Ion Channels and Transporters in Muscle Cell Differentiation
Abstract
1. Introduction
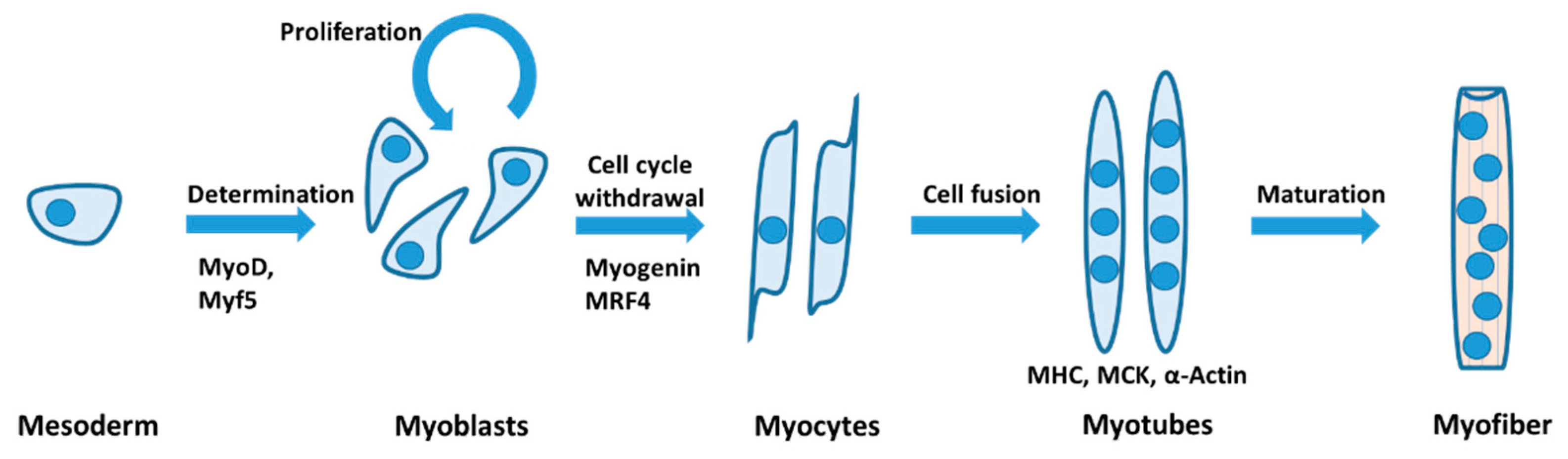
2. Ion Channels in Skeletal Myogenesis
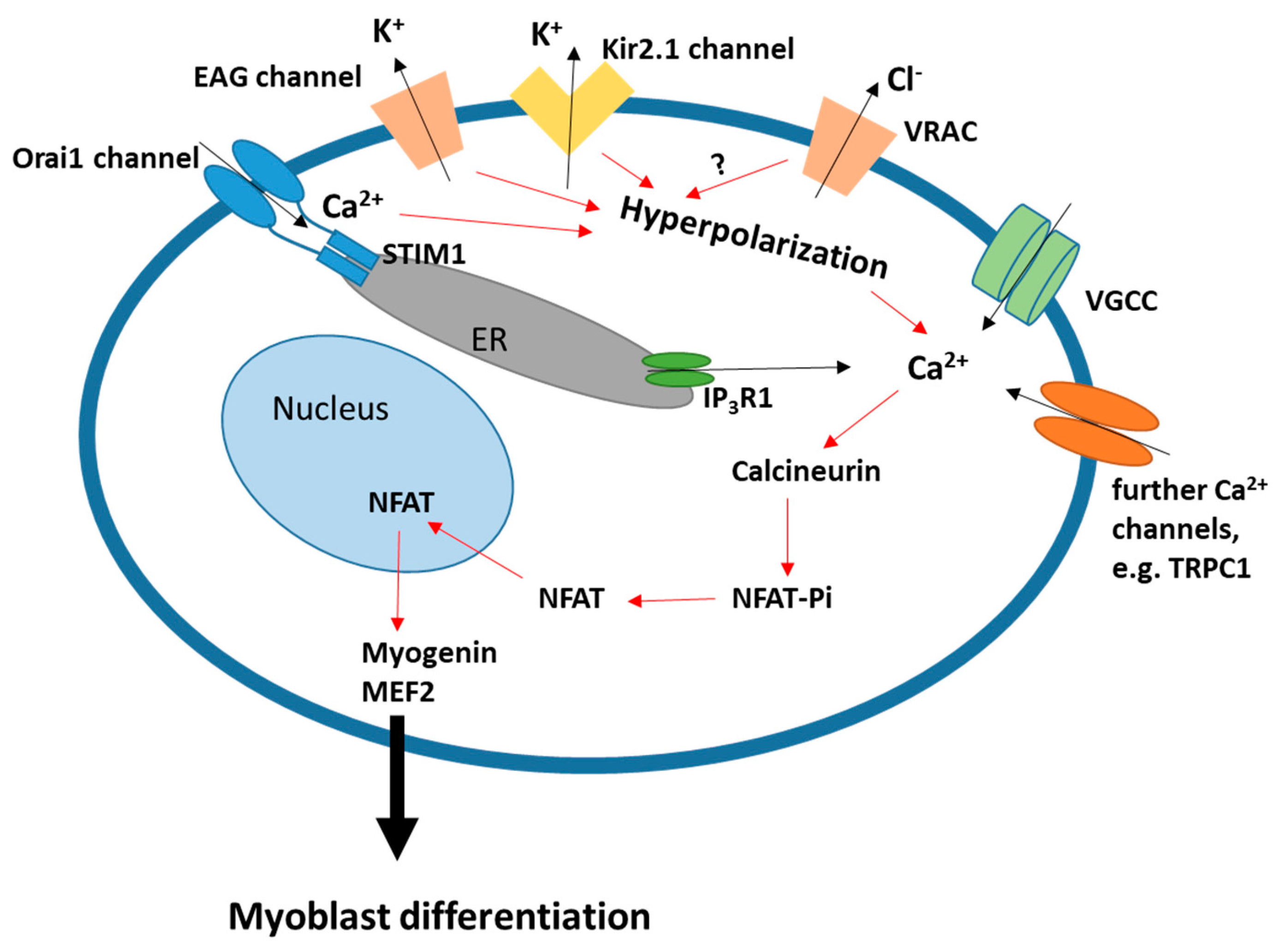
2.1. Membrane Hyperpolarization
2.2. Ca2+ Signaling
2.3. Further Molecular Mechanisms
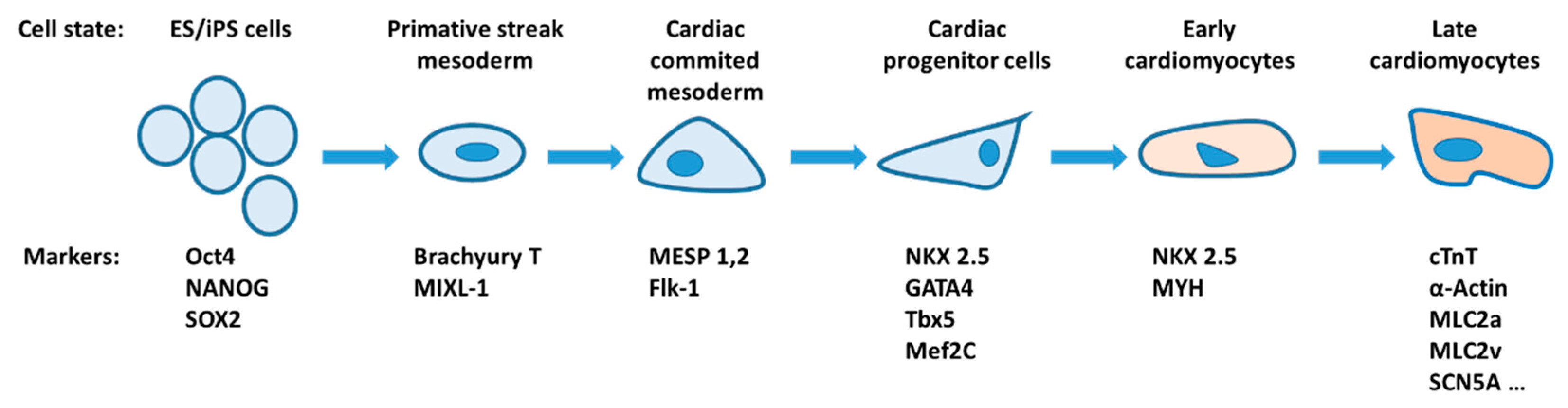
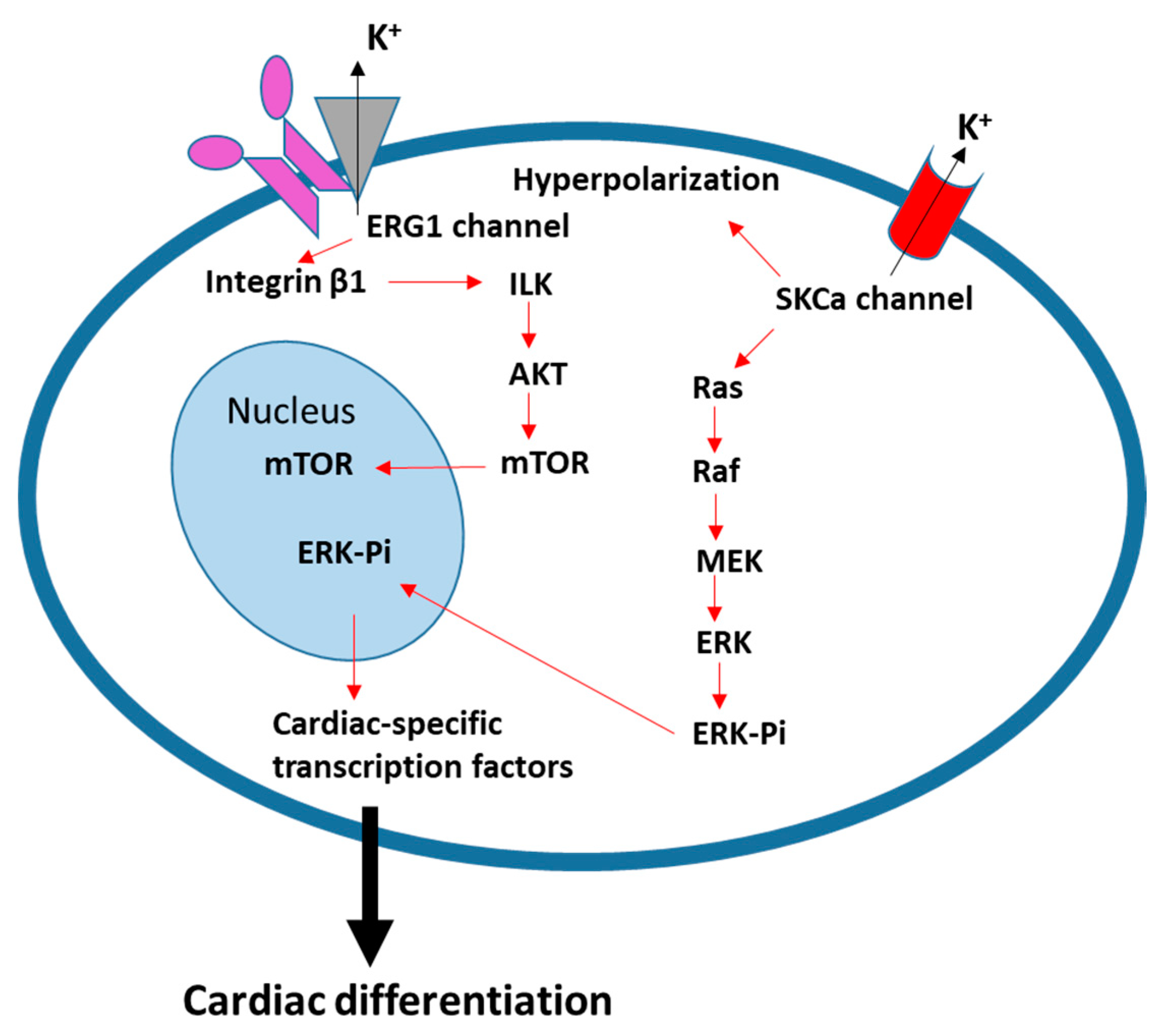
3. Ion Channels and Transporters in Cardiac Differentiation
4. Ion Channel Activity in Smooth Muscle Cell Differentiation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CaMK | Ca2+/calmodulin-dependent kinase |
| CLIC5 | Chloride intracellular channel 5 |
| cTnI | Cardiac Troponin I |
| DM1 | Myotonic dystrophy type 1 |
| EAG | Ether-à-go-go |
| ER | Endoplasmic reticulum |
| ESC | Embryonic stem cell |
| GTP | 5’-guanosine-triphosphate |
| IKCa | Intermediate-conductance Ca2+-activated K+ channel |
| IP3R | Inositol 1,4,5 trisphosphate receptor |
| iPSC | Induced pluripotent stem cell |
| LRRC8 | Leucine-rich repeat containing family 8 |
| MCK | Muscle creatine kinase |
| MEF2 | Myocyte enhancer factor 2 |
| MESP1 | Mesoderm posterior protein 1 |
| MHC | Myosin heavy chain |
| MLC2A | Myosin light chain 2a |
| Myf5 | Myogenic factor 5 |
| MyoD | Myoblast determination protein |
| NFAT | Nuclear factor of activated T-cell |
| NKCC1 | Na+/K+/2Cl− cotransporter 1 |
| NMDA | N-methyl-D-aspartate |
| PSC | Pluripotent stem cell |
| PTEN | Phosphatase and tensin homolog |
| RyR | Ryanodine receptor |
| SKCa | Small and intermediate conductance Ca2+-activated K+ channel |
