MicroRNA Profiles in Monocyte-Derived Macrophages Generated by Interleukin-27 and Human Serum: Identification of a Novel HIV-Inhibiting and Autophagy-Inducing MicroRNA
Abstract
1. Introduction
2. Results
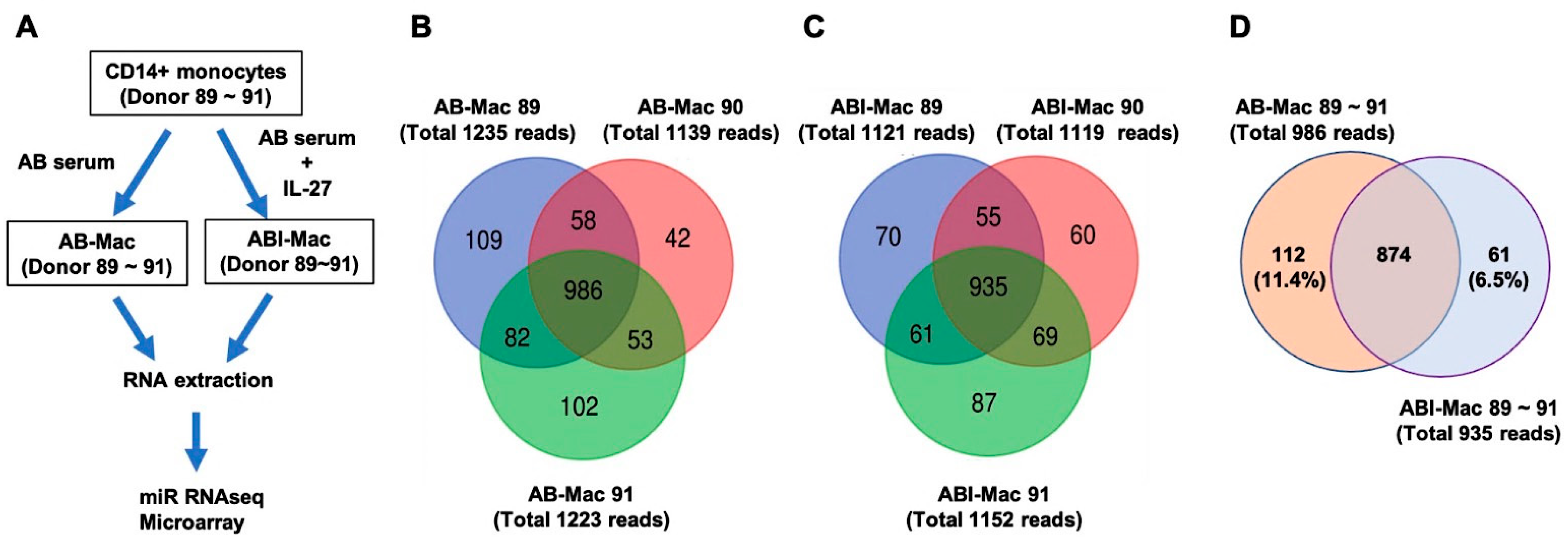
2.1. Profiles of miRNAs in the ABI-Macs
2.2. Identification of Novel miRNAs in the AB- and ABI-Mac Subsets
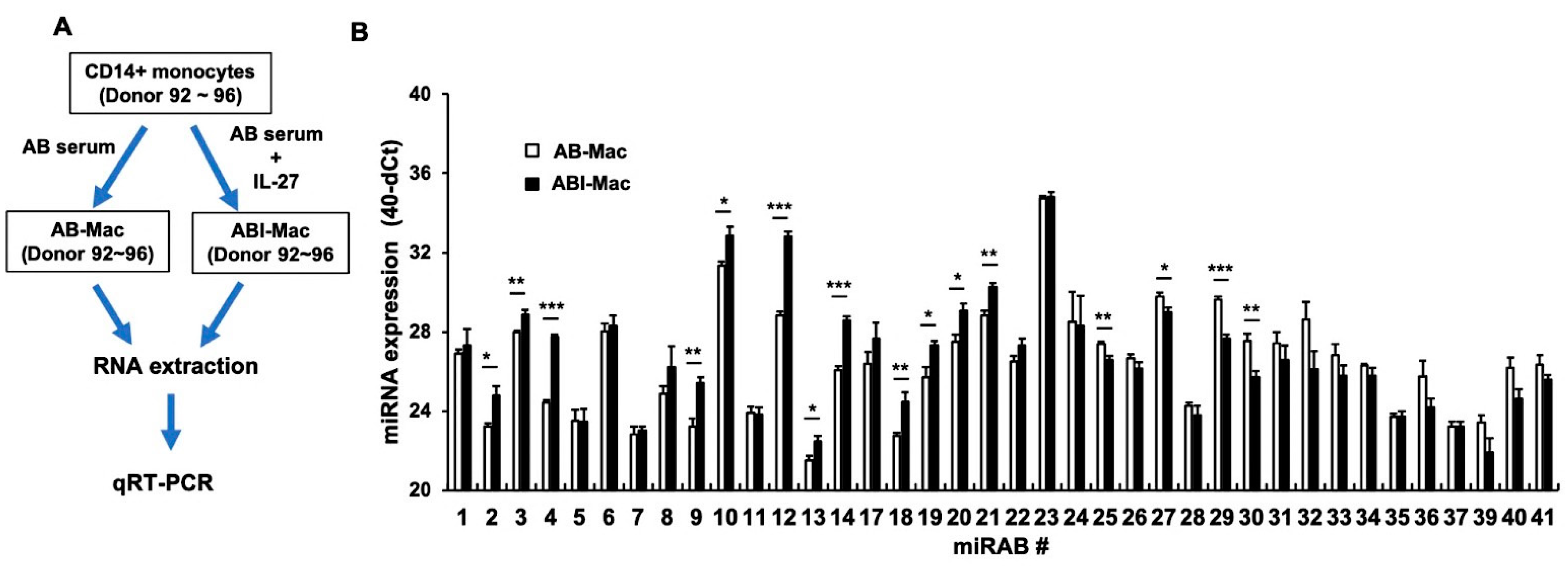
2.3. Confirmation of the Expression of Novel miRNAs Using qRT-PCR
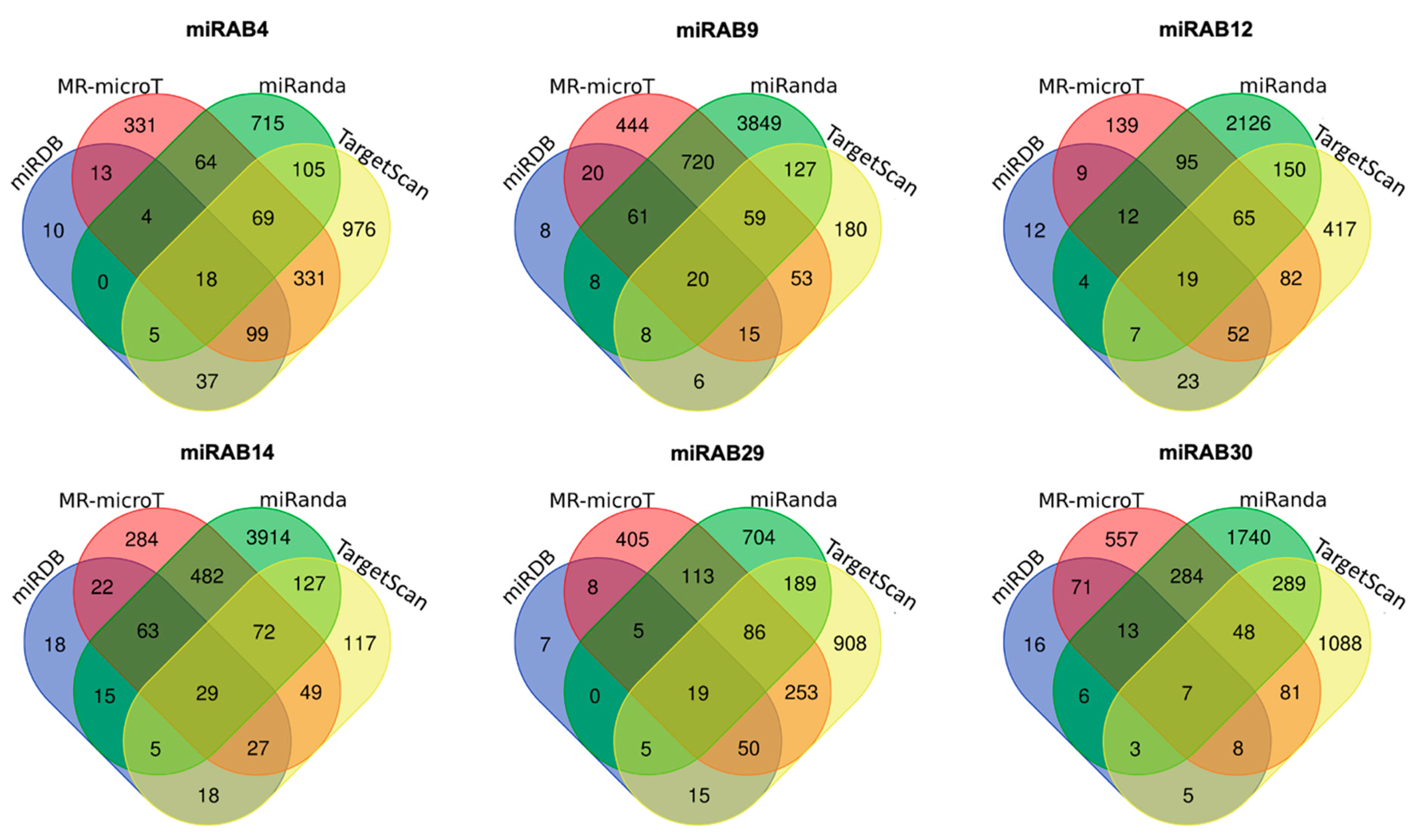
2.4. Prediction of Potential miRNA Target Genes
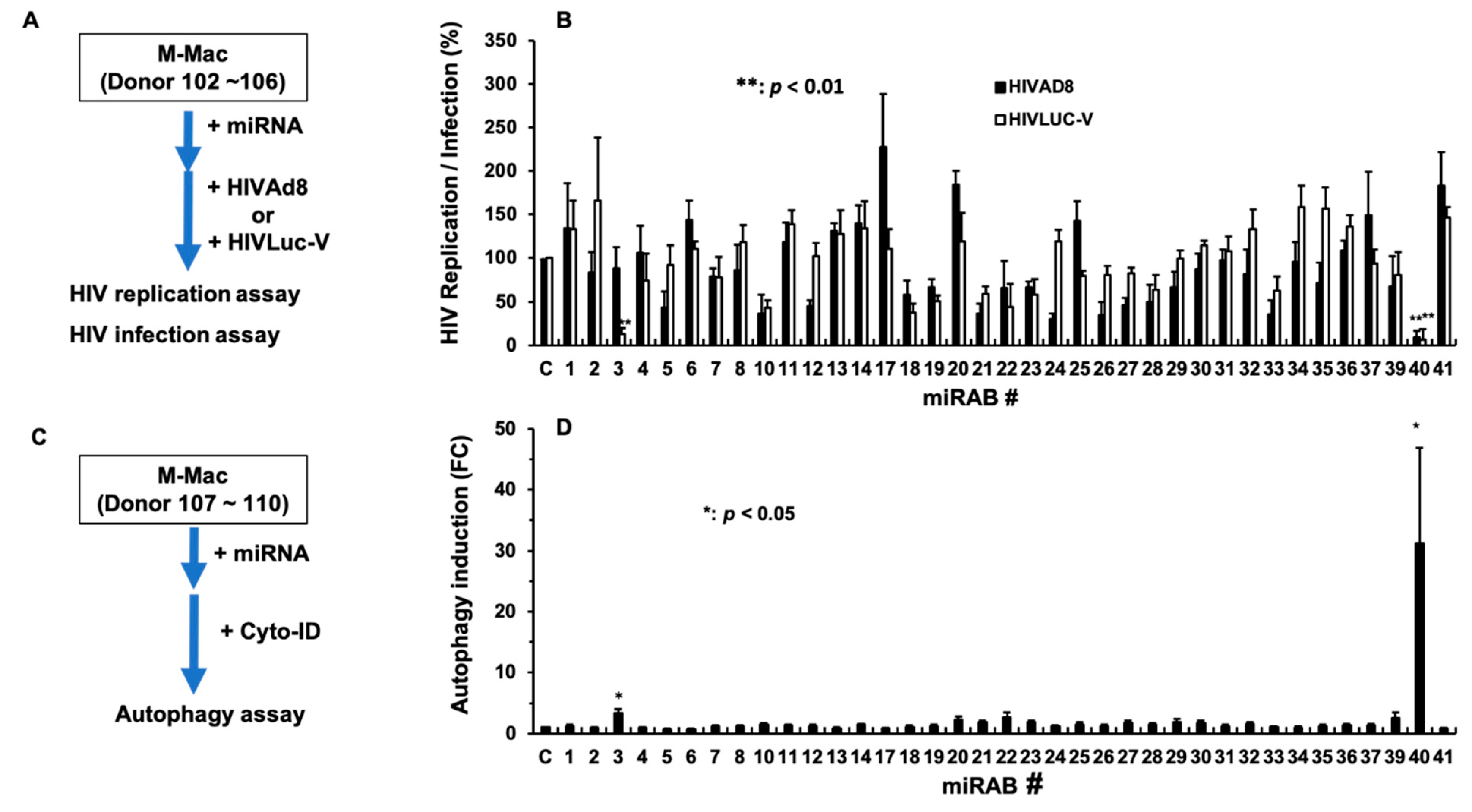
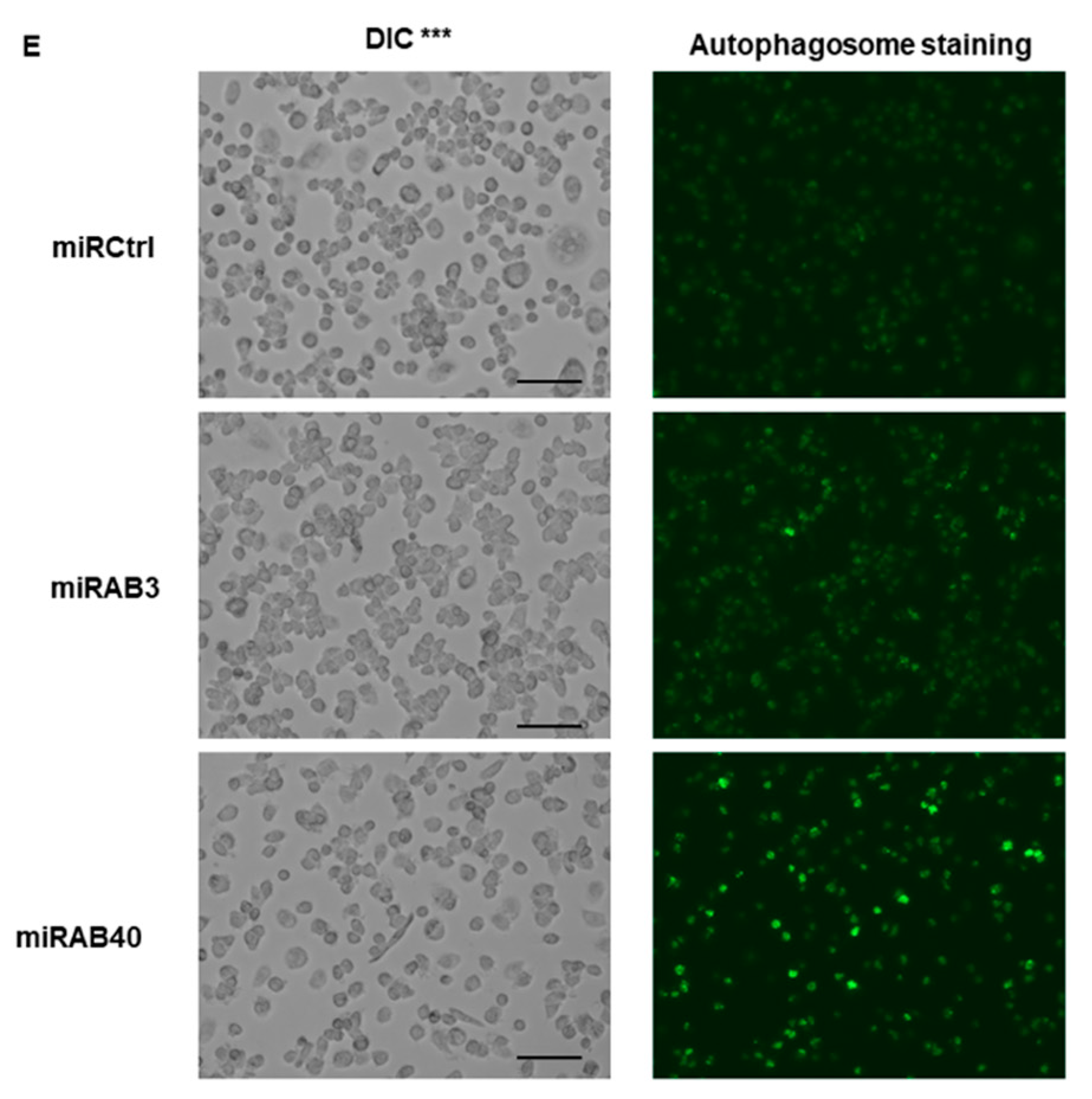
2.5. Evaluation of the Biological Functions of the Novel miRNAs
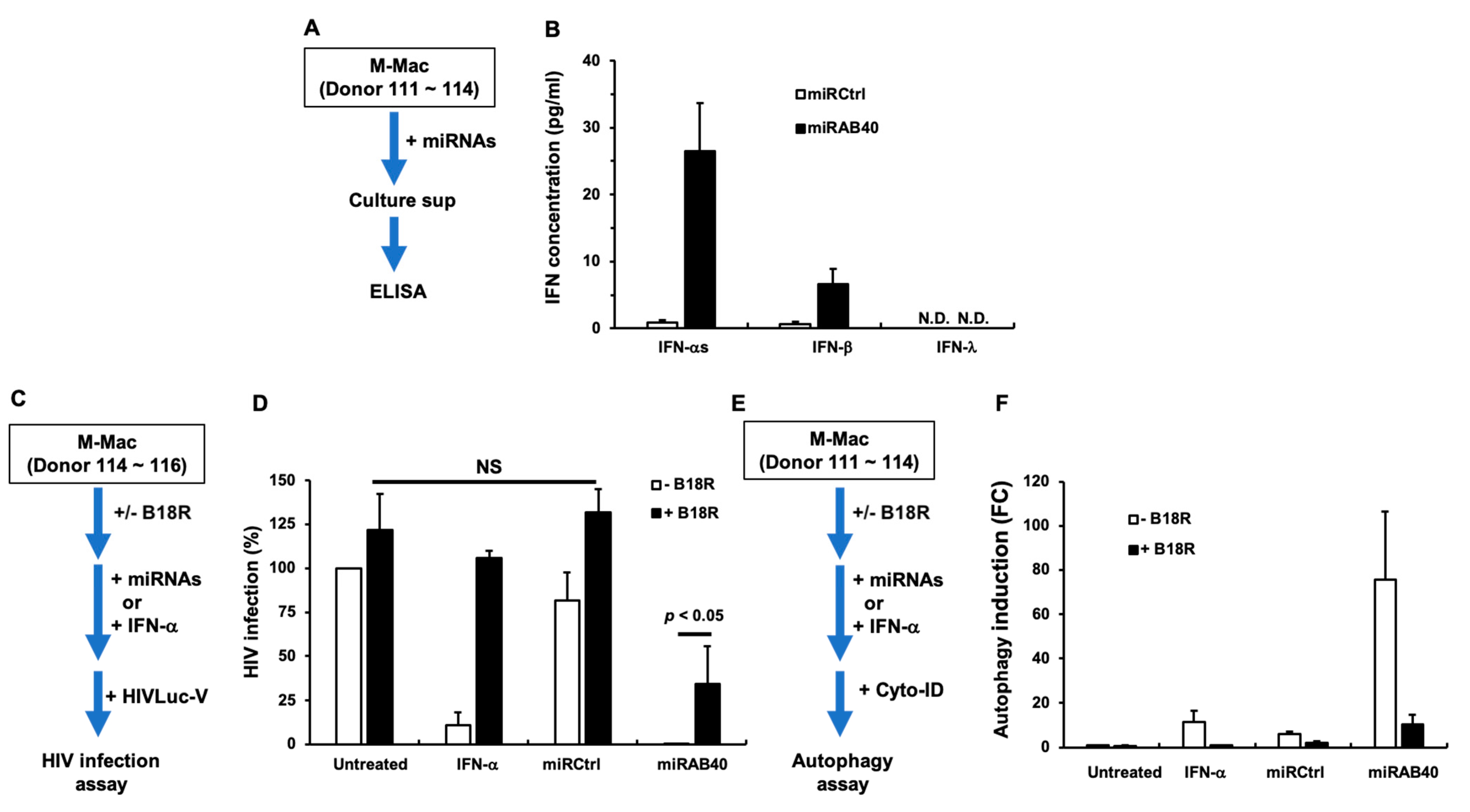
2.6. Investigation of the Mechanism Underlying miRAB40-Mediated Anti-HIV and Pro-Autophagy Properties
3. Discussion
4. Materials and Methods
4.1. Generation of Cells and Viruses
4.2. Preparation of RNA and Quality Control (QC) for RNA and RNA Seq
4.3. Read Processing and Mapping
4.4. Identification of Novel miRNAs
4.5. Prediction of Target mRNA of miRNAs
4.6. Microarray Data Analysis
4.7. Cross Analysis of Predicted miRNA Targets and Differently Expressed Genes
4.8. Real-Time PCR
4.9. Transfection of miRNA Mimic
4.10. HIV Replication Assay
4.11. HIV Infection Assay
4.12. Autophagy Assay
4.13. Enzyme-Linked Immunosorbent Assay
4.14. Neutralization of Type-I IFN Using B18R
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA 2012, 3, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Abdelmohsen, K.; Gorospe, M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014, 34, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.S.; Malakar, A.K.; Chakraborty, S. Interplay between miRNAs and human diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying circulating microRNAs as biomarkers of cardiovascular disease: A systematic review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef]
- O′Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Alexander, M.; Hu, R.; Runtsch, M.C.; Kagele, D.A.; Mosbruger, T.L.; Tolmachova, T.; Seabra, M.C.; Round, J.L.; Ward, D.M.; O’Connell, R.M. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat. Commun. 2015, 6, 7321. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, M.; Kim, Y.; Jung, H.S.; Jeoung, D. Exosomal MicroRNAs as Mediators of Cellular Interactions Between Cancer Cells and Macrophages. Front. Immunol. 