Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation
Abstract
1. Introduction
2. Results
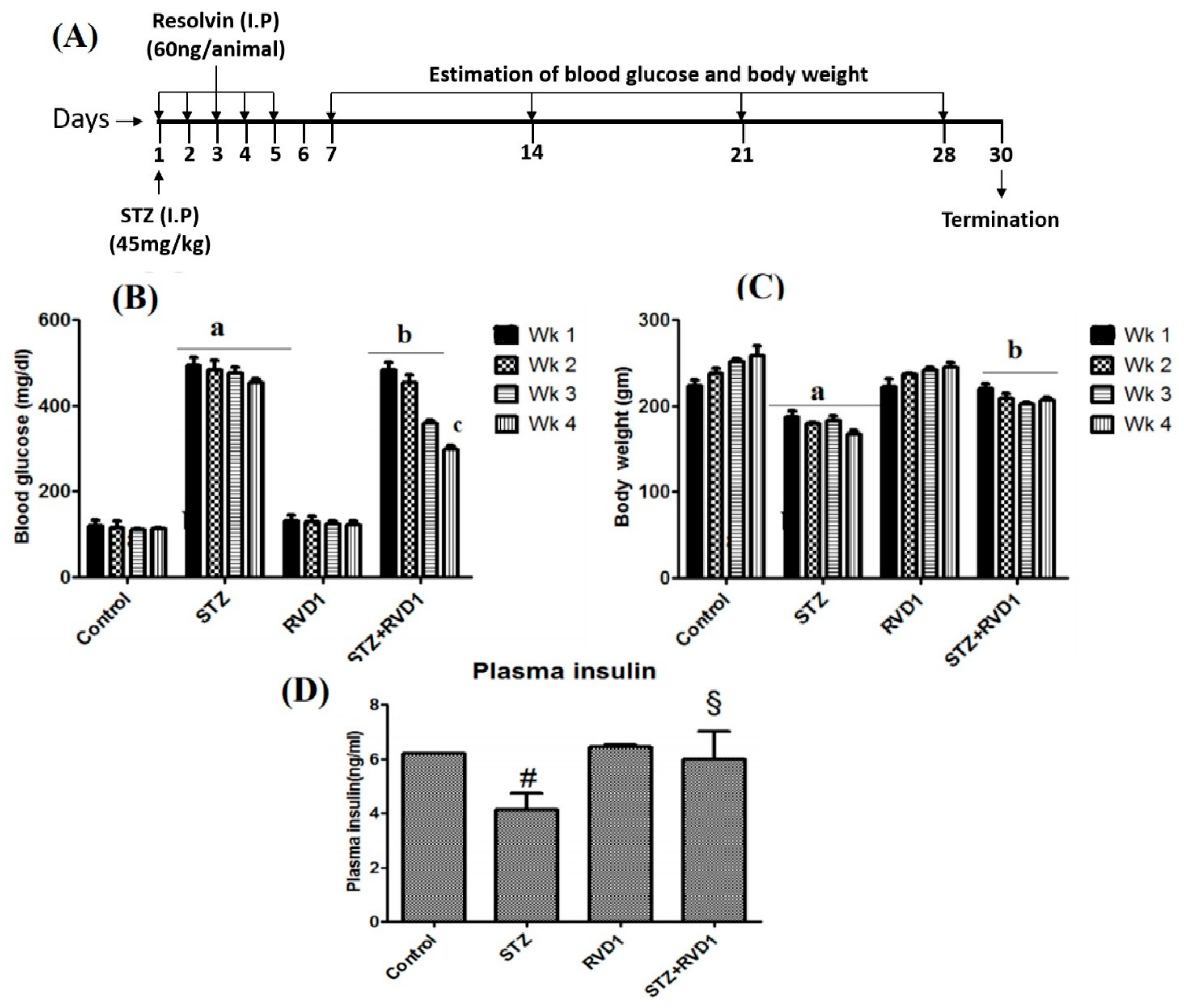
2.1. Effect of RVD1 Treatment on Plasma Glucose and Body Weight in STZ Induced T1DM
2.2. RVD1 Treatment Decreased Insulin Resistance and Enhanced Insulin Sensitivity
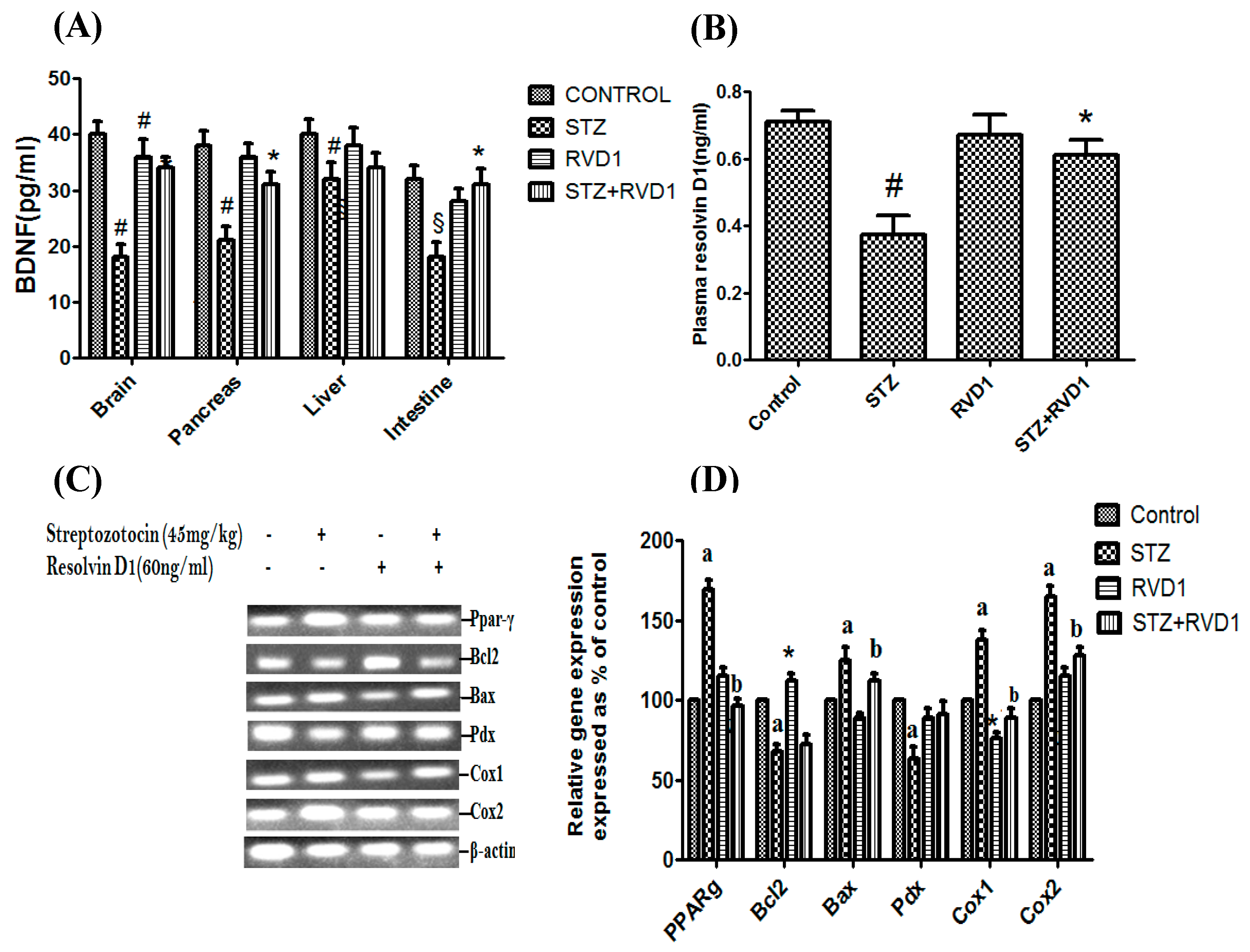
2.3. Effect of RVD1 on Tissue BDNF Concentrations and Plasma RVD1 Levels
2.4. Effect of RVD1 Treatment on STZ-Induced Changes in the Expression of Bcl2/Bax/Pdx/PPAR-γ/Cox-1/Cox-2 Genes
2.5. Effect of RVD1 on Plasma BDNF, Cytokines, and LXA4 Levels in STZ-Induced T1DM
2.6. Effect of RVD1 on STZ-Induced Changes in p65Nf-kB, PPPAR-γ, iNOS, Foxo 1, Gsk3β Proteins in the Pancreatic Tissue
2.7. RVD1 Restores the Balance between Pro- and Antioxidants Altered by STZ
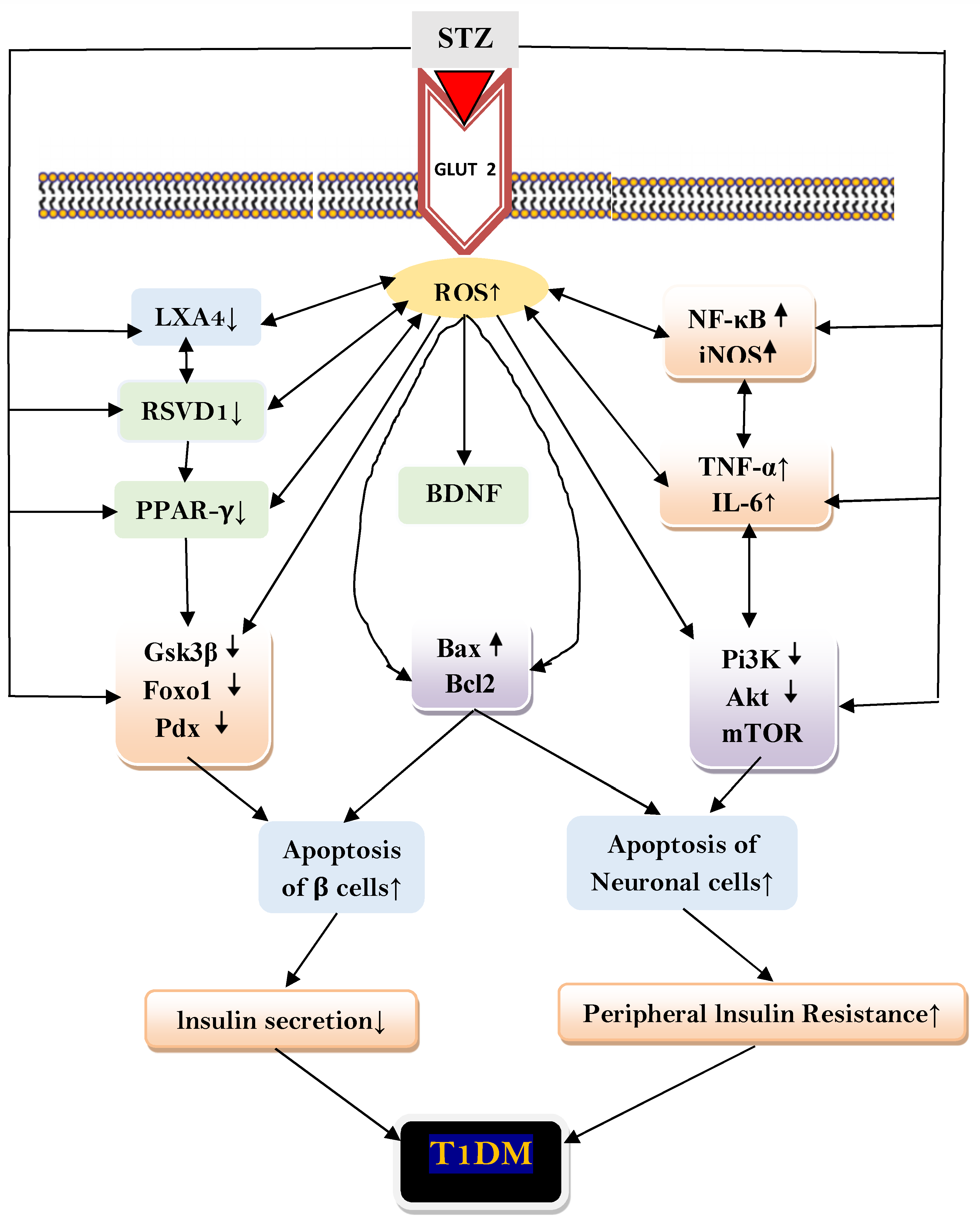
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. In Vivo Studies
4.3. Induction of Type 1 Diabetes Mellitus
4.4. Measurement of Blood Glucose, Insulin, Food Intake, and Body Weight
4.5. BDNF ELISA Studies
4.6. Estimation of Antioxidant Enzymes
4.7. Measurement of Plasma RVD1, BDNF, TNF-α, IL-6, and LXA4 Concentrations
4.8. Gene Expression Studies in Pancreas
4.8.1. Isolation of RNA and cDNA Synthesis
4.8.2. Semiquantitative PCR for Gene Expression Studies
4.8.3. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallberg, M.; Cooke, A. Immune mechanisms in type 1 diabetes. Trends Immunol. 2013, 34, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Bluestone, J.A.; Eisenbarth, G.S.; Hebrok, M.; Herold, K.C.; Accili, D.; Pietropaolo, M.; Arvan, P.R.; Von Herrath, M.; Markel, D.S.; et al. How does type 1 diabetes develop? The notion of homicide or β-cell suicide revisited. Diabetes 2011, 30, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.; Martinson, A.L.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.K.; Smart, N.G.; Cunningham, J.J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotech. 2008, 26, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.-F. Anti-CD3 antibodies for type 1 diabetes: Beyond expectations. Lancet 2011, 378, 459–460. [Google Scholar] [CrossRef]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015, 8, 181–188. [Google Scholar]
