Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats
Abstract
:1. Introduction
2. Results
2.1. Animal Characteristics
2.2. Endothelium-Dependent and Independent Vascular Relaxation
2.3. Vascular Contraction in Response to Endothelin-1
2.4. Oxidative Stress and Glycation in the Vascular Wall
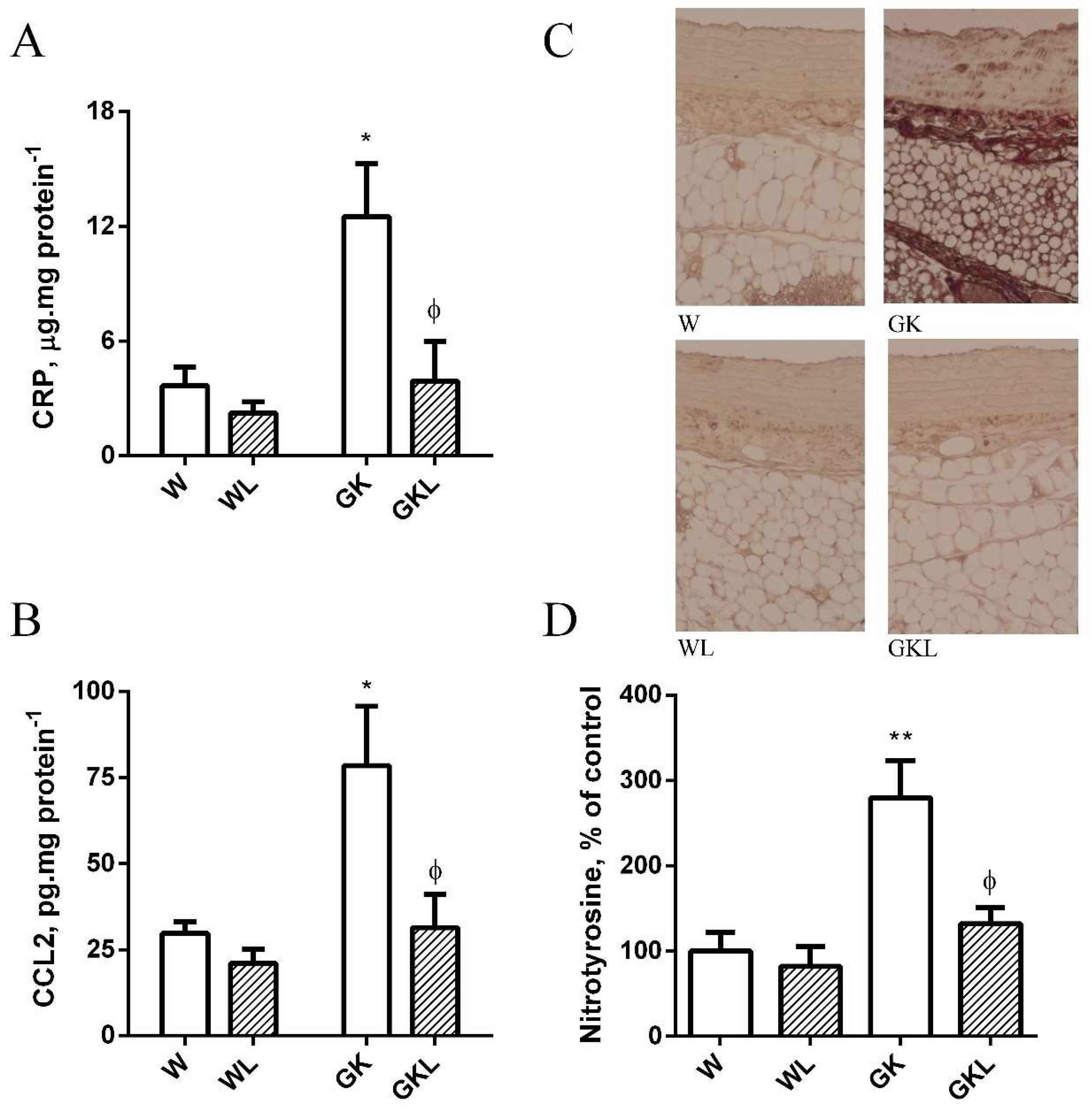
2.5. PVAT Oxidative and Inflammatory Biomarkers
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Determination of Metabolic Parameters
4.3. Isometric Tension Studies
4.4. Detection of Superoxide
4.5. Determination of Aortic Immunofluorescence
4.6. Enzymatic Assays
4.7. Measurement of Glutathione Concentration
4.8. Statistical Analysis
4.9. Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| AGEs | advanced glycation end-products |
| AUC | area under the curve |
| CCL2 | CC-chemokine ligand 2 |
| CRP | C reactive protein |
| DHE | dihydroethidium |
| eNOS | endothelial nitric oxide synthase |
| ET1 | endothelin-1 |
| GK | Goto-Kakizaki |
| IPGTT | intraperitoneal glucose tolerance test |
| ITT | insulin tolerance test |
| L-NAME | N-nitro-L-arginine- methyl ester |
| NO | nitric oxide |
| PVAT | perivascular adipose tissue |
| PPAR-γ | peroxisome proliferator-activated receptor |
| ROS | reactive oxygen species |
| SNP | sodium nitroprusside |
| T2D | type 2 diabetes |
References
- Stehouwer, C.D.; Henry, R.M.; Ferreira, I. Arterial stiffness in diabetes and the metabolic syndrome: A pathway to cardio-vascular disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Hiuge, A.; Makino, H.; Nagumo, A.; Takaki, H.; Konishi, H.; Goto, Y.; Yoshimasa, Y.; Miyamoto, Y. Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. J. Atheroscler. Thromb. 2010, 17, 828–833. [Google Scholar] [CrossRef] [Green Version]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Sena, C.M.; Pereira, A.M.; Seiça, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Fernandes, R.; Seiça, R.M. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011, 163, 424–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azul, L.; Leandro, A.; Boroumand, P.; Klip, A.; Seiça, R.; Sena, C.M. Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic. Biol. Med. 2019, 146, 264–274. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.; Papavassiliou, A.; Stefanadis, C. Flavonoids in Atherosclerosis: An Overview of Their Mechanisms of Action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Blandizzi, C.; Antonioli, L. Dietary flavonoids as a potential intervention to improve redox balance in obesity and related co-morbidities: A review. Nutr. Res. Rev. 2018, 31, 239–247. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Qian, L.B.; Wang, H.P.; Chen, Y.; Chen, F.X.; Ma, Y.Y.; Bruce, I.C.; Xia, Q. Luteolin reduces high glucose-mediated impairment of endothelium dependent relaxation in rat aorta by reducing oxidative stress. Pharmacol. Res. 2010, 61, 281–287. [Google Scholar] [CrossRef]