2020, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-cell communication: MicroRNAs as hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Samatov, T.R.; Tonevitsky, A.G.; Burwinkel, B. Circulating miRNAs: Cell-cell communication function? Front. Genet. 2013, 4, 119. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.M.; Cheng, G.; Wieland, S.; Volinia, S.; Croce, C.M.; Chisari, F.V.; David, M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature 2007, 449, 919–922. [Google Scholar] [CrossRef]
- Shabani, M.; Nasr Esfahani, B.; Sadegh Ehdaei, B.; Moghim, S.; Mirzaei, A.; Sharifi, M.; Mouhebat, L. Inhibition of herpes simplex virus type 1 replication by novel hsa-miR-7704 in vitro. Res. Pharm. Sci. 2019, 14, 167–174. [Google Scholar] [CrossRef]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Yoshida, H.; Hunter, C.A. The immunobiology of interleukin-27. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Hunter, C.A. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 2008, 117, 123–130. [Google Scholar] [CrossRef]
- Aparicio-Siegmund, S.; Garbers, C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015, 26, 579–586. [Google Scholar] [CrossRef]
- Yoshimura, T.; Takeda, A.; Hamano, S.; Miyazaki, Y.; Kinjyo, I.; Ishibashi, T.; Yoshimura, A.; Yoshida, H. Two-Sided Roles of IL-27: Induction of Th1 Differentiation on Naive CD4+ T Cells versus Suppression of Proinflammatory Cytokine Production Including IL-23-Induced IL-17 on Activated CD4+ T Cells Partially Through STAT3-Dependent Mechanism. J. Immunol. 2006, 177, 5377–5385. [Google Scholar] [CrossRef] [PubMed]
- Sowrirajan, B.; Saito, Y.; Poudyal, D.; Chen, Q.; Sui, H.; DeRavin, S.S.; Imamichi, H.; Sato, T.; Kuhns, U.B.; Noguchi, N.; et al. Interleukin-27 Enhances the Potential of Reactive Oxygen Species Generation from Monocyte-derived Macrophages and Dendritic cells by Induction of p47phox. Sci. Rep. 2017, 7, 43441. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, J.M.; Lempicki, R.A.; Gorelick, R.J.; Yang, J.; Adelsberger, J.W.; Garcia-Pineres, A.J.; Pinto, L.A.; Lane, H.C.; Imamichi, T. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: Implications for immune control of HIV-1 infection by IL-27. Blood 2007, 109, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Greenwell-Wild, T.; Vázquez, N.; Jin, W.; Rangel, Z.; Munson, P.J.; Wahl, S.M. