UV-Induced Reduction of ACVR1C Decreases SREBP1 and ACC Expression by the Suppression of SMAD2 Phosphorylation in Normal Human Epidermal Keratinocytes
Abstract
1. Introduction
2. Results
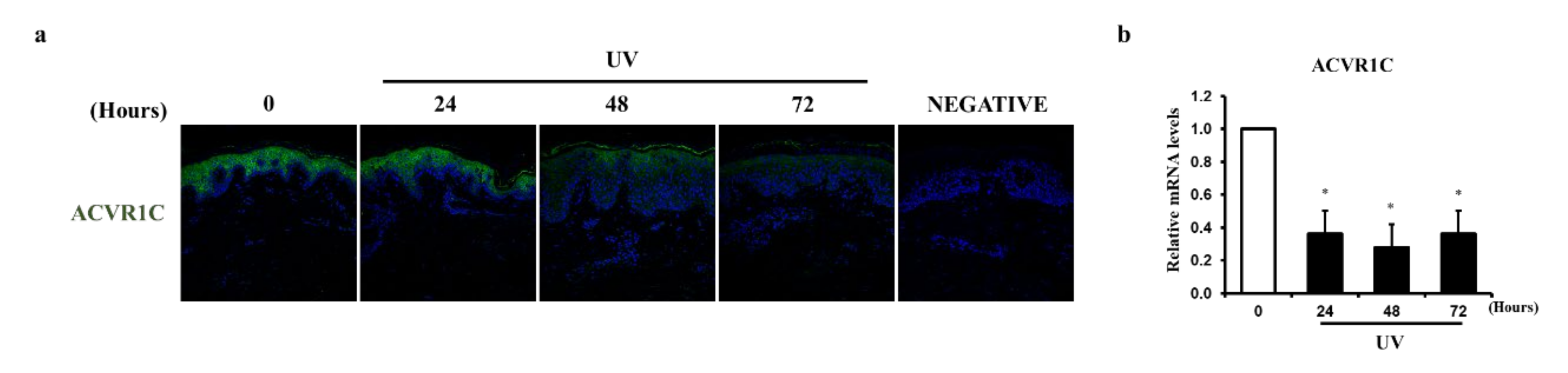
2.1. UV Irradiation Decreases the Expression of ACVR1C in the Epidermis
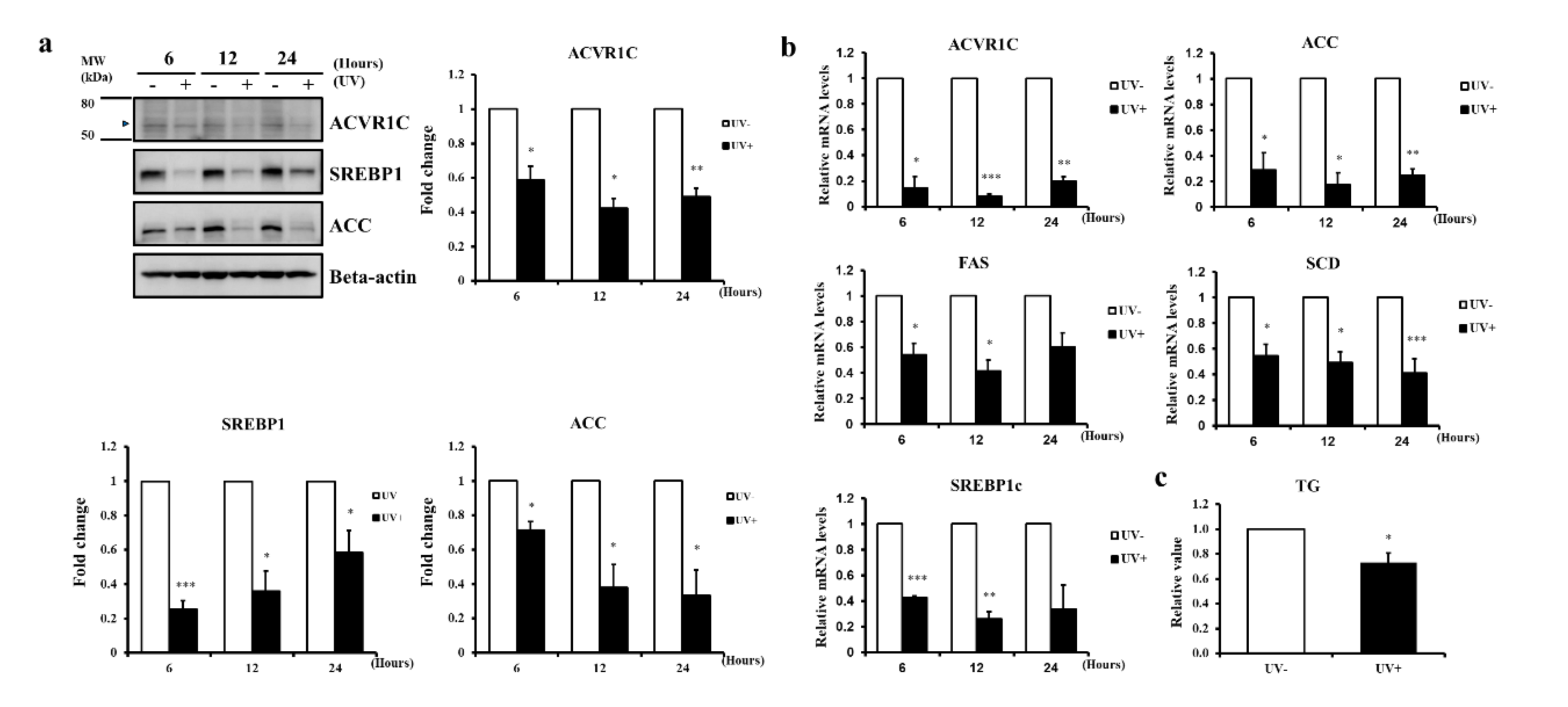
2.2. UV Irradiation Decreases the Expression of ACVR1C and Lipogenic Genes in Primary NHEK
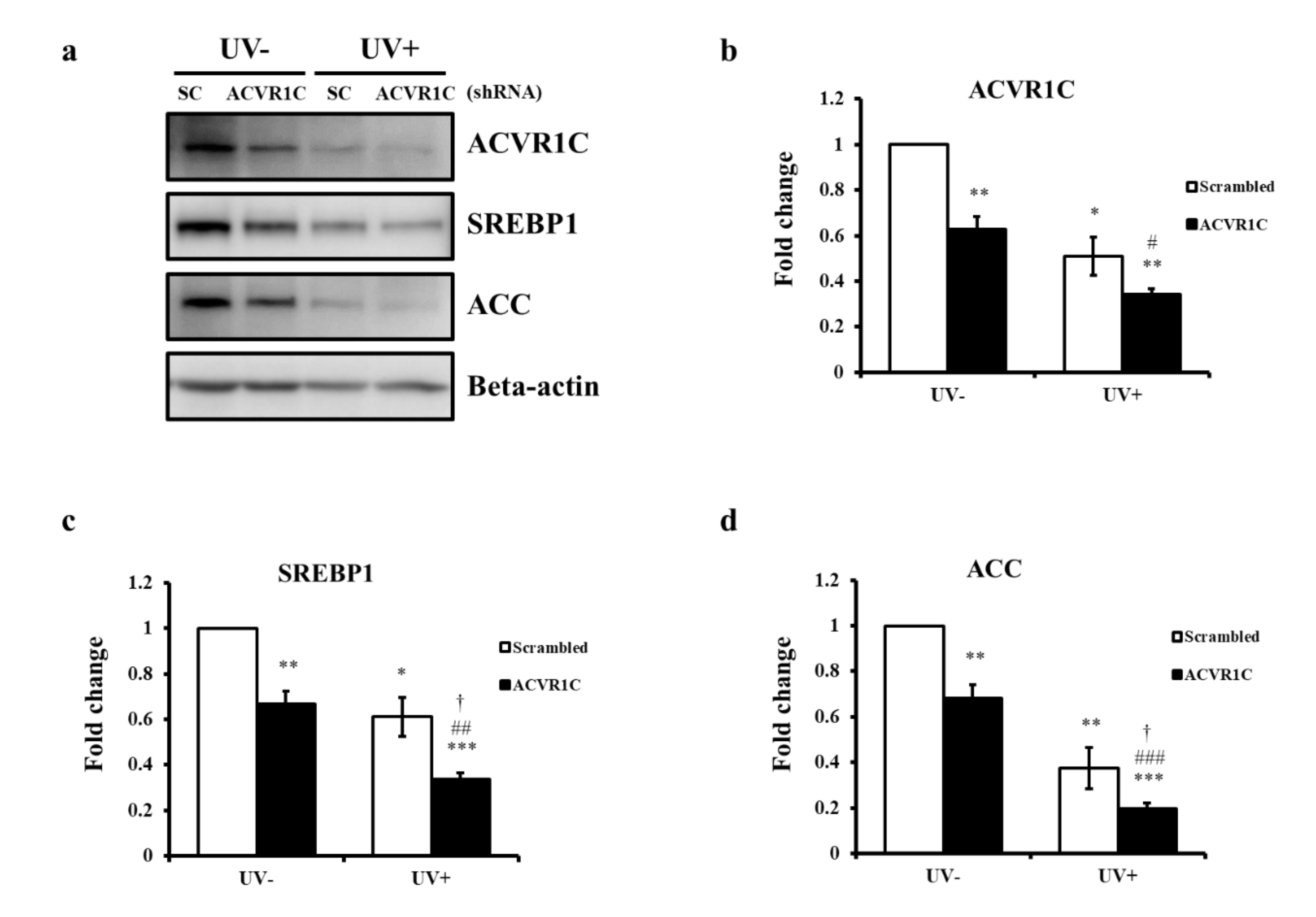
2.3. Knockdown of ACVR1C Decreases the Expression of SREBP1 and ACC in Both Non-Irradiated and UV-Irradiated NHEK
2.4. The Overexpression of ACVR1C Ameliorates UV-Induced Decreases in SREBP1 and ACC Protein Expression in Primary NHEK
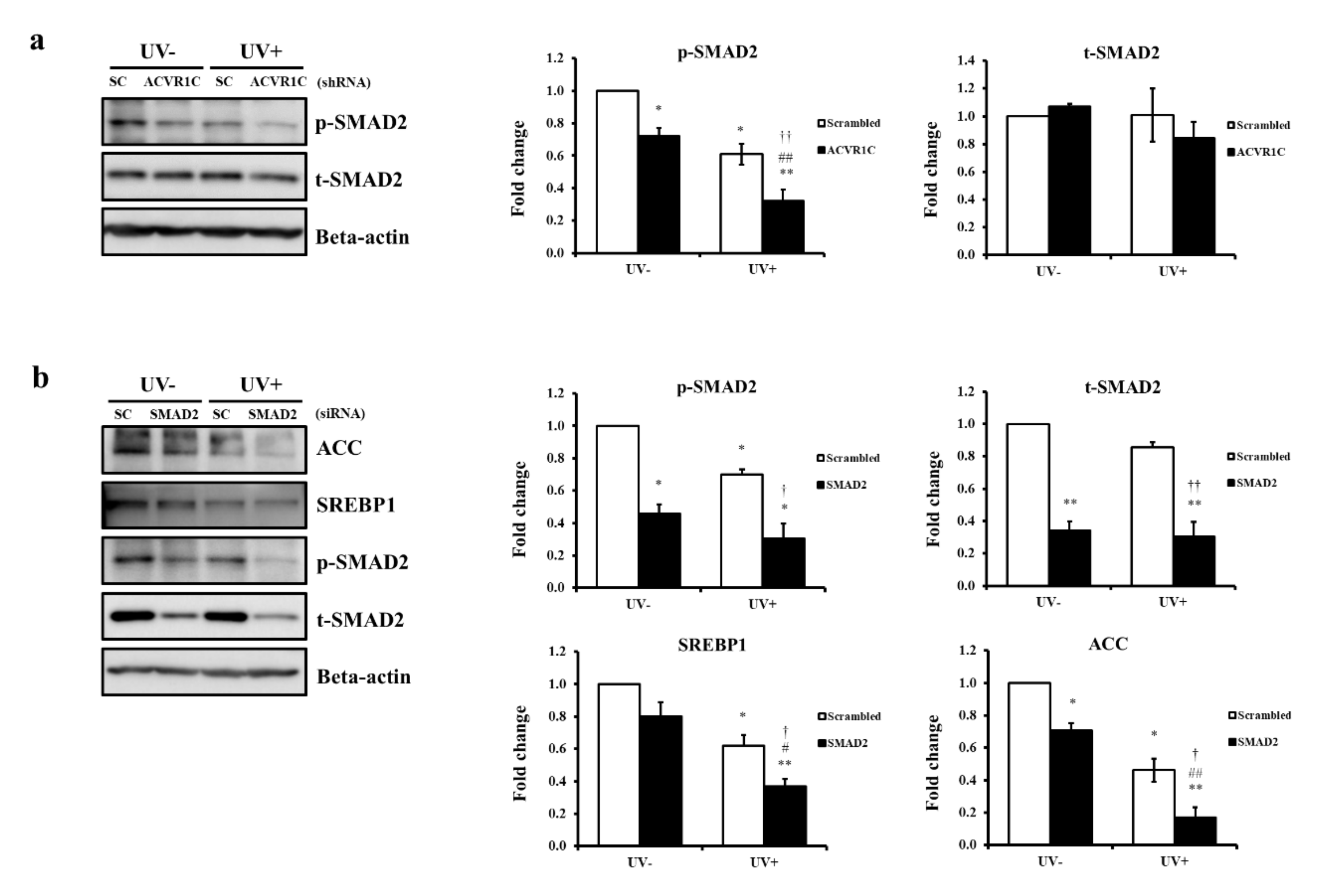
2.5. ACVR1C Regulates the Expression of SREBP1 and ACC Protein via the Suppression of SMAD2 Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Human Skin Samples and UV Irradiation
4.2. Cell Culture and UV Irradiation
4.3. Plasmid Transfection
4.4. RNA Interference
4.5. Western Blot Analysis and Immunofluorescence Staining
4.6. Real-Time and Semi-Quantitative PCR (RT-PCR)
4.7. Triglyceride Contents
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACVR1C | Activin A receptor type 1C |
| ACC | acetyl CoA carboxylase |
| MED | Minimal erythema dose |
| NHEK | Normal human epidermal keratinocytes |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| SREBP1 | Sterol regulatory element-binding protein1 |
| TG | Triglycerides |
| UV | Ultraviolet |
References