- Si, H.; Wyeth, R.P.; Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur. J. Nutr. 2013, 53, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Nallasamy, P.; Liu, D.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-alpha induced monocyte adhesion to endothelial cells via suppressing IKBa/NF-kB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhardt, R. Prevention of Taurolithocholate-Induced Hepatic Bile Canalicular Distortions by HPLC-Characterized Extracts of Artichoke (Cynara scolymus) Leaves. Planta Med. 2002, 68, 776–779. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin attenuate diabetes-induced impairment in endothelial-dependent relaxation: Effect on lipid profile, AGEs and NO generation. Phytother. Res. 2013, 27, 1678–1684. [Google Scholar] [CrossRef]
- Kwon, E.-Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.-S. Luteolin Attenuates Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue in Mice with Diet-Induced Obesity. Diabetes 2015, 64, 1658–1669. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Zhang, L.; Dong, J.; Zhang, X.; Chen, Y.-G.; Bao, B.; Liu, J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014, 58, 1258–1268. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.; Fernandes, R.; Crisóstomo, J.; Seiça, R.; Sena, C.M. The Sulforaphane and pyridoxamine supplementation normalize endothelial dysfunction associated with type 2 diabetes. Sci. Rep. 2017, 7, 14357. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an Anti-Inflammatory and Neuroprotective Agent: A Brief Review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Lan, N.; Li, S.; Zhang, J.; Wang, S.; Li, C.; Shang, Y.; Huang, T.; Zhang, L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav. Brain Res. 2014, 267, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Jin, D.; Chen, X. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J. Nutr. Biochem. 2010, 21, 941–947. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.-Q.; Song, X.-X.; Ge, J.-P.; Xu, Y.-C. Luteolin protect against diabetic cardiomyopathy in rat model via regulating the AKT/GSK-3β signalling pathway. Biomed. Res. 2017, 28, 1359–1363. [Google Scholar]
- Nepali, S.; Son, J.S.; Poudel, B.; Lee, J.-H.; Lee, Y.M.; Kim, D.K. Luteolin is a bioflavonoid that attenuates adipocyte-derived inflammatory responses via suppression of nuclear factor-κB/mitogen-activated protein kinases pathway. Pharmacogn. Mag. 2015, 11, 627–635. [Google Scholar]