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood 2009, 114, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lidie, K.B.; Chen, Q.; Adelsberger, J.W.; Zheng, X.; Huang, D.; Yang, J.; Lempicki, R.A.; Rehman, T.; Dewar, R.L.; et al. IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation. J. Exp. Med. 2013, 210, 517–534. [Google Scholar] [CrossRef]
- Swaminathan, S.; Dai, L.; Lane, H.C.; Imamichi, T. Evaluating the potential of IL-27 as a novel therapeutic agent in HIV-1 infection. Cytokine Growth Factor Rev. 2013, 24, 571–577. [Google Scholar] [CrossRef]
- Swaminathan, S.; Hu, X.; Zheng, X.; Kriga, Y.; Shetty, J.; Zhao, Y.; Stephens, R.; Tran, B.; Baseler, M.W.; Yang, J.; et al. Interleukin-27 treated human macrophages induce the expression of novel microRNAs which may mediate anti-viral properties. Biochem. Biophys. Res. Commun. 2013, 434, 228–234. [Google Scholar] [CrossRef]
- Laverdure, S.; Wang, Z.; Nagashima, K.; Lane, H.C.; Imamichi, T. IL-27 induces autophagy during monocyte-to-macrophage differentiation through a novel LC3-independent pathway. J. Immunol. 2018, 200 (Suppl. 1), 164. [Google Scholar]
- Hu, X.; Chen, Q.; Sowrirajan, B.; Bosche, M.; Imamichi, T.; Sherman, B.T. Genome-Wide Analyses of MicroRNA Profiling in Interleukin-27 Treated Monocyte-Derived Human Dendritic Cells Using Deep Sequencing: A Pilot Study. Int. J. Mol. Sci. 2017, 18, 925. [Google Scholar] [CrossRef]
- Goswami, S.; Hu, X.; Chen, Q.; Qiu, J.; Yang, J.; Poudyal, D.; Sherman, B.T.; Chang, W.; Imamichi, T. Profiles of MicroRNAs in Interleukin-27-Induced HIV-Resistant T cells: Identification of a Novel Anti-Viral MicroRNA. J. Acquir. Immune Defic. Syndr. 2020. Online Ahead of Print. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Kanellos, I.; Vergoulis, T.; Sacharidis, D.; Dalamagas, T.; Hatzigeorgiou, A.; Sartzetakis, S.; Sellis, T. MR-microT: A MapReduce-based MicroRNA target prediction method. In Proceedings of the 26th International Conference on Scientific and Statistical Database Management, Aalborg, Denmark, 30 June–2 July 2014; ACM: New York, NY, USA, 2014; pp. 1–4. [Google Scholar]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human MicroRNA Targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; De Vries, N.; Tak, P.P.; et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Berg, N.; Lee, J.W.; Le, T.T.; Neudecker, V.; Jing, N.; Eltzschig, H. MicroRNA miR-223 as regulator of innate immunity. J. Leukoc. Biol. 2018, 104, 515–524. [Google Scholar] [CrossRef]