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Athenstaedt, K.; Daum, G. The life cycle of neutral lipids: Synthesis, storage and degradation. Cell. Mol. Life Sci. 2006, 63, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Cases, S.; Jensen, D.R.; Chen, H.C.; Sande, E.; Tow, B.; Sanan, D.A.; Raber, J.; Eckel, R.H.; Farese, R.V., Jr. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking. Dgat. Nat. Genet. 2000, 25, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar]
- Radner, F.P.; Fischer, J. The important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier function. Biochim. Biophys. Acta 2014, 1841, 409–415. [Google Scholar] [CrossRef]
- Kim, E.J.; Jin, X.J.; Kim, Y.K.; Oh, I.K.; Kim, J.E.; Park, C.H.; Chung, J.H. UV decreases the synthesis of free fatty acids and triglycerides in the epidermis of human skin in vivo, contributing to development of skin photoaging. J. Dermatol. Sci. 2010, 57, 19–26. [Google Scholar] [CrossRef]
- Coleman, R.A.; Mashek, D.G. Mammalian triacylglycerol metabolism: Synthesis, lipolysis, and signaling. Chem. Rev. 2011, 111, 6359–6386. [Google Scholar] [CrossRef]
- Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Hasty, A.H.; Osuga, J.; Tamura, Y.; Shionoiri, F.; Iizuka, Y.; Ohashi, K.; Harada, K.; et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem. 1999, 274, 35832–35839. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Ericsson, J.; Jackson, S.M.; Kim, J.B.; Spiegelman, B.M.; Edwards, P.A. Identification of glycerol-3-phosphate acyltransferase as an adipocyte determination and differentiation factor 1- and sterol regulatory element-binding protein-responsive gene. J. Biol. Chem. 1997, 272, 7298–7305. [Google Scholar] [CrossRef]
- Meguro, S.; Arai, Y.; Masukawa, K.; Uie, K.; Tokimitsu, I. Stratum corneum lipid abnormalities in UVB-irradiated skin. Photochem. Photobiol. 1999, 69, 317–321. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Trautinger, F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin. Exp. Dermatol. 2001, 26, 573–577. [Google Scholar] [CrossRef]