| SOCE | Store-operated Ca2+ entry |
| STIM1 | Stromal interaction molecule 1 |
| TPC2 | Two-pore channel type 2 |
| TRPC | Transient receptor potential canonical channel |
| TRPV1 | Transient receptor potential vanilloid 1 |
| VGCC | Voltage-gated Ca2+ channel |
| VRAC | Volume-regulated anion channel |
| VSMC | Vascular smooth muscle cell |
References
- Camerino, D.C.; Tricarico, D.; Desaphy, J.-F. Ion channel pharmacology. Neurotherapeutics 2007, 4, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Jentsch, T.J. Chloride channelopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 173–189. [Google Scholar] [CrossRef]
- Enkvetchakul, D. Genetic Disorders of Ion Channels. Mo. Med. 2010, 107, 270–275. [Google Scholar] [PubMed]
- Liu, Y.; Wang, K. Exploiting the Diversity of Ion Channels: Modulation of Ion Channels for Therapeutic Indications. In Concepts and Principles of Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 260, pp. 187–205. [Google Scholar] [CrossRef]
- Koch, M.C.; Steinmeyer, K.; Lorenz, C.; Ricker, K.; Wolf, F.; Otto, M.; Zoll, B.; Lehmann-Horn, F.; Grzeschik, K.-H.; Jentsch, T.J. The Skeletal Muscle Chloride Channel in Dominant and Recessive Human Myotonia. Science 1992, 257, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.H.; Riisager, A.; de Paoli, F.V.; Chen, T.-Y.; Nielsen, O.B. Role of physiological ClC-1 Cl− ion channel regulation for the excitability and function of working skeletal muscle. J. Gen. Physiol. 2016, 147, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Jeng, C.