- Sioud, M. Overcoming the challenges of siRNA activation of innate immunity: Design better therapeutic siRNAs. Methods Mol. Biol. 2015, 1218, 301–319. [Google Scholar] [CrossRef]
- Sui, H.; Zhou, M.; Chen, Q.; Lane, H.C.; Imamichi, T. siRNA enhances DNA-mediated interferon lambda-1 response through crosstalk between RIG-I and IFI16 signalling pathway. Nucleic Acids Res. 2014, 42, 583–598. [Google Scholar] [CrossRef]
- Harris, J. Autophagy and cytokines. Cytokine 2011, 56, 140–144. [Google Scholar] [CrossRef]
- Symons, J.A.; Alcamí, A.; Smith, G.L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 1995, 81, 551–560. [Google Scholar] [CrossRef]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of host proteins required for HIV infection through a functional genomic screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, M.; Huang, Q.; Gates, A.T.; Zhang, X.D.; Castle, J.C.; Stec, E.; Ferrer, M.; Strulovici, B.; Hazuda, D.J.; et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 2008, 4, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Konig, R.; Zhou, Y.; Elleder, D.; Diamond, T.L.; Bonamy, G.M.; Irelan, J.T.; Chiang, C.-Y.; Tu, B.P.; De Jesus, P.D.; Lilley, C.E.; et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 2008, 135, 49–60. [Google Scholar] [CrossRef]
- Chen, Q.; Swaminathan, S.; Yang, D.; Dai, L.; Sui, H.; Yang, J.; Hornung, R.L.; Wang, Y.; Huang, D.W.; Hu, X.; et al. Interleukin-27 is a potent inhibitor of cis HIV-1 replication in monocyte-derived dendritic cells via a type I interferon-independent pathway. PLoS ONE 2013, 8, e59194. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.C.; Zhang, X.; Katsounas, A.; Bharucha, J.P.; Kottilil, S.; Imamichi, T. Interleukin-27, an anti-HIV-1 cytokine, inhibits replication of hepatitis C virus. J. Interferon Cytokine Res. 2010, 30, 427–431. [Google Scholar] [CrossRef]
- Poudyal, D.; Yang, J.; Chen, Q.; Goswami, S.; Adelsberger, J.W.; Das, S.; Herman, A.; Hornung, R.L.; Andresson, T.; Imamichi, T. IL-27 posttranslationally regulates Y-box binding protein-1 to inhibit HIV-1 replication in human CD4+ T cells. AIDS 2019, 33, 1819–1830. [Google Scholar] [CrossRef]