- Mossman, B.T.; Ireland, C.M.; Filipak, M.; LeDoux, S.; Wilso, G.L. Comparative interactions of streptozotocin and chlorozotocin with DNA of an insulin-secreting cell line (RINr). Diabetologia 1986, 29, 186–191. [Google Scholar] [CrossRef]
- Pettepher, C.C.; LeDoux, S.P.; Bohr, A.V.; Wilson, G.L. Repair of alkali-labile sites within the mitochondrial DNA of RINr 38 cells after exposure to the nitrosourea streptozotocin. J. Biol. Chem. 1991, 266, 3113–3117. [Google Scholar] [CrossRef]
- Kullin, M.; Li, Z.; Hansen, J.B.; Björk, E.; Sandler, S.; Karlsson, F.A. K(ATP) channel openers protect rat islets against the toxic effect of streptozotocin. Diabetes 2000, 4, 1131–1136. [Google Scholar] [CrossRef]
- Matsuda, M.; Kawasaki, F.; Mikami, Y.; Takeuchi, Y.; Saito, M.; Eto, M.; Kaku, K. Rescue of beta-cell exhaustion by diazoxide after the development of diabetes mellitus in rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 2002, 453, 141–148. [Google Scholar] [CrossRef]
- Grossman, E.J.; Lee, D.D.; Tao, J.; Wilson, R.A.; Park, S.Y.; Bell, G.I.; Chong, A.S. Glycemic control promotes pancreatic beta-cell regeneration in streptozotocin-induced diabetic mice. PLoS ONE 2010, 5, e8749. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.; Ables, E.T.; Crawford, L.; Lowe, D.; Offield, M.F.; Magnuson, M.A.; Wright, C.V. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev. Biol. 2008, 314, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, U.; Jonsson, J.; Jonsson, L.; Simu, K.; Edlund, H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998, 12, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Ahmed, N.T.; Luciani, D.S.; Han, Z.; Tran, H.; Fujita, J.; Misler, S.; Edlund, H.; Polonsky, K.S. Increased islet apoptosis in Pdx1+/- mice. J. Clin. Investig. 2003, 111, 1147–1160. [Google Scholar] [CrossRef]
- Johnson, J.D.; Bernal-Mizrachi, E.; Alejandro, E.U.; Han, Z.; Kalynyak, T.B.; Li, H.; Beith, J.L.; Gross, J.; Warnock, G.L.; Townsend, R.R.; et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc. Natl. Acad. Sci. USA 2006, 103, 19575–19580. [Google Scholar] [CrossRef]