-J.; Fu, S.-J.; You, C.-Y.; Peng, Y.-J.; Hsiao, C.-T.; Chen, T.-Y.; Tang, C.-Y. Defective Gating and Proteostasis of Human ClC-1 Chloride Channel: Molecular Pathophysiology of Myotonia Congenita. Front. Neurol. 2020, 11, 76. [Google Scholar] [CrossRef]
- Cannon, S.C. Sodium Channelopathies of Skeletal Muscle. Mediat. Drugs Gastrointest. Motil. I 2017, 246, 309–330. [Google Scholar] [CrossRef]
- Mantegazza, M.; Cestèle, S.; Catterall, W.A. Sodium channelopathies of skeletal muscle and brain. Physiol. Rev. 2021, 101, 1633–1689. [Google Scholar] [CrossRef]
- Cannon, S. Channelopathies of Skeletal Muscle Excitability. Muscle 2012, 5, 1013–1022. [Google Scholar] [CrossRef]
- Phillips, L.; Trivedi, J.R. Skeletal Muscle Channelopathies. Neurotherapeutics 2018, 15, 954–965. [Google Scholar] [CrossRef]
- Kline, J.; Costantini, O. Inherited Cardiac Arrhythmias and Channelopathies. Med Clin. North Am. 2019, 103, 809–820. [Google Scholar] [CrossRef]
- Wallace, E.; Howard, L.; Liu, M.; O’Brien, T.; Ward, D.; Shen, S.; Prendiville, T. Long QT Syndrome: Genetics and Future Perspective. Pediatr. Cardiol. 2019, 40, 1419–1430. [Google Scholar] [CrossRef]
- Binggeli, R.; Weinstein, R.C. Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 1986, 123, 377–401. [Google Scholar] [CrossRef]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; Perlman, H. Cell cycle exit upon myogenic differentiation. Curr. Opin. Genet. Dev. 1997, 7, 597–602. [Google Scholar] [CrossRef]
- Sampath, S.C.; Sampath, S.C.; Millay, D.P. Myoblast fusion confusion: The resolution begins. Skeletal Muscle 2018, 8, 3. [Google Scholar] [CrossRef]