- Ambjørn, M.; Ejlerskov, P.; Liu, Y.; Lees, M.; Jäättelä, M.; Issazadeh-Navikas, S. IFNB1/interferon-β-induced autophagy in MCF-7 breast cancer cells counteracts its proapoptotic function. Autophagy 2013, 9, 287–302. [Google Scholar] [CrossRef]
- Lee, H.K.; Lund, J.M.; Ramanathan, B.; Mizushima, N.; Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 2007, 315, 1398–1401. [Google Scholar] [CrossRef]
- Ablasser, A.; Hur, S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 2020, 21, 17–29. [Google Scholar] [CrossRef]
- Forster, S.C.; Tate, M.D.; Hertzog, P.J. MicroRNA as Type I Interferon-Regulated Transcripts and Modulators of the Innate Immune Response. Front. Immunol. 2015, 6, 334. [Google Scholar] [CrossRef]
- Ishibashi, O.; Ali, M.M.; Luo, S.S.; Ohba, T.; Katabuchi, H.; Takeshita, T.; Takizawa, T. Short RNA duplexes elicit RIG-I-mediated apoptosis in a cell type- and length-dependent manner. Sci. Signal. 2011, 4, ra74. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Farazi, T.A.; Hoell, J.I.; Morozov, P.; Tuschl, T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013, 774, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Saha, B. IL-27 in tumor immunity and immunotherapy. Trends Mol. Med. 2013, 19, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fabbi, M.; Carbotti, G.; Ferrini, S. Dual Roles of IL-27 in Cancer Biology and Immunotherapy. Mediat. Inflamm. 2017, 2017, 3958069. [Google Scholar] [CrossRef] [PubMed]
- Theodore, T.S.; Englund, G.; Buckler-White, A.; Buckler, C.E.; Martin, M.A.; Peden, K.W. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res. Hum. Retrovir. 1996, 12, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.I.; Chen, B.K.; Choe, S.; Landau, N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 1995, 206, 935–944. [Google Scholar] [CrossRef]
- He, J.; Choe, S.; Walker, R.; Di Marzio, P.; Morgan, D.O.; Landau, N.R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 1995, 69, 6705–6711. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Tsao, M.S.; McPherson, J.D. Optimization of miRNA-seq data preprocessing. Brief. Bioinform. 2015, 16, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Burge, S.W.; Bateman, A.; Daub, J.; Eberhardt, R.Y.; Eddy, S.R.; Floden, E.W.; Gardner, P.P.; Jones, T.A.; Tate, J.; et al. Rfam 12.0: Updates to the RNA families database. Nucleic Acids Res. 2015, 43, D130–D137. [Google Scholar] [CrossRef] [PubMed]