- Singla, R.K.; Kumar, R.; Khan, S.; Mohit; Kumari, K.; Garg, A. Natural Products: Potential Source of DPP-IV Inhibitors. Curr. Protein Pept. Sci. 2019, 20, 1218–1225. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, X.; Shuai, X.; Xu, Y.; Liu, Y.; Liang, X.; Wei, D.; Su, D. Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J. Biomed. Res. 2014, 28, 292–298. [Google Scholar]
- Hretoğlu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Li, H.-T.; Wu, X.-D.; Davey, A.K.; Wang, J. Antihyperglycemic effects of baicalin on streptozotocin—Nicotinamide induced diabetic rats. Phytother. Res. 2011, 25, 189–194. [Google Scholar] [CrossRef]
- Mosquera, D.M.G.; Ortega, Y.H.; By, B.; Muro, L.V.; Hernandez, Y.S.; Ábalos, R.G.; Dehaen, W.; Pieters, L.; Apers, S. Antihyperglycemic Activity of Extracts from Boldoa purpurascens Leaves in Alloxan-Induced Diabetic Rats. Phytother. Res. 2012, 27, 721–724. [Google Scholar] [CrossRef]
- Liang, Y.C.; Tsai, S.H.; Tsai, D.C.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor- gamma by flavonoids in mouse macrophages. FEBS Lett. 2001, 496, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Ferreira, I.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. The Flavone Luteolin Inhibits Liver X Receptor Activation. J. Nat. Prod. 2016, 79, 1423–1428. [Google Scholar] [CrossRef]
- Liang, Y.C.; Tsai, S.H.; Tsai, D.C.; Lin-Shiau, S.Y.; Lin, J.K. Luteolin induces vasorelaxion in rat thoracic aorta via calcium and potassium channels. Pharmazie 2005, 60, 444–447. [Google Scholar]
- Chen, H.-I.; Hu, W.-S.; Hung, M.-Y.; Ou, H.-C.; Huang, S.-H.; Hsu, P.-T.; Day, C.-H.; Lin, K.-H.; Viswanadha, V.P.; Kuo, W.-W.; et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1032–1043. [Google Scholar] [CrossRef]
- Yang, J.T.; Qian, L.B.; Zhang, F.J.; Wang, J.; Ai, H.; Tang, L.H.; Wang, H.P. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J. Cardiovasc. Pharmacol. 2015, 65, 349–356. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.F.; Gao, Q.; Ye, Z.G.; Lu, X.J.; Wang, H.P.; Jiang, H.D.; Bruce, I.C.; Xia, Q. Inhibition of superoxide anion-mediated impairment of endothelium by treatment with luteolin and apigenin in rat mesenteric artery. Life Sci. 2008, 83, 110–117. [Google Scholar] [CrossRef]
- Deqiu, Z.; Kang, L.; Jiali, Y.; Baolin, L. Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie 2011, 93, 506–512. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, H.-Y.; Quispe, Y.N.G.; Wang, Z.; Zuo, G.; Lim, S.S. Aldose Reductase, Protein Glycation Inhibitory and Antioxidant of Peruvian Medicinal Plants: The Case of Tanacetum parthenium L. and Its Constituents. Molecules 2019, 24, 2010. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, G.; Lakshmanan, D.K.; Murugesan, S.; Elangovan, A.; Rajasekaran, N.S.; Thilagar, S. Attenuation of protein glycation by functional polyphenolics of dragon fruit (Hylocereus polyrhizus); an In Vitro and In Silico evaluation. Food Res. Int. 2020, 140, 110081. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.-T.; Sang, S. Essential Structural Requirements and Additive Effects for Flavonoids to Scavenge Methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef]