- Konig, S.; Hinard, V.; Arnaudeau, S.; Holzer, N.; Potter, G.; Bader, C.R.; Bernheim, L. Membrane Hyperpolarization Triggers Myogenin and Myocyte Enhancer Factor-2 Expression during Human Myoblast Differentiation. J. Biol. Chem. 2004, 279, 28187–28196. [Google Scholar] [CrossRef] [PubMed]
- Konig, S.; Béguet, A.; Bader, C.R.; Bernheim, L. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development 2006, 133, 3107–3114. [Google Scholar] [CrossRef]
- Fennelly, C.; Wang, Z.; Criswell, T.; Soker, S. Sustained Depolarization of the Resting Membrane Potential Regulates Muscle Progenitor Cell Growth and Maintains Stem Cell Properties In Vitro. Stem Cell Rev. Rep. 2016, 12, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Bernheim, L.; Liu, J.H.; Hamann, M.; Haenggeli, C.A.; Fischer-Lougheed, J.; Bader, C.R. Contribution of a non-inactivating potassium current to the resting membrane potential of fusion-competent human myoblasts. J. Physiol. 1996, 493, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bijlenga, P.; Occhiodoro, T.; Liu, J.-H.; Bader, C.R.; Bernheim, L.; Fischer-Lougheed, J. An ether-à-go-go K+ current, Ih-eag, contributes to the hyperpolarization of human fusion-competent myoblasts. J. Physiol. 1998, 512, 317–323. [Google Scholar] [CrossRef]
- Liu, J.-H.; Bijlenga, P.; Fischer-Lougheed, J.; Occhiodoro, T.; Kaelin, A.; Bader, C.R.; Bernheim, L. Role of an inward rectifier K+ current and of hyperpolarization in human myoblast fusion. J. Physiol. 1998, 510, 467–476. [Google Scholar] [CrossRef]