- Mirgayazova, R.; Khadiullina, R.; Mingaleeva, R.; Chasov, V.; Gomzikova, M.; Garanina, E.; Rizvanov, A.; Bulatov, E. Novel Isatin-based activator of p53 transcriptional functions in tumor cells. Mol. Biol. Res. Commun. 2019, 8, 119–128. [Google Scholar] [PubMed]
- Xu, G.; Zhong, Y.; Munir, S.; Yang, B.B.; Tsang, B.K.; Peng, C. Nodal induces apoptosis and inhibits proliferation in human epithelial ovarian cancer cells via activin receptor-like kinase 7. J. Clin. Endocrinol. Metab. 2004, 89, 5523–5534. [Google Scholar] [CrossRef]
- Bu, Y.; Okunishi, K.; Yogosawa, S.; Mizuno, K.; Irudayam, M.J.; Brown, C.W.; Izumi, T. Insulin Regulates Lipolysis and Fat Mass by Upregulating Growth/Differentiation Factor 3 in Adipose Tissue Macrophages. Diabetes 2018, 67, 1761–1772. [Google Scholar] [CrossRef]
- Bernard, D.J.; Lee, K.B.; Santos, M.M. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod. Biol. Endocrinol. 2006, 4, 52. [Google Scholar] [CrossRef]
- Tsuchida, K.; Nakatani, M.; Hitachi, K.; Uezumi, A.; Sunada, Y.; Ageta, H.; Inokuchi, K. Activin signaling as an emerging target for therapeutic interventions. Cell Commun. Signal. 2009, 7, 15. [Google Scholar] [CrossRef]
- Jornvall, H.; Blokzijl, A.; ten Dijke, P.; Ibanez, C.F. The orphan receptor serine/threonine kinase ALK7 signals arrest of proliferation and morphological differentiation in a neuronal cell line. J. Biol. Chem. 2001, 276, 5140–5146. [Google Scholar] [CrossRef]
- Xu, G.; Zhou, H.; Wang, Q.; Auersperg, N.; Peng, C. Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol. Cancer Res. 2006, 4, 235–246. [Google Scholar] [CrossRef]
