ERK-Directed Phosphorylation of mGlu5 Gates Methamphetamine Reward and Reinforcement in Mouse
Abstract
1. Introduction
2. Results
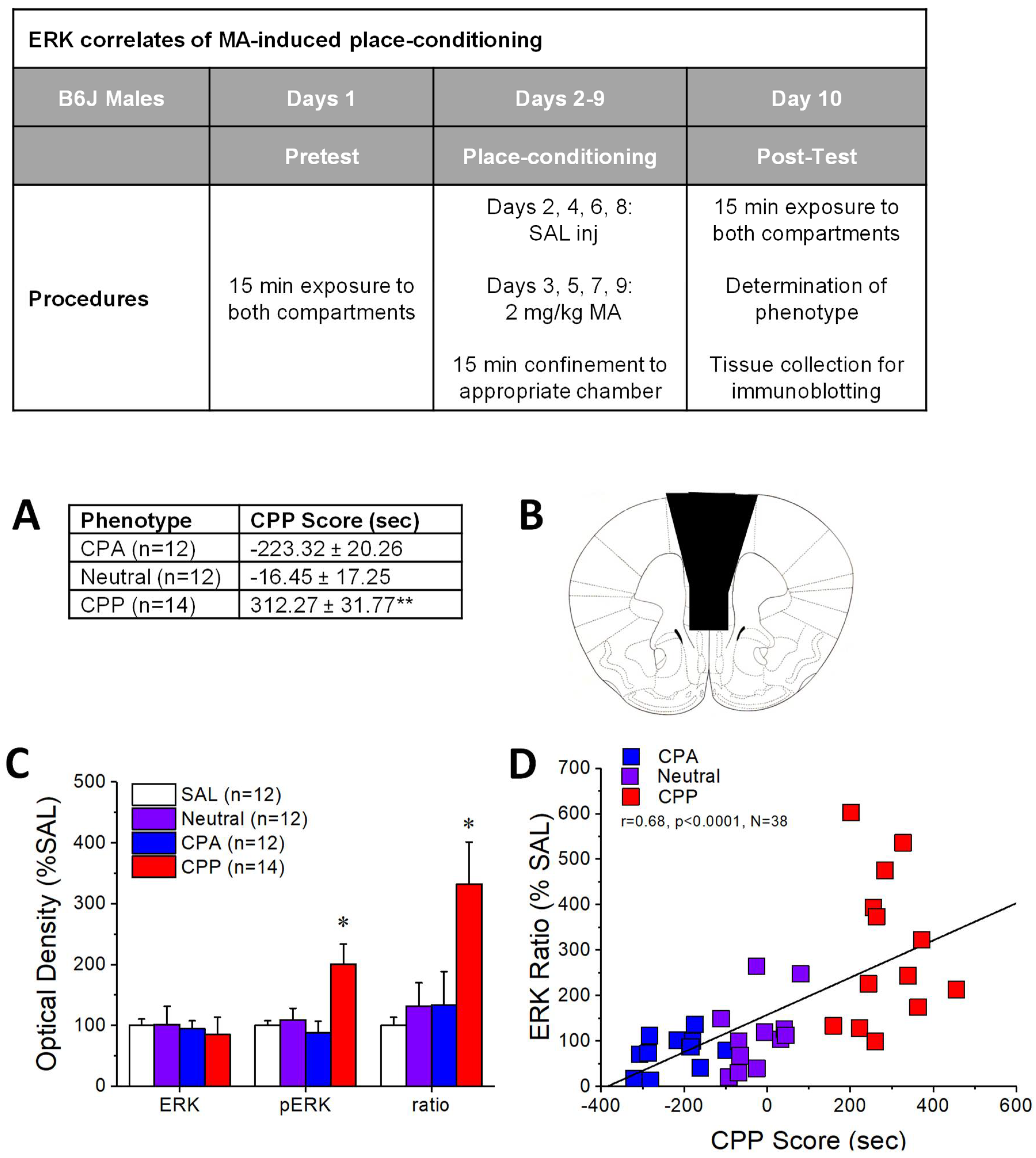
2.1. pERK Expression within mPFC Predicts the Positive Affective Valence of MA
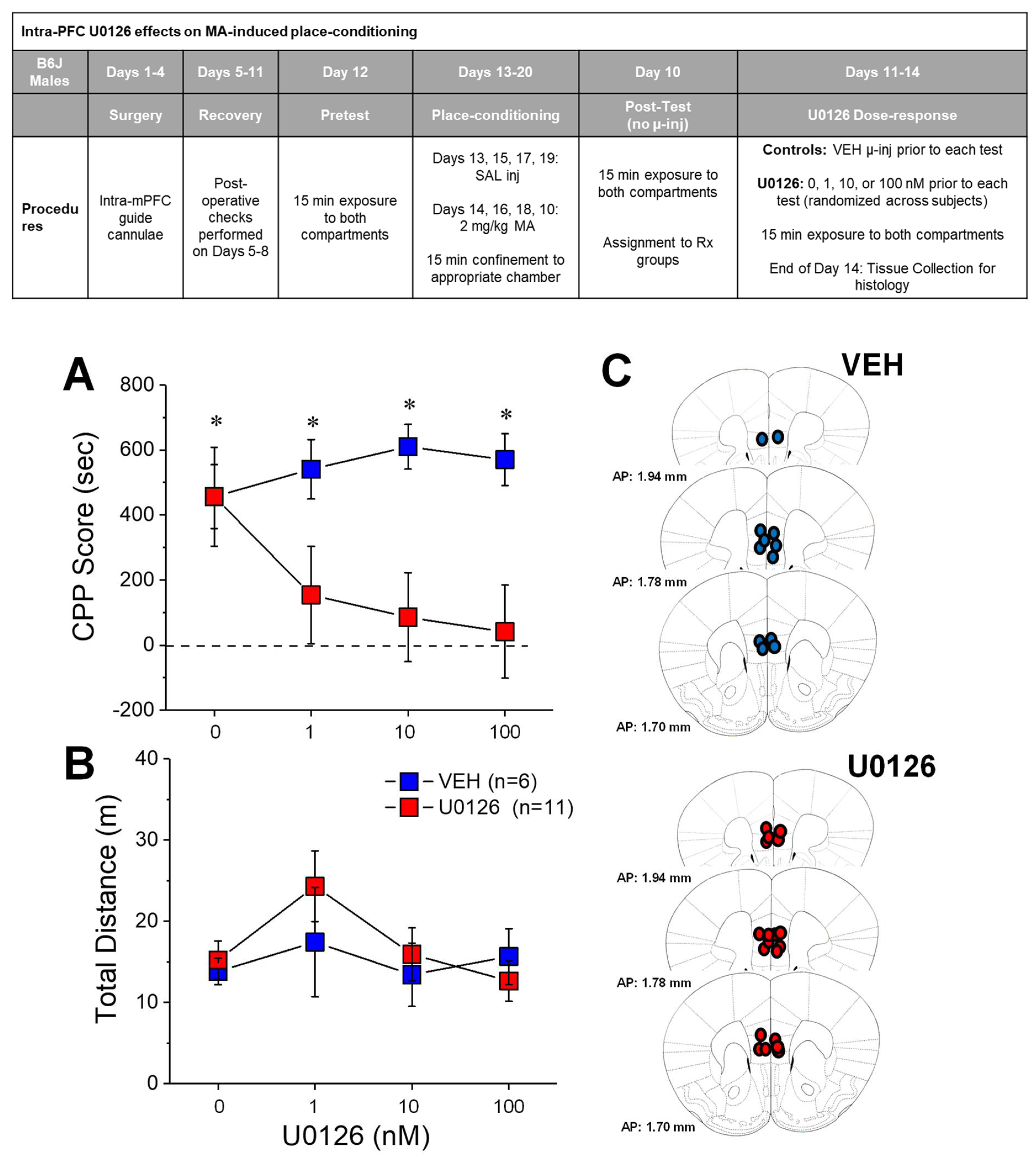
2.2. U0126 Reduces MA-Induced Place-Preference
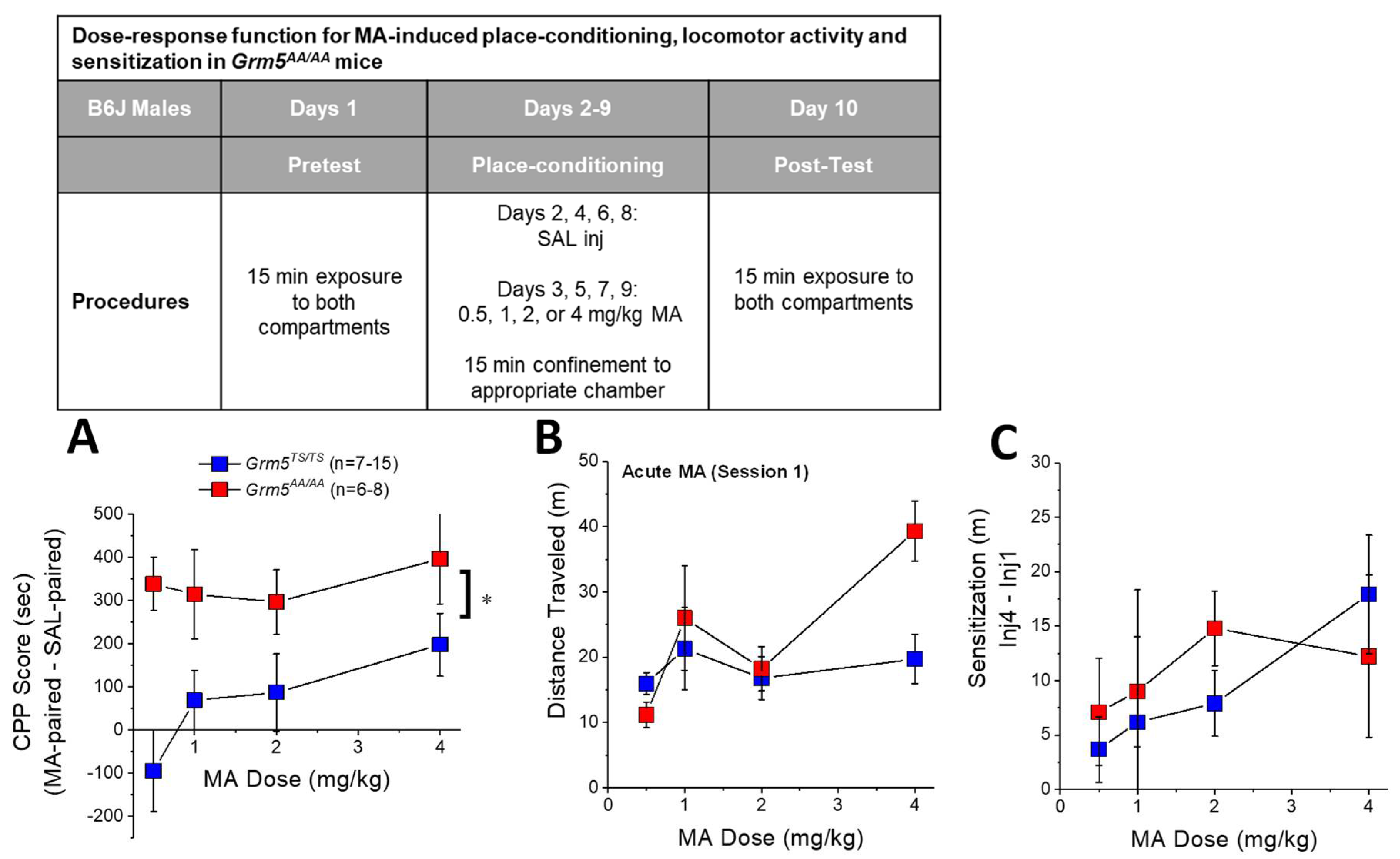
2.3. Transgenic Disruption of ERK-Dependent Phosphorylation of (T1123/S1126)mGlu5 Augments MA-Conditioned Reward