- Ando, C.; Takahashi, N.; Hirai, S.; Nishimura, K.; Lin, S.; Uemura, T.; Goto, T.; Yu, R.; Nakagami, J.; Murakami, S.; et al. Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 2009, 583, 3649–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz, M.; Sena, C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020, 59, 101040. [Google Scholar] [CrossRef]
- Muruzabal, F.J.; Frühbeck, G.; Gomez-Ambrosi, J.; Archanco, M.; A Burrell, M. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002, 128, 149–152. [Google Scholar] [CrossRef]
- Monika, P.; Geetha, A. The modulating effect of Persea americana fruit extract on the level of expression of fatty acid synthase complex, lipoprotein lipase, fibroblast growth factor-21 and leptin—A biochemical study in rats subjected to experimental hyperlipidemia and obesity. Phytomedicine 2015, 22, 939–945. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; Paini, A.; De Ciuceis, C.; Withers, S.; Greenstein, A.; Heagerty, A.M.; Rizzoni, D. Modulation of Vascular Reactivity by Perivascular Adipose Tissue (PVAT). Curr. Hypertens. Rep. 2018, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gomez-Ambrosi, J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell. Signal. 2001, 13, 827–833. [Google Scholar] [CrossRef]
- Torrado-Salmerón, C.; Guarnizo-Herrero, V.; Henriques, J.; Seiça, R.; Sena, C.M.; Torrado-Santiago, S. Multiparticulate systems of ezetimibe micellar system and atorvastatin solid dispersion efficacy of low-dose ezetimibe/atorvastatin on high-fat di-et-induced hyperlipidemia and hepatic steatosis in diabetic rats. Pharmaceutics 2021, 13, 421. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Seiça, R.M. Effects of atorvastatin and insulin in vascular dysfunction associated with type 2 diabetes. Physiol. Res. 2014, 63, 189–197. [Google Scholar] [CrossRef]
- Sena, C.; Barosa, C.; Nunes, E.; Seiça, R.; Jones, J. Sources of endogenous glucose production in the Goto–Kakizaki diabetic rat. Diabetes Metab. 2007, 33, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Nunes, E.; Louro, T.; Proença, T.; Fernandes, R.; Boarder, M.R.; Seiça, R. Effects of α-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br. J. Pharmacol. 2008, 153, 894–906. [Google Scholar] [CrossRef] [Green Version]





| W | WL | GK | GKL | |
|---|---|---|---|---|
| BW (g) | 432.1 ± 7.9 | 401.7 ± 5.3 *** | 375 ± 10.9 *** | 350.6 ± 7.9 ***, ϕϕϕ |