- Reiche, K.; Stadler, P.F. RNAstrand: Reading direction of structured RNAs in multiple sequence alignments. Algorithms Mol. Biol. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, D.; Herman, A.; Adelsberger, J.W.; Yang, J.; Hu, X.; Chen, Q.; Bosche, M.; Sherman, B.T.; Imamichi, T. A novel microRNA, hsa-miR-6852 differentially regulated by Interleukin-27 induces necrosis in cervical cancer cells by downregulating the FoxM1 expression. Sci. Rep. 2018, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Imamichi, T.; Berg, S.C.; Imamichi, H.; Lopez, J.C.; Metcalf, J.A.; Falloon, J.; Lane, H.C. Relative replication fitness of a high-level 3′-azido-3′-deoxythymidine-resistant variant of human immunodeficiency virus type 1 possessing an amino acid deletion at codon 67 and a novel substitution (Thr-->Gly) at codon 69. J. Virol. 2000, 74, 10958–10964. [Google Scholar] [CrossRef]
| miRNA ID | Accession # * | Mature Sequence 5′-3′ | Precursor Genomic Location |
|---|---|---|---|
| miRAB1 | MF281454 | cagagagaggaagagagcugcu | chr3:38142537..38142613:+ |
| miRAB2 | MF281455 | auguagugcuucuugggacuga | chr3:142310544..142310610:− |
| miRAB3 | MF281456 | aauugagguuuuaucugaggggau | chr11:94184662..94184742:+ |
| miRAB4 | MF281457 | agcacauuuaggaauaggggaa | chr9:5509899..5509964:− |
| miRAB5 | MF281458 | ucuauguauggauauguguguau | chr8:22688994..22689070:− |
| miRAB6 | MF281459 | agggucucacuguugccagga | chr8:11864247..11864305:− |
| miRAB7 | MF281460 | ccaaggucugacucauggguaga | chr7:32869838..32869887:− |
| miRAB8 | MF281461 | ucuaccccagggagaaucugaga | chr12:13216143..13216224:+ |
| miRAB9 | MF281462 | aaggaggaagacugggcauagu | chr5:132487413..132487471:− |
| miRAB10 | MF281463 | cugccugugugggacugagaugc | chr6_GL000252v2_alt:2526871..2526961:− |
| miRAB11 | MF281464 | aagaguaauugugguuuuuguu | chr4:8218466..8218522:+ |
| MiRAB12 | MF281465 | uagcccuuccggauccugcgc | chr2:191013985..191014043:− |
| miRAB13 | MF281466 | uuuguuuucuaguuaccucu | chr11:6094879..6094943:+ |
| miRAB14 | MF281467 | ucagggaacagcaacagggcugc | chr1:159800217..159800282:− |
| miRAB15 ** | KY994057 | aggacuggaugucgggcugcaugu | chr4:141707940..141708015:+ |
| miRAB16 ** | KY994062 | acguggacuccagacucucugu | chr17:42311190..42311258:+ |