- Kogame, M.; Matsuo, S.; Nakatani, M.; Kurisaki, A.; Nishitani, H.; Tsuchida, K.; Sugino, H. ALK7 is a novel marker for adipocyte differentiation. J. Med. Investig. 2006, 53, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Kumar, M.; Xu, G.; Ju, W.; Yoon, T.; Xu, E.; Huang, X.; Gaisano, H.; Peng, C.; Wang, Q. Activin receptor-like kinase 7 induces apoptosis of pancreatic beta cells and beta cell lines. Diabetologia 2006, 49, 506–518. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertolino, P.; Holmberg, R.; Reissmann, E.; Andersson, O.; Berggren, P.O.; Ibanez, C.F. Activin B receptor ALK7 is a negative regulator of pancreatic beta-cell function. Proc. Natl. Acad. Sci. USA 2008, 105, 7246–7251. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Xu, G.; Zhou, T.; Yang, C.; Wang, X.; Peng, C.; Zhou, H. Reduced expression of activin receptor-like kinase 7 in breast cancer is associated with tumor progression. Med. Oncol. 2012, 29, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tang, Y.; Wu, G.; Mei, Y.; Liu, W.; Liu, X.; Wan, N.; Liu, Y.; Huang, C. ALK7 protects against pathological cardiac hypertrophy in mice. Cardiovasc. Res. 2015, 108, 50–61. [Google Scholar] [CrossRef]
- Li, W.B.; Zhao, J.; Liu, L.; Wang, Z.H.; Han, L.; Zhong, M.; Zhang, Y.; Zhang, W.; Tang, M.X. Silencing of activin receptor-like kinase 7 alleviates aortic stiffness in type 2 diabetic rats. Acta Diabetol. 2015, 52, 717–726. [Google Scholar] [CrossRef]
- Michael, I.P.; Saghafinia, S.; Tichet, M.; Zangger, N.; Marinoni, I.; Perren, A.; Hanahan, D. ALK7 Signaling Manifests a Homeostatic Tissue Barrier That Is Abrogated during Tumorigenesis and Metastasis. Dev. Cell. 2019, 49, 409–424.e6. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, D.H.; Kim, Y.K.; Lee, Y.M.; Eun, H.C.; Chung, J.H. Decreased expression of activin A receptor 1C may result in Ca(2+) -induced aberrant skin hypersensitivity. Exp. Dermatol. 2016, 25, 402–404. [Google Scholar] [CrossRef]