2.4. Transgenic Disruption of ERK-Dependent Phosphorylation of (T1123/S1126)mGlu5 Does Not Affect MA-Induced Locomotion or Locomotor Sensitization
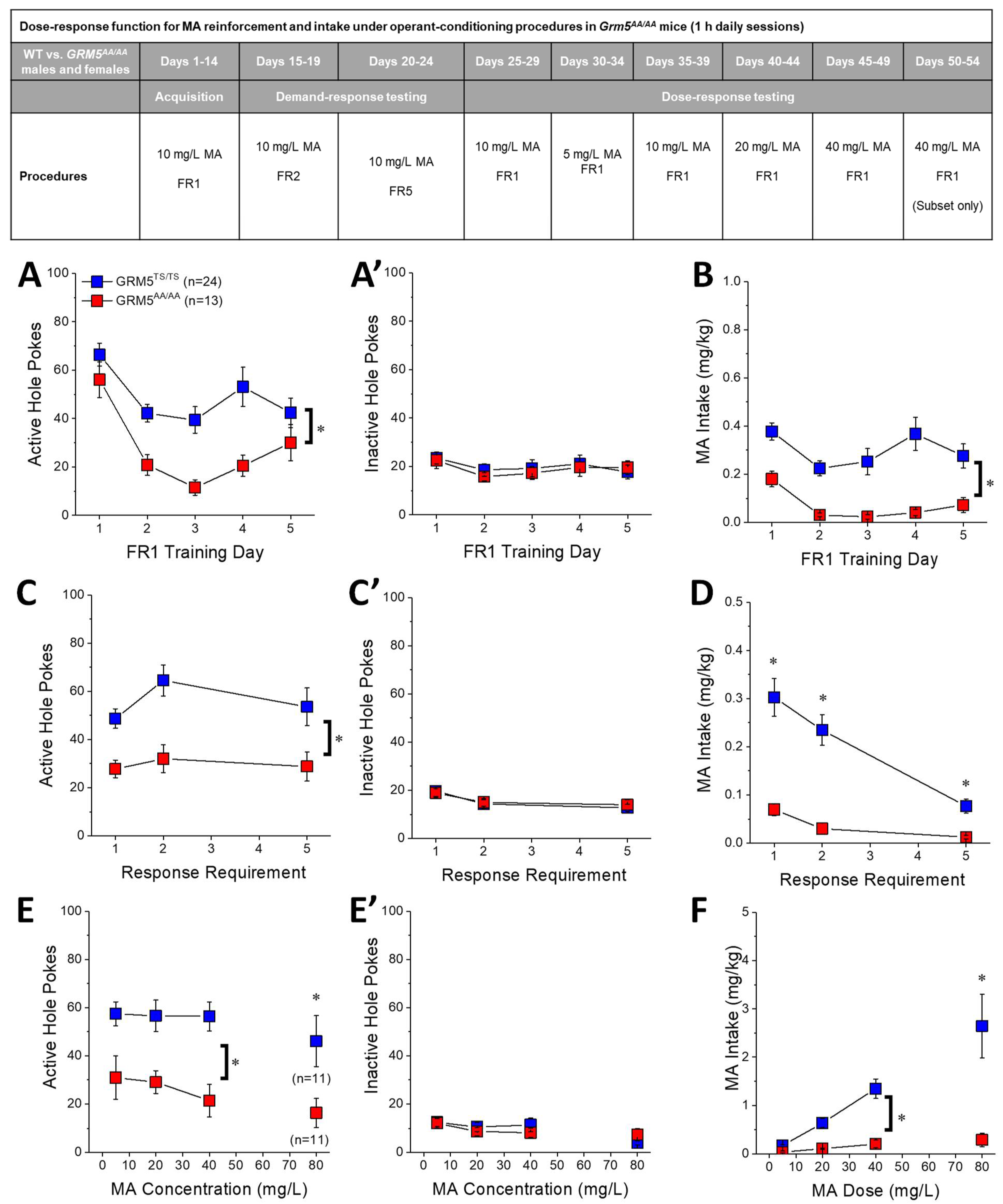
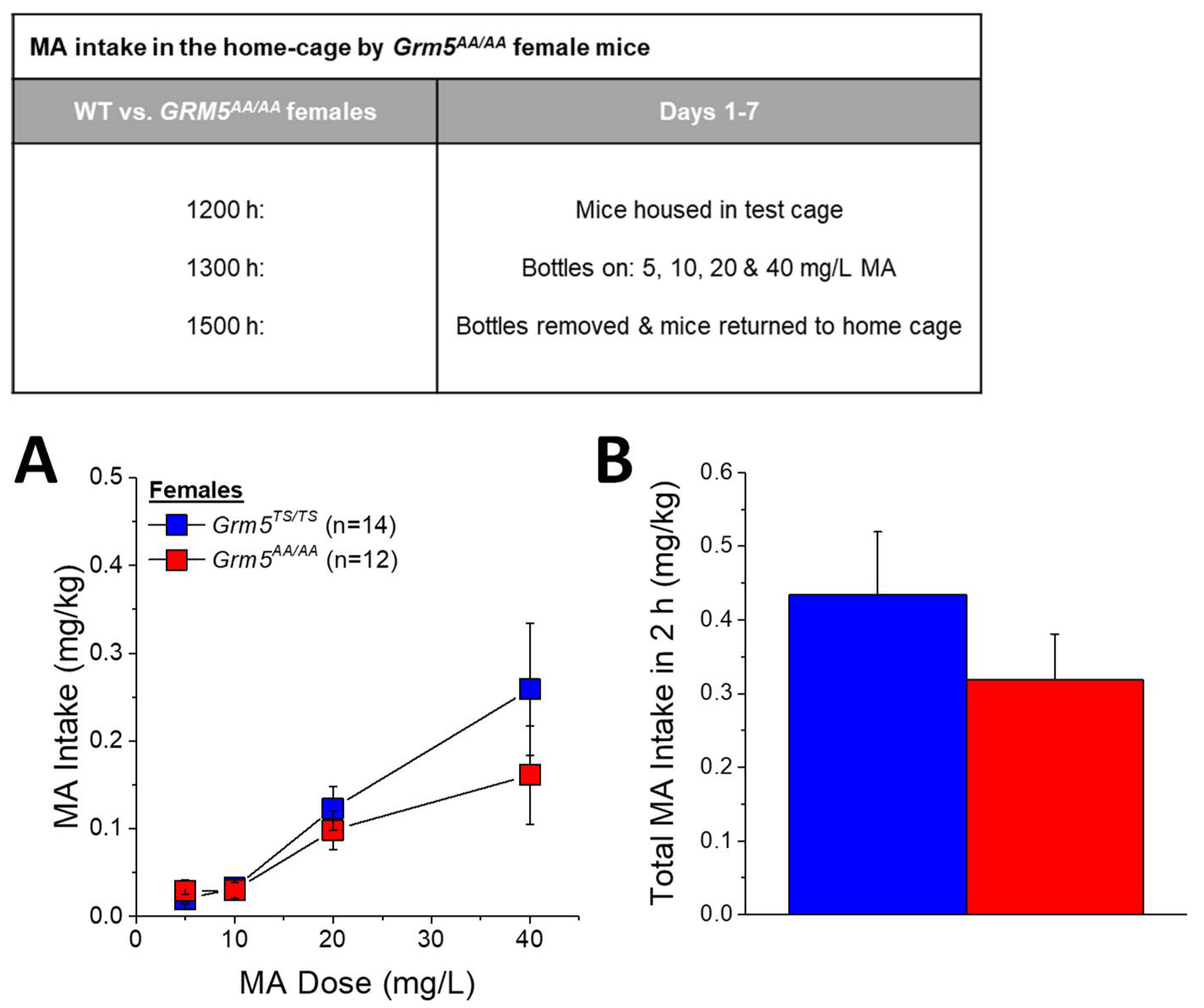
2.5. Transgenic Disruption of ERK-Dependent Phosphorylation of T1123/S1126-mGlu5 Robustly Attenuates Oral MA Reinforcement and Intake
2.6. Acquisition of Self-Administration
2.7. MA Demand under Increasing Response Requirement
2.8. Dose-Response Function for Oral MA Reinforcement
2.9. Transgenic Disruption of ERK-Dependent Phosphorylation of (T1123/S1126)mGlu5 Blunts Oral MA Intake under DID Procedures
3. Discussion
3.1. Individual Differences in MA-Preference Are Positively Correlated with ERK Hyper-Activity within PFC
3.2. Local Inhibition of mPFC ERK Phosphorylation Eliminates an Established MA-CPP Phenotype
3.3. Grm5AA/AA Mice Exhibit More Robust MA-CPP
3.4. Grm5AA/AA Mice Exhibit Reduced Oral MA Self-Administration
4. Materials and Methods
4.1. Subjects
4.2. Place-Conditioning and Locomotor Activity
4.3. Operant-Conditioning for Oral Methamphetamine Reinforcement
4.4. Methamphetamine Intake under Drinking-in-the-Dark Procedures
4.5. Stereotaxic Surgery, U0126 Microinjection, and Histology
4.6. Immunoblotting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drug Enforcement Administration. National Drug Threat Assessment; Document No. DEA-DCT-DIR-007-20; Strategic Intelligence Section: Washington, DC, USA, 2019.