| Adiposity index (%) | 2.77 ± 0.14 | 2.05 ± 0.12 *** | 2.4 ± 0.09 *** | 1.95 ± 0.11 ***, ϕϕϕ |
| Triglycerides (mmol·L−1) | 0.6 ± 0.03 | 0.45 ± 0.06 | 1.07 ± 0.04 *** | 1.21 ± 0.07 *** |
| Total cholesterol (mmol·L−1) | 1.92 ± 0.05 | 1.91 ± 0.04 | 3.5 ± 0.06 *** | 2.97 ± 0.1 ***, ϕϕϕ |
| Non-HDL cholesterol (mmol·L−1) | 0.89 ± 0.04 | 0.8 ± 0.03 * | 1.36 ± 0.04 *** | 0.9 ± 0.04 ϕϕϕ |
| W − PVAT | W + PVAT | WL − PVAT | WL + PVAT | GK − PVAT | GK + PVAT | GKL − PVAT | GKL + PVAT | |
|---|---|---|---|---|---|---|---|---|
| ACh | ||||||||
| pEC50 | 6.58 ± 0.1 | 6.78 ± 0.1 | 8.25 ± 0.5 ***,### | 7.06 ± 0.2 **,§§§ | 6.5 ± 0.14 §§§,$$$ | 6.3 ± 0.21 ##,§§§,$$$ | 6.43 ± 0.14 §§§,$$$ | 7.78 ± 0.5 ***,###,§§,$$$, ϕϕϕ, ∆∆∆, &&& |
| Maximal relaxation (%) | 86.3 ± 2.1 | 91.3 ± 2.3 | 91.8 ± 4.8 * | 93 ± 2.8 ** | 53.2 ± 4.7 ***,###,§§§,$$$ | 35.4 ± 4.6 ***,###,§§§,$$$, ϕϕϕ | 71.5 ± 3.9 ***,###,§§§,$$$, ϕϕϕ, ∆∆∆ | 78.0 ± 2.7 ***,###,§§§,$$$, ϕϕϕ, ∆∆∆, && |
| SNP | ||||||||
| pEC50 | 7.68 ± 0.03 | 7.91 ± 0.05 *** | 8.05 ± 0.12 ***,## | 8.1 ± 0.07 ***,### | 7.76 ± 0.07 ##,§§§,$$$ | 7.78 ± 0.08 ##,§§§,$$$ | 8.2 ± 0.1 ***,###,§§, ϕϕϕ, ∆∆∆ | 8.2 ± 0.06 ***,###,§§, ϕϕϕ, ∆∆∆ |
| Maximal relaxation (%) | 99.1 ± 1.2 | 96.9 ± 1.47 | 100.1 ± 2.9 | 99.7 ± 2.1 | 97.6 ± 3.3 | 99.5 ± 2.07 | 99.8 ± 3.7 | 96.3 ± 2.66 |
| ET1 | ||||||||
| pEC50 | 7. 16 ± 0.14 | 6.99 ±0.09 *** | 7.06 ± 0.07 | 7.57 ± 0.1 ***,###,§§§ | 7.01 ± 0.03 **,$$$ | 6.99 ± 0.07 ***,$$$ | 6.97 ±0.04 ***,§§,$$$, ϕ | 7.18 ± 0.05 ###,§,$$$, ϕϕϕ, ∆∆∆,&&& |
| Maximal contraction (g) | 5.43 ± 0.23 | 3.72 ± 0.23 *** | 4.54 ± 0.25 ***,### | 2.33 ± 0.3 ***,###,§§§ | 4.51 ± 0.25 ***,###,$$$ | 4.78 ± 0.4 ***,###,$$$ | 4.41 ± 0.2 ***,###,$$$, ∆ | 1.73 ± 0.17 ***,###,§§§,$$, ϕϕϕ, ∆∆∆,&&& |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, M.; Leandro, A.; Azul, L.; Figueirinha, A.; Seiça, R.; Sena, C.M. Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 13671. https://doi.org/10.3390/ijms222413671
Queiroz M, Leandro A, Azul L, Figueirinha A, Seiça R, Sena CM. Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats. International Journal of Molecular Sciences. 2021; 22(24):13671. https://doi.org/10.3390/ijms222413671
Chicago/Turabian StyleQueiroz, Marcelo, Adriana Leandro, Lara Azul, Artur Figueirinha, Raquel Seiça, and Cristina M. Sena. 2021. "Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats" International Journal of Molecular Sciences 22, no. 24: 13671. https://doi.org/10.3390/ijms222413671
APA StyleQueiroz, M., Leandro, A., Azul, L., Figueirinha, A., Seiça, R., & Sena, C. M. (2021). Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats. International Journal of Molecular Sciences, 22(24), 13671. https://doi.org/10.3390/ijms222413671






