memory B Cell Generation in Humoral Immunity. J. Immunol. 2018, 200, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pruitt, M.; Tran, D.; Du Bois, W.; Zhang, K.; Patel, R.; Hoover, S.; Simpson, R.M.; Simmons, J.; Gary, J.; et al. B Cell–Specific Deficiencies in MTOR Limit Humoral Immune Responses. J. Immunol. 2013, 191, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Teater, M.; Dominguez, P.M.; Redmond, D.; Chen, Z.; Ennishi, D.; Scott, D.W.; Cimmino, L.; Ghione, P.; Chaudhuri, J.; Gascoyne, R.D.; et al. AICDA Drives Epigenetic Heterogeneity and Accelerates Germinal Center-Derived Lymphomagenesis. Nat. Commun. 2018, 9, 222. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhao, M.; Hu, H.; Huang, H. Activation-Induced Cytidine Deaminase Overexpression in Double-Hit Lymphoma: Potential Target for Novel Anticancer Therapy. Sci. Rep. 2020, 10, 14164. [Google Scholar] [CrossRef]
- Sall, F.B.; El Amine, R.; Markozashvili, D.; Tsfasman, T.; Oksenhendler, E.; Lipinski, M.; Vassetzky, Y.; Germini, D. HIV-1 Tat Protein Induces Aberrant Activation of AICDA in Human B-lymphocytes from Peripheral Blood. J. Cell. Physiol. 2019, 234, 15678–15685. [Google Scholar] [CrossRef]
- Gorbacheva, M.A.; Tikhomirova, M.A.; Potashnikova, D.M.; Akbay, B.; Sheval, E.V.; Musinova, Y.R. Production of Stable Cell Lines on the Basis of the Cultured RPMI 8866 B-Cells with Constant and Inducible Expression of the Human Immunodeficiency Virus Tat Protein. Russ. J. Dev. Biol. 2019, 50, 275–280. [Google Scholar] [CrossRef]
- Buccigrossi, V.; Laudiero, G.; Nicastro, E.; Miele, E.; Esposito, F.; Guarino, A. The HIV-1 Transactivator Factor (Tat) Induces Enterocyte Apoptosis through a Redox-Mediated Mechanism. PLoS ONE 2011, 6, e29436. [Google Scholar] [CrossRef]
- Rozzi, S.J.; Borelli, G.; Ryan, K.; Steiner, J.P.; Reglodi, D.; Mocchetti, I.; Avdoshina, V. PACAP27 Is Protective Against Tat-Induced Neurotoxicity. J. Mol. Neurosci. 2014, 54, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Keating, R.; McGargill, M.A. MTOR Regulation of Lymphoid Cells in Immunity to Pathogens. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Blanchet, F.P.; Moris, A.; Nikolic, D.S.; Lehmann, M.; Cardinaud, S.; Stalder, R.; Garcia, E.; Dinkins, C.; Leuba, F.; Wu, L.; et al. Human Immunodeficiency Virus-1 Inhibition of Immunoamphisomes in Dendritic Cells Impairs Early Innate and Adaptive Immune Responses. Immunity 2010, 32, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Cinti, A.; Le Sage, V.; Milev, M.P.; Valiente-Echeverría, F.; Crossie, C.; Miron, M.-J.; Panté, N.; Olivier, M.; Mouland, A.J. HIV-1 Enhances MTORC1 Activity and Repositions Lysosomes to the Periphery by Co-Opting Rag GTPases. Sci. Rep. 2017, 7, 5515. [Google Scholar] [CrossRef]
- Kumar, B.; Arora, S.; Ahmed, S.; Banerjea, A.C. Hyperactivation of Mammalian Target of Rapamycin Complex 1 by HIV-1 Is Necessary for Virion Production and Latent Viral Reactivation. FASEB J. 2017, 31, 180–191. [Google Scholar] [CrossRef]
- Planas, D.; Routy, J.; Ancuta, P.; Planas, D.; Zhang, Y.; Monteiro, P.; Goulet, J.; Gosselin, A. HIV-1 Selectively Targets Gut-Homing Mechanisms Find the Latest Version: HIV-1 Selectively Targets Gut-Homing Mechanisms. JCI Insight 2017, 2, 1–21. [Google Scholar]
- Rehman, S.; Husain, M.; Yadav, A.; Kasinath, B.S.; Malhotra, A.; Singhal, P.C. HIV-1 Promotes Renal Tubular Epithelial Cell Protein Synthesis: Role of MTOR Pathway. PLoS ONE 2012, 7, e30071. [Google Scholar] [CrossRef]
- Zhang, H.-S.; Zhang, Z.-G.; Zhou, Z.; Du, G.-Y.; Li, H.; Yu, X.-Y.; Huang, Y.-H. PKM2-Mediated Inhibition of Autophagy Facilitates Tat’s Inducing HIV-1 Transactivation. Arch. Biochem. Biophys. 2017, 625–626, 17–23. [Google Scholar] [CrossRef]