- United Nations Office on Drugs and Crime: World Drug Report 2019; Sales No. E.19.XI.8; United Nations Publication: New York, NY, USA, 2019.
- Rothman, R.B.; Baumann, M.H. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003, 479, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Hattori, H.; Asano, M.; Oya, M.; Katsumata, Y. Inhibition of monoamine oxidase by d-methamphetamine. Biochem. Pharmacol. 1980, 29, 2071–2073. [Google Scholar] [CrossRef]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Iyo, M.; Ouchi, Y.; Matsunaga, T.; Tsukada, H.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Takei, N.; Mori, N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am. J. Psychiatry 2001, 158, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Franceschi, D.; Sedler, M.; Gatley, S.J.; Miller, E.; Hitzemann, R.; Ding, Y.S.; et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001, 21, 9414–9418. [Google Scholar] [CrossRef]
- Volkow, N.D.; Chang, L.; Wang, G.J.; Fowler, J.S.; Ding, Y.S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R.; et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry 2001, 158, 2015–2021. [Google Scholar] [CrossRef]
- Johanson, C.E.; Frey, K.A.; Lundahl, L.H.; Keenan, P.; Lockhart, N.; Roll, J.; Galloway, G.P.; Koeppe, R.A.; Kilbourn, M.R.; Robbins, T.; et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 2006, 185, 327–338. [Google Scholar] [CrossRef]
- McCann, U.D.; Ricaurte, G.A. Amphetamine neurotoxicity: Accomplishments and remaining challenges. Neurosci. Biobehav. Rev. 2004, 27, 821–826. [Google Scholar] [CrossRef]
- Yamamoto, B.K.; Moszczynska, A.; Gudelsky, G.A. Amphetamine toxicities: Classical and emerging mechanisms. Ann. N. Y. Acad. Sci. 2010, 1187, 101–121. [Google Scholar] [CrossRef]
- Steinkellner, T.; Freissmuth, M.; Sitte, H.H.; Montgomery, T. The ugly side of amphetamines: Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol. Chem. 2011, 392, 103–115. [Google Scholar] [CrossRef]
- Phillips, T.J.; Mootz, J.R.; Reed, C. Identification of Treatment Targets in a Genetic Mouse Model of Voluntary Methamphetamine Drinking. Int. Rev. Neurobiol. 2016, 126, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Grilly, D.M.; Loveland, A. What is a “low dose” of D-amphetamine for inducing behavioral effects in laboratory rats? Psychopharmacology 2001, 153, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Schuckit, M.A.; Tipp, J.E.; Smith, T.L.; Wiesbeck, G.A.; Kalmijn, J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J. Stud. Alcohol. 1997, 58, 397–404. [Google Scholar] [CrossRef]
- DiFranza, J.R.; Savageau, J.A.; Fletcher, K.; Ockene, J.K.; Rigotti, N.A.; McNeill, A.D.; Coleman, M.; Wood, C. Recollections and repercussions of the first inhaled cigarette. Addict. Behav. 2004, 29, 261–272. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Horwood, L.J.; Lynskey, M.T.; Madden, P.A. Early reactions to cannabis predict later dependence. Arch. Gen. Psychiatry 2003, 60, 1033–1039. [Google Scholar] [CrossRef]
- Davidson, E.S.; Finch, J.F.; Schenk, S. Variability in subjective responses to cocaine: Initial experiences of college students. Addict. Behav. 1993, 18, 445–453. [Google Scholar] [CrossRef]
- Chait, L.D. Factors influencing the reinforcing and subjective effects of d-amphetamine in humans. Behav. Pharmacol. 1993, 4, 191–199. [Google Scholar] [CrossRef]
- De Wit, H.; Uhlenhuth, E.H.; Johanson, C.E. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986, 16, 341–360. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Reed, C.; Burkhart-Kasch, S.; Li, N.; Cunningham, C.L.; Janowsky, A.; Franken, F.H.; Wiren, K.M.; Hashimoto, J.G.; Scibelli, A.C.; et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009, 8, 758–771. [Google Scholar] [CrossRef]