- Gundala, N.K.; Naidu, V.G.; Das, U.N. Arachidonic acid and lipoxin A4 attenuate alloxan induced cytotoxicity to RIN5F cells in vitro and type 1 diabetes mellitus in vivo. BioFactors 2017, 43, 251–271. [Google Scholar] [CrossRef]
- Gundala, N.K.; Naidu, V.G.; Das, U.N. Arachidonic acid and lipoxin A4 attenuate streptozotocin-induced cytotoxicity to RIN5F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition 2017, 35, 61–80. [Google Scholar] [CrossRef]
- Gundala, N.K.; Naidu, V.G.; Das, U.N. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochem. Biophys. Res. Commun. 2018, 496, 105–113. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual antiinflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.S.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Haworth, O.; Croze, R.; Oh, S.F.; Uddin, M.; Carlo, T.; Pfeffer, M.A.; Priluck, R.; Serhan, C.N.; Levy, B.D. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 2012, 189, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, R.B.; Gündüz, Ö.S.; Özdemir, A.T.; Akgül, Ö. Low levels of pro-resolving lipid mediators lipoxin-A4, resolvin-D1 and resolvin-E1 in patients with rheumatoid arthritis. Immunol. Lett. 2020, 227, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Norling, L.V.; Headland, S.E.; Dalli, J.; Arnardottir, H.H.; Haworth, O.; Jones, H.R.; Irimia, D.; Serhan, C.N.; Perretti, M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 2016, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.; Romero, E.; Goetzmann, J.; Hasselschwert, D.L.; Dray, B.; Vanchiere, J.; Fontenot, J.; Yun, J.W.; Norris, P.C.; White, L.; et al. Endogenous specialized proresolving mediator profiles in a novel experimental model of lymphatic obstruction and intestinal inflammation in African Green Monkeys. Am. J. Pathol. 2019, 189, 1953–1972. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, S.; Esteban-Muñoz, A.; Giménez-Martínez, R.; Aguilar-Cordero, M.J.; Miralles-Buraglia, B.; Olalla-Herrera, M. A Comparison of Changes in the Fatty Acid Profile of Human Milk of Spanish Lactating Women during the First Month of Lactation Using Gas Chromatography-Mass Spectrometry. A Comparison with Infant Formulas. Nutrients 2019, 11, 3055. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Lammardo, A.; Bonvissuto, M.; Giovannini, M.; Galli, C.; Riva, E. Polyunsaturated fatty acids in maternal plasma and in breast milk. Prostaglandins Leukot. Essent. Fatty Acids 2002, 66, 535–540. [Google Scholar] [CrossRef]