- Swindell, W.R.; Stuart, P.E.; Sarkar, M.K.; Voorhees, J.J.; Elder, J.T.; Johnston, A.; Gudjonsson, J.E. Cellular dissection of psoriasis for transcriptome analyses and the post-GWAS era. BMC Med. Genom. 2014, 7, 27. [Google Scholar] [CrossRef]
- Yogosawa, S.; Mizutani, S.; Ogawa, Y.; Izumi, T. Activin receptor-like kinase 7 suppresses lipolysis to accumulate fat in obesity through downregulation of peroxisome proliferator-activated receptor gamma and C/EBPalpha. Diabetes 2013, 62, 115–123. [Google Scholar] [CrossRef]
- Saint-Martory, C.; Roguedas-Contios, A.M.; Sibaud, V.; Degouy, A.; Schmitt, A.M.; Misery, L. Sensitive skin is not limited to the face. Br. J. Dermatol. 2008, 158, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Yogosawa, S.; Izumi, T. Roles of activin receptor-like kinase 7 signaling and its target, peroxisome proliferator-activated receptor gamma, in lean and obese adipocytes. Adipocyte 2013, 2, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Choi, H.R.; Won, C.H.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Alteration of the TGF-beta/SMAD pathway in intrinsically and UV-induced skin aging. Mech. Ageing Dev. 2005, 126, 560–567. [Google Scholar] [CrossRef]
- Andersson, O.; Korach-Andre, M.; Reissmann, E.; Ibanez, C.F.; Bertolino, P. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc. Natl. Acad. Sci. USA 2008, 105, 7252–7256. [Google Scholar] [CrossRef]