- Shabani, S.; McKinnon, C.S.; Reed, C.R.; Cunningham, C.L.; Phillips, T.J. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011, 10, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Mckinnon, C.S.; Cunningham, C.L.; Phillips, T.J. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology 2012, 62, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.; Dobbs, L.K.; Ford, M.M.; Mark, G.P.; Finn, D.A.; Phillips, T.J. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology 2012, 62, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Szumlinski, K.K.; Lominac, K.D.; Campbell, R.R.; Cohen, M.; Fultz, E.K.; Brown, C.N.; Miller, B.W.; Quadir, S.G.; Martin, D.; Thompson, A.B.; et al. Methamphetamine addiction vulnerability: The glutamate, the bad, and the ugly. Biol. Psychiatry 2017, 81, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Lominac, K.D.; Quadir, S.G.; Barrett, H.M.; McKenna, C.L.; Schwartz, L.M.; Ruiz, P.N.; Wroten, M.G.; Campbell, R.R.; Miller, B.W.; Holloway, J.J.; et al. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur. J. Neurosci. 2016, 43, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.N.; Fultz, E.K.; Ferdousian, S.; Rogers, S.; Lustig, E.; Page, A.; Shahin, J.R.; Flaherty, D.M.; Von Jonquieres, G.; Bryant, C.D.; et al. Transgenic Analyses of Homer2 Function Within Nucleus Accumbens Subregions in the Regulation of Methamphetamine Reward and Reinforcement in Mice. Front. Psychiatry 2020, 11, 11. [Google Scholar] [CrossRef]
- Lominac, K.D.; Sacramento, A.D.; Szumlinski, K.K.; Kippin, T.E. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology 2012, 37, 707–722. [Google Scholar] [CrossRef]
- Chesworth, R.; Brown, R.M.; Kim, J.H.; Lawrence, A.J. The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine-seeking in mice. PLoS ONE 2013, 8, e68371. [Google Scholar] [CrossRef]
- Miyatake, M.; Narita, M.; Shibasak, M.; Nakamura, A.; Suzuki, T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur. J. Neurosci. 2005, 22, 1476–1488. [Google Scholar] [CrossRef]
- Herrold, A.A.; Voigt, R.M.; Napier, T.C. mGluR5 is necessary for maintenance of methamphetamine-induced associative learning. Eur. Neuropsychopharmacol. 2013, 23, 691–696. [Google Scholar] [CrossRef]
- Gass, J.T.; Osborne, M.P.; Watson, N.L.; Brown, J.L.; Olive, M.F. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology 2009, 34, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.P.; Olive, M.F. A role for mGluR5 receptors in intravenous methamphetamine self-administration. Ann. N. Y. Acad. Sci. 2008, 1139, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Kufahl, P.R.; Hood, L.E.; Nemirovsky, N.E.; Barabas, P.; Halstengard, C.; Villa, A.; Moore, E.; Watterson, L.R.; Olive, M.F. Positive allosteric modulation of mGluR5 accelerates extinction learning but not relearning following methamphetamine self-administration. Front. Pharmacol. 2012, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Widholm, J.J.; Gass, J.T.; Cleva, R.M.; Olive, M.F. The mGluR5 positive allosteric modulator CDPPB does not alter extinction or contextual reinstatement of methamphetamine-seeking behavior in rats. J. Addict. Res. Ther. 2011, S1. [Google Scholar] [CrossRef] [PubMed]
- Crocker, C.E.; Bernier, D.C.; Hanstock, C.C.; Lakusta, B.; Purdon, S.E.; Seres, P.; Tibbo, P.G. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: A (1)H MRS study at 3 Tesla. Schizophr. Res. 2014, 157, 231–237. [Google Scholar] [CrossRef]
- Ernst, T.; Chang, L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J. Neuroimmune Pharmacol. 2008, 3, 165–172. [Google Scholar] [CrossRef]
- O’Neill, J.; Tobias, M.C.; Hudkins, M.; London, E.D. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef][Green Version]
- Zamora-Martinez, E.R.; Edwards, S. Neuronal extracellular signal-regulated kinase (ERK) activity as marker and mediator of alcohol and opioid dependence. Front. Integr. Neurosci. 2014, 8, 24. [Google Scholar] [CrossRef]
- Lu, L.; Koya, E.; Zhai, H.; Hope, B.T.; Shaham, Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006, 29, 695–703. [Google Scholar] [CrossRef]
- Sun, W.L.; Quizon, P.M.; Zhu, J. Molecular Mechanism: ERK Signaling, Drug Addiction, and Behavioral Effects. Prog. Mol. Biol. Trans. Sci. 2015, 137, 1–40. [Google Scholar] [CrossRef]