- Wang, L.; Shimizu, Y.; Kaneko, S.; Hanaka, S.; Abe, T.; Shimasaki, H.; Hisaki, H.; Nakajima, H. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatr. Int. 2000, 42, 14–20. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Herzig, P.; Giani, G. Early infant feeding and risk of type 1 diabetes mellitus-a nationwide population-based case-control study in pre-school children. Diabetes Metab. Res. Rev. 2008, 24, 211–222. [Google Scholar] [CrossRef]
- Sadauskaitė-Kuehne, V.; Ludvigsson, J.; Padaiga, Z.; Jašinskienė, E.; Samuelsson, U. Samuelsson. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab. Res. Rev. 2004, 20, 150–157. [Google Scholar] [CrossRef]
- Lund-Blix, N.A.; Sander, S.D.; Størdal, K.; Andersen, A.M.; Rønningen, K.S.; Joner, G.; Skrivarhaug, T.; Njølstad, P.R.; Husby, S.; Stene, L.C. Infant Feeding and Risk of Type 1 Diabetes in Two Large Scandinavian Birth Cohorts. Diabetes Care 2017, 40, 920–927. [Google Scholar] [CrossRef]
- Stene, L.C.; Ulriksen, J.; Magnus, P.; Joner, G. Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring. Diabetologia 2000, 43, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.; Orr, S.K.; Dalli, J.; Serhan, C.N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016, 9, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Troxler, H.; Klinke, G.; Rogler, D.; Braegger, C.P.; Hersberger, M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Gundala, N.K.; Rhenghachar, P.; Polavarapu, S.; Hari, A.D.; Sadananda, M.; Das, U.N. Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Mohan, I.K.; Das, U.N. Prevention of chemically-induced diabetes mellitus in experimental animals by polyunsaturated fatty acids. Nutrition 2001, 17, 126–151. [Google Scholar] [CrossRef]
- Suresh, Y.; Das, U. Protective action of arachidonic acid against alloxan-induced cytotoxicity and diabetes mellitus. Prostaglandins Leukot. Essent. Fatty Acids 2007, 64, 37–52. [Google Scholar] [CrossRef]