| miRAB17 | MF281468 | aaggguuugggucugagcuguau | chr2:85318324..85318388:− |
| miRAB18 | MF281469 | cuccccugauguaccugaacaagag | chr3:27374770..27374839:− |
| miRAB19 | MF281470 | agcggaacuugaggagccgaga | chr1:161446654..161446715:− |
| miRAB20 | MF281471 | auccuagcuugccugagacugu | chr2:71526827..71526913:+ |
| miRAB21 | MF281472 | ugacuuguguuucuuuuucccaa | chr4:70946713..70946766:− |
| miRAB22 | MF281473 | uuagagcuucaaccuccaguguga | chr8:115534466..115534532:− |
| miRAB23 | MF281474 | guuaggucaagguguagcccaug | chr5:80651341..80651395:− |
| miRAB24 | MF281475 | cuuccccacccucuccugcagc | chr19:12952627..12952689:+ |
| miRAB25 | MF281476 | cucgggcgcuccggcuguaagg | chr5:96936079..96936137:+ |
| miRAB26 | MF281477 | agacggaucaggcucuccuc | chr1:44805291..44805352:+ |
| miRAB27 | MF281478 | ucaguaagagugggcucugucga | chr3:196326926..196326980:− |
| miRAB28 | MF281479 | uccggauccagcuucuugucu | chr22:37878013..37878073:+ |
| miRAB29 | MF281480 | ugcugugugauuuugagugac | chr5:151255955..151256014:− |
| miRAB30 | MF281481 | uguccuugggccucuuuguuu | chrX:109740259..109740316:+ |
| miRAB31 | MF281482 | uuuguacuccggccgcugcuga | chr2:42092534..42092630:+ |
| miRAB32 | MF281483 | aaagagcuuggucuuuggagcca | chr10:71422254..71422310:+ |
| miRAB33 | MF281484 | cucggccuuugcucgcagcacucg | chr15:56365282..56365340:− |
| miRAB34 | MF281485 | ucuauuucuuguguucccugc | chr8:130528069..130528129:− |
| miRAB35 | MF281486 | aucugcucgccggagcucacucu | chr15:75648027..75648082:+ |
| miRAB36 | MF281487 | cgcgcucgcgggggcucugaga | chr1:178725112..178725166:− |
| miRAB37 | MF281488 | cugaggagaacaggagcucucuu | chr22:22085831..22085887:− |
| miRAB38 ** | MF281431 | uccccaguacccccacca | chr1:156887613..156887686:+ |
| miRAB39 | MF281489 | uccauaucccaaccugucagagu | chr10:89197656..89197715:+ |
| miRAB40 | MF281490 | uaacuucucauuaugccuucugga | chr1:221076658..221076720:+ |
| miRAB41 | MF281491 | ucaggggaugggagugacauggc | chr17:74522904..74522966:− |
| miRNA ID | Gene Name ** | Symbol | FC *** | p Value **** |
|---|---|---|---|---|
| miRAB4 | dipeptidyl peptidase 4 | DPP4 | −3.96 | p < 0.001 |
| miRAB9 | RAB6B, member RAS oncogene family | RAB6B | −2.14 | p < 0.05 |
| myosin ID | MYO1D | −2.96 | p < 0.01 | |
| miRAB12 | actin binding LIM protein family member 3 | ABLIM3 | −2.29 | p < 0.01 |
| miRAB14 | C-X-C motif chemokine ligand 9 | CXCL9 | 15.08 | p < 0.05 |
| RAB6B, member RAS oncogene family | RAB6B | −2.14 | p < 0.05 | |