- Xue, M.; Yao, S.; Hu, M.; Li, W.; Hao, T.; Zhou, F.; Zhu, X.; Lu, H.; Qin, D.; Yan, Q.; et al. HIV-1 Nef and KSHV Oncogene K1 Synergistically Promote Angiogenesis by Inducing Cellular MiR-718 to Regulate the PTEN/AKT/MTOR Signaling Pathway. Nucleic Acids Res. 2014, 42, 9862–9879. [Google Scholar] [CrossRef]
- Poggi, A.; Carosio, R.; Fenoglio, D.; Brenci, S.; Murdaca, G.; Setti, M.; Indiveri, F.; Scabini, S.; Ferrero, E.; Zocchi, M.R. Migration of V Delta 1 and V Delta 2 T Cells in Response to CXCR3 and CXCR4 Ligands in Healthy Donors and HIV-1-Infected Patients: Competition by HIV-1 Tat. Blood 2004, 103, 2205–2213. [Google Scholar] [CrossRef]
- Kurnaeva, M.A.; Sheval, E.V.; Musinova, Y.R.; Vassetzky, Y.S. Tat Basic Domain: A “Swiss Army Knife” of HIV-1 Tat? Rev. Med. Virol. 2019, 29, e2031. [Google Scholar] [CrossRef]
- Epeldegui, M.; Breen, E.C.; Hung, Y.P.; Boscardin, W.J.; Detels, R.; Martínez-Maza, O. Elevated Expression of Activation Induced Cytidine Deaminase in Peripheral Blood Mononuclear Cells Precedes AIDS-NHL Diagnosis. AIDS 2007, 21, 2265–2270. [Google Scholar] [CrossRef]
- Kawamura, K.; Wada, A.; Wang, J.-Y.; Li, Q.; Ishii, A.; Tsujimura, H.; Takagi, T.; Itami, M.; Tada, Y.; Tatsumi, K.; et al. Expression of Activation-Induced Cytidine Deaminase Is Associated with a Poor Prognosis of Diffuse Large B Cell Lymphoma Patients Treated with CHOP-Based Chemotherapy. J. Cancer Res. Clin. Oncol. 2016, 142, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mdletshe, N.; Nel, A.; Shires, K.; Mowla, S. HIV Nef Enhances the Expression of Oncogenic C-MYC and Activation-Induced Cytidine Deaminase in Burkitt Lymphoma Cells, Promoting Genomic Instability. Infect Agents Cancer 2020, 15, 54. [Google Scholar] [CrossRef]
- Chiu, H.; Jackson, L.V.; Oh, K.I.; Mai, A.; Ronai, Z.A.; Ruggero, D.; Fruman, D.A. The MTORC1/4E-BP/EIF4E Axis Promotes Antibody Class Switching in B Lymphocytes. J. Immunol. 2019, 202, 579–590. [Google Scholar] [CrossRef]
- Wu, C.; Fu, Q.; Guo, Q.; Chen, S.; Goswami, S.; Sun, S.; Li, T.; Cao, X.; Chu, F.; Chen, Z.; et al. Lupus-Associated Atypical Memory B Cells Are MTORC1-Hyperactivated and Functionally Dysregulated. Ann. Rheum. Dis. 2019, 78, 1090–1100. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbay, B.; Germini, D.; Bissenbaev, A.K.; Musinova, Y.R.; Sheval, E.V.; Vassetzky, Y.; Dokudovskaya, S. HIV-1 Tat Activates Akt/mTORC1 Pathway and AICDA Expression by Downregulating Its Transcriptional Inhibitors in B Cells. Int. J. Mol. Sci. 2021, 22, 1588. https://doi.org/10.3390/ijms22041588
Akbay B, Germini D, Bissenbaev AK, Musinova YR, Sheval EV, Vassetzky Y, Dokudovskaya S. HIV-1 Tat Activates Akt/mTORC1 Pathway and AICDA Expression by Downregulating Its Transcriptional Inhibitors in B Cells. International Journal of Molecular Sciences. 2021; 22(4):1588. https://doi.org/10.3390/ijms22041588
Chicago/Turabian StyleAkbay, Burkitkan, Diego Germini, Amangeldy K. Bissenbaev, Yana R. Musinova, Evgeny V. Sheval, Yegor Vassetzky, and Svetlana Dokudovskaya. 2021. "HIV-1 Tat Activates Akt/mTORC1 Pathway and AICDA Expression by Downregulating Its Transcriptional Inhibitors in B Cells" International Journal of Molecular Sciences 22, no. 4: 1588. https://doi.org/10.3390/ijms22041588
APA StyleAkbay, B., Germini, D., Bissenbaev, A. K., Musinova, Y. R., Sheval, E. V., Vassetzky, Y., & Dokudovskaya, S. (2021). HIV-1 Tat Activates Akt/mTORC1 Pathway and AICDA Expression by Downregulating Its Transcriptional Inhibitors in B Cells. International Journal of Molecular Sciences, 22(4), 1588. https://doi.org/10.3390/ijms22041588