- Suresh, Y.; Das, U.N. Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus: Effect of ω-3 fatty acids. Nutrition 2003, 19, 213–228. [Google Scholar] [CrossRef]
- Suresh, Y.; Das, U.N. Differential effect of saturated, monounsaturated, and polyunsaturated fatty acids on alloxan-induced diabetes mellitus. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 199–213. [Google Scholar] [CrossRef]
- Suresh, Y.; Das, U.N. Long-chain polyunsaturated fatty acids and chemically induced diabetes mellitus: Effect of omega-6 fatty acids. Nutrition 2003, 19, 93–114. [Google Scholar] [CrossRef]
- Bathina, S.; Srinivas, N.; Das, U.N. BDNF protects pancreatic β cells (RIN5F) against cytotoxic action of alloxan, streptozotocin, doxorubicin and benzo(a)pyrene in vitro. Metabolism 2016, 65, 667–684. [Google Scholar] [CrossRef]
- Tonra, J.R.; Ono, M.; Liu, X.; Garcia, K.; Jackson, C.; Yancopoulos, G.D.; Wiegand, S.J.; Wong, V. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes 1999, 48, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tsuchida, A.; Itakura, Y.; Nonomura, T.; Ono, M.; Hirota, F.; Inoue, T.; Nakayama, C.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 2000, 49, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ono-Kishino, M.; Sugaru, E.; Yamanaka, M.; Taiji, M.; Noguchi, H. Brain-derived neurotrophic factor (BDNF) regulates glucose and energy metabolism in diabetic mice. Diabetes Metab. Res. Rev. 2002, 18, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G.; Boizel, R.; Bessard, J.; Cracowski, J.L.; Bessard, G.; Halimi, S.; Stanke-Labesque, F. Urinary leukotriene E4 excretion is increased in type 1 diabetic patients: A quantification by liquid chromatography-tandem mass spectrometry. Prostaglandins Lipid Mediat. 2005, 78, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Larsson, A.; Vessby, J.; Vessby, B.; Berne, C. Type 1 diabetes is associated with increased cyclooxygenase- and cytokine-mediated inflammation. Diabetes Care 2005, 28, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Arisaka, M.; Fukuda, Y.; Yamashiro, Y. Prostaglandin metabolism in children with diabetes mellitus. I. Plasma prostaglandin E2, F2 alpha, TXB2, and serum fatty acid levels. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.P.; Williams, R.L.; Dupont, J. Increased prostaglandin synthesis in childhood diabetes mellitus. J. Pediatr. 1979, 94, 185–189. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 201–210. [Google Scholar] [CrossRef]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef]

| Summary of the Analysis of Various Anti-Oxidant/Lipid Peroxides and Nitric Oxide Levels in Pancreas of Resolvin-D1/Stz-Treated Diabetic Rats | |||||||
|---|---|---|---|---|---|---|---|
| Tissue | Group | SOD (Units/mg protein) | CAT (mM H2O2/min/mg protein | GST (mM/min/gm protein) | GPX (mM/min/gm protein) | LPO (µM TMOP) | Nitric oxide (µM Nitrite) |
| Pancreas | Control | 36.6 ± 1.1 | 889 ± 69.1 | 17.7 ± 1.9 | 116.7 ± 5.7 | 1.22 ± 0.01 | 1.06 ± 0.04 |
| STZ (65 mg/kg) | 73.1 ± 3.4 * | 1291 ± 32 * | 51.2 ± 4.5 * | 193 ± 3.2 * | 1.48 ± 0.21 * | 1.22 ± 0.08 * | |
| RVD1 | 45.3 ± 2.4 | 732 ± 81.9 | 27.3 ± 1.5 | 98.5 ± 2.1 § | 0.96 ± 0.10 | 0.96 ± 0.03 § | |
| STZ + RVD1 | 41.2 ± 2.9 ** | 703 ± 60.8 ** | 21 ± 1.09 ** | 96.2 ± 5.2 ** | 0.88 ± 0.06 ** | 1.08 ± 0.07 ** | |
| Assay/Reagents | Kit/Analysis | Source |
|---|---|---|
| 17(S)-Resolvin D1 (RVD1) | Analysis | Cayman Chemical Company (Ann Arbor, MI, USA). |
| Plasma insulin levels | ultrasensitive rat insulin ELISA kit | Crystal Chemical Inc (Belvidere, IL, USA) |
| Plasma BDNF levels | BDNF ELISA kit | Chemikine Sandwich ELISA kit (Millipore, Burlington, MA, USA) (CYT306) |
| RVD1 levels | RVD1 ELISA kit | Cayman Chemical Company (Ann Arbor, MI, USA, 500,380) |
| IL-6 levels | IL-6 ELISA kit | Kendall Square (Cambridge, MA, USA) |
| TNF-α levels | Quantikine TNF-α Immunoassay ELISA kit | RTA00, R&D Systems (Minneapolis, MN, USA) |
| LXA4 levels | Lipoxin A4 ELISA kit | Oxford Biomedical Research Company (Rochester Hills, MI, USA) (EA45) |
| Gene Expression Studies by Semi Quantitative PCR | Size | Primer Sequences | Steps Involved |
|---|---|---|---|
| Forward/Reverse Primer | Number of Cycles | ||