- Rosen, L.G.; Sun, N.; Rushlow, W.; Laviolette, S.R. Molecular and neuronal plasticity mechanisms in the amygdala-prefrontal cortical circuit: Implications for opiate addiction memory formation. Front. Neurosci. 2015, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yan, C.; Yang, X.; Zhou, X.; Lv, W.; Guo, N.; Li, Y.; Bai, J. Thioredoxin-1 downregulation in the nucleus accumbens promotes methamphetamine-primed reinstatement in mice. Neuropharmacology 2018, 139, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Hui, R.; Liu, Y.; Luo, Y.; Wang, J.; Shen, X.; Xie, B.; Yu, F.; Cong, B.; Ma, C. Molecular hydrogen attenuates methamphetamine-induced behavioral sensitization and activation of ERK-ΔFosB signaling in the mouse nucleus accumbens. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 97, 109781. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Hu, J.H.; Milshteyn, A.; Zhang, P.W.; Moore, C.G.; Park, S.; Datko, M.C.; Domingo, R.D.; Reyes, C.M.; Wang, X.J.; et al. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell 2013, 154, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.R.; Domingo, R.D.; Williams, A.R.; Wroten, M.G.; McGregor, H.A.; Waltermire, R.S.; Greentree, D.I.; Goulding, S.P.; Thompson, A.B.; Lee, K.M.; et al. Increased Alcohol-Drinking Induced by Manipulations of mGlu5 Phosphorylation within the Bed Nucleus of the Stria Terminalis. J. Neurosci. 2019, 39, 2745–2761. [Google Scholar] [CrossRef]
- Ruan, Q.T.; Yazdani, N.; Blum, B.C.; Beierle, J.A.; Lin, W.; Coelho, M.A.; Fultz, E.K.; Healy, A.F.; Shahin, J.R.; Kandola, A.K.; et al. A Mutation in Hnrnph1 That Decreases Methamphetamine-Induced Reinforcement, Reward, and Dopamine Release and Increases Synaptosomal hnRNP H and Mitochondrial Proteins. J. Neurosci. 2020, 40, 107–130. [Google Scholar] [CrossRef]
- Fultz, E.K.; Martin, D.L.; Hudson, C.N.; Kippin, T.E.; Szumlinski, K.K. Methamphetamine-alcohol interactions in murine models of sequential and simultaneous oral drug-taking. Drug Alcohol Depend. 2017, 177, 178–186. [Google Scholar] [CrossRef]
- Shab, G.; Fultz, E.K.; Page, A.; Coelho, M.A.; Brewin, L.W.; Stailey, N.; Brown, C.N.; Bryant, C.D.; Kippin, T.E.; Szumlinski, K.K. The motivational valence of methamphetamine relates inversely to subsequent methamphetamine self-administration in female C57BL/6J mice. Behav. Brain Res. 2021, 398, 112959. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Yamada, K.; Mizuno, M.; Mizuno, T.; Nitta, A.; Noda, Y.; Nabeshima, T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharmacol. 2004, 65, 1293–1301. [Google Scholar] [CrossRef]
- McDaid, J.; Graham, M.P.; Napier, T.C. Methamphetamine-induced sensitization differentially alters pCREB and DeltaFosB throughout the limbic circuit of the mammalian brain. Mol. Pharmacol. 2006, 70, 2064–2074. [Google Scholar] [CrossRef]
- Su, H.; Sun, T.; Wang, X.; Du, Y.; Zhao, N.; Zhu, J.; Yan, J.; Chen, T.; Yun, K. Levo-tetrahydropalmatine attenuates methamphetamine reward behavior and the accompanying activation of ERK phosphorylation in mice. Neurosci. Lett. 2020, 714, 134416. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Dai, Y.; Zhang, C.; Yang, C.; Huang, Q.; Hao, W.; Shen, H. Higher impulsivity and lower grey matter volume in the bilateral prefrontal cortex in long-term abstinent individuals with severe methamphetamine use disorder. Drug Alcohol Depend. 2020, 212, 108040. [Google Scholar] [CrossRef]
- Malcolm, R.; Myrick, H.; Li, X.; Henderson, S.; Brady, K.T.; George, M.S.; See, R.E. Regional Brain Activity in Abstinent Methamphetamine Dependent Males Following Cue Exposure. J. Drug Abuse 2016, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- London, E.D.; Simon, S.L.; Berman, S.M.; Mandelkern, M.A.; Lichtman, A.M.; Bramen, J.; Shinn, A.K.; Miotto, K.; Learn, J.; Dong, Y.; et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch. Gen. Psychiatry 2004, 61, 73–84. [Google Scholar] [CrossRef]
- Rubinfeld, H.; Seger, R. The ERK cascade: A prototype of MAPK signaling. Mol. Biotechnol. 2005, 31, 151–174. [Google Scholar] [CrossRef]
- Salzmann, J.; Marie-Claire, C.; Le Guen, S.; Roques, B.P.; Noble, F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br. J. Pharmacol. 2003, 140, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Valjent, E.; Corvol, J.C.; Pages, C.; Besson, M.J.; Maldonado, R.; Caboche, J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000, 20, 8701–8709. [Google Scholar] [CrossRef]
- Valjent, E.; Caboche, J.; Vanhoutte, P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: A molecular substrate for learning and memory? Mol. Neurobiol. 2001, 23, 83–99. [Google Scholar] [CrossRef]
- Valjent, E.; Corvol, J.C.; Trzaskos, J.M.; Girault, J.A.; Hervé, D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006, 7, 20. [Google Scholar] [CrossRef]