| miRAB29 | dipeptidyl peptidase 4 | DPP4 | −3.96 | p < 0.01 |
| RAB6B, member RAS oncogene family | RAB6B | −2.14 | p < 0.05 | |
| miRAB30 | Podocalyxin-like | PODXL | −2.45 | p < 0.05 |
| Host-Dependency Factors (HDFs) for HIV Infection | ||
| Gene | FC * | p Values ** |
| HIBCH | 1.7 ± 0.2 (4) | p < 0.01 |
| TBL1X | 1.5 ± 0.2 (4) | p < 0.05 |
| SLC24A1 | 2.5 ± 0.5 (4) | p < 0.05 |
| MAP3K7 | 1.8 ± 0.4 (4) | NS *** |
| ETS2 | 2.0 ± 1.2 (4) | NS |
| DHX15 | 1.5 ± 0.2 (4) | p < 0.05 |
| Autophagy Regulatory Factors (ARFs) | ||
| Gene | FC | p Values |
| PIK3C | 1.6 ± 0.2 (4) | p < 0.05 |
| RAB9A | 2.5 ± 0.6 (4) | n.s |
| NRAS | 1.3 ± 0.2 (4) | n.s |
| SIK1 | 5.2 ± 1.7 (4) | n.s |
| ITGb1 | 1.1 ± 0.1 (4) | n.s |
| CIP2A/KIAA1524 | 1.0 ± 0.2 (3) | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamichi, T.; Goswami, S.; Hu, X.; Laverdure, S.; Yang, J.; Qiu, J.; Chen, Q.; Sherman, B.T.; Chang, W. MicroRNA Profiles in Monocyte-Derived Macrophages Generated by Interleukin-27 and Human Serum: Identification of a Novel HIV-Inhibiting and Autophagy-Inducing MicroRNA. Int. J. Mol. Sci. 2021, 22, 1290. https://doi.org/10.3390/ijms22031290
Imamichi T, Goswami S, Hu X, Laverdure S, Yang J, Qiu J, Chen Q, Sherman BT, Chang W. MicroRNA Profiles in Monocyte-Derived Macrophages Generated by Interleukin-27 and Human Serum: Identification of a Novel HIV-Inhibiting and Autophagy-Inducing MicroRNA. International Journal of Molecular Sciences. 2021; 22(3):1290. https://doi.org/10.3390/ijms22031290
Chicago/Turabian StyleImamichi, Tomozumi, Suranjana Goswami, Xiaojun Hu, Sylvain Laverdure, Jun Yang, Ju Qiu, Qian Chen, Brad T. Sherman, and Weizhong Chang. 2021. "MicroRNA Profiles in Monocyte-Derived Macrophages Generated by Interleukin-27 and Human Serum: Identification of a Novel HIV-Inhibiting and Autophagy-Inducing MicroRNA" International Journal of Molecular Sciences 22, no. 3: 1290. https://doi.org/10.3390/ijms22031290
APA StyleImamichi, T., Goswami, S., Hu, X., Laverdure, S., Yang, J., Qiu, J., Chen, Q., Sherman, B. T., & Chang, W. (2021). MicroRNA Profiles in Monocyte-Derived Macrophages Generated by Interleukin-27 and Human Serum: Identification of a Novel HIV-Inhibiting and Autophagy-Inducing MicroRNA. International Journal of Molecular Sciences, 22(3), 1290. https://doi.org/10.3390/ijms22031290