| Bax | 105 bp | F: 5′-CACCAGCTCTGAACAGATCATGA-3′ R: 5′-TCAGCCCATCTTCTTCCAGATGT-3′ | For Bax/Bcl2 genes, Initial denaturation 94 °C for 2 min, 95 °C for 30 s denaturation, 59 °C for 30 s annealing was employed. Later, 72 °C for 30 s extension and 72 °C for 5 min final extension, and overall, 34 cycles for Bcl2/Bax. |
| Bcl2 | 110 bp | F: 5′-CACCCCTGGCATCTTCTCCTT-3′ R: 5′-AGCGTCTTCAGAGACAGCCAG-3′ | |
| Pdx | 528 bp | F: 5′-GTAGTAGCGGGACAACGAGC-3′ R: 5′-CAGTTGGGAGCCTGATTCTC-3′ | For β-actin 94 °C for 2 min initial denaturation, 94 °C for 30 s denaturation, 64 °C and 59 °C for 30 s annealing for Pdx and β-actin, respectively, and 72 °C for 30 s extension with 72 °C for 5 min final extension and overall, 35 cycles were performed. |
| β-actin | 617 bp | F: 5′-CGTGGGCCGCCCTAGGCACCA-3′ R: 5′-TTGGCCTTAGGGTTCAGGGGG-3′ | |
| PPAR-γ | 859 bp | F: 5′-TGATATCGACCAGCTGAACC-3′ R: 5′-TCAGCGACTGG GACTTTTCT-3′ | For Cox-1, Cox-2, PPAR-γ 94 °C for 2 min initial denaturation, 94 °C for 30 s denaturation, 69 °C, 52.5 °C, and for 30 s annealing, respectively. Later, 72 °C for 30 s extension and 72 °C for 5 min final extension, and overall, 35 cycles were performed. |
| Cox-1 | 450 bp | F: 5′-ACTCACTCAGTTTGAGTCATTC-3′ R: 5′-TTTGATTAGTACTGTAGGGTTAATG-3′ | |
| Cox-2 | 583 bp | F: 5′-TGCATGTGGCTGTGGATGTCATCAA-3′ R: 5′-CACTAAGACAGACCCGTC ATCTCCA-3′ | |
| Polymerase chain reaction (PCR) reagents were obtained from Genei (Bangalore, India). PCR primers were purchased from Bioserve (Hyderabad, India). | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bathina, S.; Das, U.N. Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. Int. J. Mol. Sci. 2021, 22, 1516. https://doi.org/10.3390/ijms22041516
Bathina S, Das UN. Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. International Journal of Molecular Sciences. 2021; 22(4):1516. https://doi.org/10.3390/ijms22041516
Chicago/Turabian StyleBathina, Siresha, and Undurti N. Das. 2021. "Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation" International Journal of Molecular Sciences 22, no. 4: 1516. https://doi.org/10.3390/ijms22041516
APA StyleBathina, S., & Das, U. N. (2021). Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. International Journal of Molecular Sciences, 22(4), 1516. https://doi.org/10.3390/ijms22041516