- Valjent, E.; Bertran-Gonzalez, J.; Hervé, D.; Fisone, G.; Girault, J.A. Looking BAC at striatal signaling: Cell-specific analysis in new transgenic mice. Trends Neurosci. 2009, 32, 538–547. [Google Scholar] [CrossRef]
- Gerdjikov, T.V.; Ross, G.M.; Beninger, R.J. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav. Neurosci. 2004, 118, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Jeong, Y.C.; Kwon, Y.B. Regulatory effect of bee venom on methamphetamine-induced cellular activities in prefrontal cortex and nucleus accumbens in mice. Biol. Pharm. Bull. 2015, 38, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Fultz, E.K.; Szumlinski, K.K. Prior binge-drinking history promotes the positive affective valence of methamphetamine in mice. Drug Alcohol Depend. 2018, 183, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Sern, K.R.; Fultz, E.K.; Coelho, M.A.; Bryant, C.D.; Szumlinski, K.K. A prior history of binge-drinking increases sensitivity to the motivational valence of methamphetamine in female C57BL/6J mice. Subst. Abuse 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Penzner, J.H.; Thompson, D.L.; Arth, C.; Fowler, J.K.; Ary, A.W.; Szumlinski, K.K. Protracted ‘anti-addictive’ effects of adolescent phenylpropanolamine exposure in C57BL/6J mice. Addict. Biol. 2008, 13, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Stafford, A.M.; Mootz, J.R.K.; Baba, H.; Erk, J.; Phillips, T.J. A breeding strategy to identify modifiers of high genetic risk for methamphetamine intake. Genes Brain Behav. 2020, 18, e12667. [Google Scholar] [CrossRef]
- Harkness, J.H.; Shi, X.; Janowsky, A.; Phillips, T.J. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology 2015, 40, 2175–2184. [Google Scholar] [CrossRef]
- Hu, J.H.; Yang, L.; Kammermeier, P.J.; Moore, C.G.; Brakeman, P.R.; Tu, J.; Yu, S.; Petralia, R.S.; Li, Z.; Zhang, P.W.; et al. Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat. Neurosci. 2012, 15, 836–844. [Google Scholar] [CrossRef]
- Lominac, K.D.; McKenna, C.L.; Schwartz, L.M.; Ruiz, P.N.; Wroten, M.G.; Miller, B.W.; Holloway, J.J.; Travis, K.O.; Rajasekar, G.; Maliniak, D.; et al. Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front. Syst. Neurosci. 2014, 8, 70. [Google Scholar] [CrossRef]
- Lominac, K.D.; Oleson, E.B.; Pava, M.; Klugmann, M.; Schwarz, M.K.; Seeburg, P.H.; During, M.J.; Worley, P.F.; Kalivas, P.W.; Szumlinski, K.K. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J. Neurosci. 2005, 25, 11586–11594. [Google Scholar] [CrossRef]
- Ary, A.W.; Lominac, K.D.; Wroten, M.G.; Williams, A.R.; Campbell, R.R.; Ben-Shahar, O.; von Jonquieres, G.; Klugmann, M.; Szumlinski, K.K. Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J. Neurosci. 2013, 33, 8101–8113. [Google Scholar] [CrossRef] [PubMed]
- Favata, M.F.; Horiuchi, K.Y.; Manos, E.J.; Daulerio, A.J.; Stradley, D.A.; Feeser, W.S.; Van Dyk, D.E.; Pitts, W.J.; Earl, R.A.; Hobbs, F.; et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998, 273, 18623–18632. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fultz, E.K.; Quadir, S.G.; Martin, D.; Flaherty, D.M.; Worley, P.F.; Kippin, T.E.; Szumlinski, K.K. ERK-Directed Phosphorylation of mGlu5 Gates Methamphetamine Reward and Reinforcement in Mouse. Int. J. Mol. Sci. 2021, 22, 1473. https://doi.org/10.3390/ijms22031473
Fultz EK, Quadir SG, Martin D, Flaherty DM, Worley PF, Kippin TE, Szumlinski KK. ERK-Directed Phosphorylation of mGlu5 Gates Methamphetamine Reward and Reinforcement in Mouse. International Journal of Molecular Sciences. 2021; 22(3):1473. https://doi.org/10.3390/ijms22031473
Chicago/Turabian StyleFultz, Elissa K., Sema G. Quadir, Douglas Martin, Daniel M. Flaherty, Paul F. Worley, Tod E. Kippin, and Karen K. Szumlinski. 2021. "ERK-Directed Phosphorylation of mGlu5 Gates Methamphetamine Reward and Reinforcement in Mouse" International Journal of Molecular Sciences 22, no. 3: 1473. https://doi.org/10.3390/ijms22031473
APA StyleFultz, E. K., Quadir, S. G., Martin, D., Flaherty, D. M., Worley, P. F., Kippin, T. E., & Szumlinski, K. K. (2021). ERK-Directed Phosphorylation of mGlu5 Gates Methamphetamine Reward and Reinforcement in Mouse. International Journal of Molecular Sciences, 22(3), 1473. https://doi.org/10.3390/ijms22031473