- Kim, M.; Han, J.H.; Kim, J.H.; Park, T.J.; Kang, H.Y. Secreted Frizzled-Related Protein 2 (sFRP2) Functions as a Melanogenic Stimulator; the Role of sFRP2 in UV-Induced Hyperpigmentary Disorders. J. Investig. Dermatol. 2016, 136, 236–244. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, J.E.; Kim, S.; Kim, M.K.; Park, C.H.; Chung, J.H. UV modulation of subcutaneous fat metabolism. J. Investig. Dermatol. 2011, 131, 1720–1726. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.-D.; Chung, M.H.; Quan, Q.-L.; Lee, D.H.; Kim, E.J.; Chung, J.H. UV-Induced Reduction of ACVR1C Decreases SREBP1 and ACC Expression by the Suppression of SMAD2 Phosphorylation in Normal Human Epidermal Keratinocytes. Int. J. Mol. Sci. 2021, 22, 1101. https://doi.org/10.3390/ijms22031101
Tian Y-D, Chung MH, Quan Q-L, Lee DH, Kim EJ, Chung JH. UV-Induced Reduction of ACVR1C Decreases SREBP1 and ACC Expression by the Suppression of SMAD2 Phosphorylation in Normal Human Epidermal Keratinocytes. International Journal of Molecular Sciences. 2021; 22(3):1101. https://doi.org/10.3390/ijms22031101
Chicago/Turabian StyleTian, Yu-Dan, Min Hwa Chung, Qing-Ling Quan, Dong Hun Lee, Eun Ju Kim, and Jin Ho Chung. 2021. "UV-Induced Reduction of ACVR1C Decreases SREBP1 and ACC Expression by the Suppression of SMAD2 Phosphorylation in Normal Human Epidermal Keratinocytes" International Journal of Molecular Sciences 22, no. 3: 1101. https://doi.org/10.3390/ijms22031101
APA StyleTian, Y.-D., Chung, M. H., Quan, Q.-L., Lee, D. H., Kim, E. J., & Chung, J. H. (2021). UV-Induced Reduction of ACVR1C Decreases SREBP1 and ACC Expression by the Suppression of SMAD2 Phosphorylation in Normal Human Epidermal Keratinocytes. International Journal of Molecular Sciences, 22(3), 1101. https://doi.org/10.3390/ijms22031101