- Fischer-Lougheed, J.; Liu, J.-H.; Espinos, E.; Mordasini, D.; Bader, C.R.; Belin, D.; Bernheim, L. Human Myoblast Fusion Requires Expression of Functional Inward Rectifier Kir2.1 Channels. J. Cell Biol. 2001, 153, 677–686. [Google Scholar] [CrossRef]
- Liu, J.-H.; König, S.; Michel, M.; Arnaudeau, S.; Fischer-Lougheed, J.; Bader, C.R.; Bernheim, L. Acceleration of human myoblast fusion by depolarization: Graded Ca2+ signals involved. Development 2003, 130, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- Hinard, V.; Belin, D.; Konig, S.; Bader, C.R.; Bernheim, L. Initiation of human myoblast differentiation via dephosphorylation of Kir2.1 K+ channels at tyrosine 242. Development 2008, 135, 859–867. [Google Scholar] [CrossRef]
- Jang, Y.-N.; Baik, E.J. JAK-STAT pathway and myogenic differentiation. JAK-STAT 2013, 2, e23282. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; König, S.; Jousset, H.; Bader, C.; Demaurex, N.; Bernheim, L. STIM1- and Orai1-dependent Store-operated Calcium Entry Regulates Human Myoblast Differentiation. J. Biol. Chem. 2009, 284, 5370–5380. [Google Scholar] [CrossRef]
- Chen, L.; Becker, T.M.; Koch, U.; Stauber, T. The LRRC8/VRAC anion channel facilitates myogenic differentiation of murine myoblasts by promoting membrane hyperpolarization. J. Biol. Chem. 2019, 294, 14279–14288. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; König, B.; Stauber, T. LRRC8 channel activation and reduction in cytosolic chloride concentration during early differentiation of C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2020, 532, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, B.; Pietrangelo, T.; Catacuzzeno, L.; Franciolini, F. Intermediate-conductance Ca2+-activated K+ channel is expressed in C2C12 myoblasts and is downregulated during myogenesis. Am. J. Physiol. Physiol. 2005, 289, C89–C96. [Google Scholar] [CrossRef]
- Pietrangelo, T.; Fioretti, B.; Mancinelli, R.; Catacuzzeno, L.; Franciolini, F.; Fanò, G.; Fulle, S. Extracellular guanosine-5′-triphosphate modulates myogenesis via intermediate Ca2+-activated K+ currents in C2C12 mouse cells. J. Physiol. 2006, 572, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ono, Y.; Sakamoto, K. DCEBIO facilitates myogenic differentiation via intermediate conductance Ca2+ activated K+ channel activation in C2C12 myoblasts. J. Pharmacol. Sci. 2017, 133, 276–279. [Google Scholar] [CrossRef]
- Chen, L.; König, B.; Liu, T.; Pervaiz, S.; Razzaque, Y.S.; Stauber, T. More than just a pressure relief valve: Physiological roles of volume-regulated LRRC8 anion channels. Biol. Chem. 2019, 400, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Kolobkova, Y.; Pervaiz, S.; Stauber, T. The expanding toolbox to study the LRRC8-formed volume-regulated anion channel VRAC. Curr. Top. Membr. 2021, 88, 119–163. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Stauber, T. Biophysics and Structure-Function Relationships of LRRC8-Formed Volume-Regulated Anion Channels. Biophys. J. 2019, 116, 1185–1193. [Google Scholar] [CrossRef]
- Osei-Owusu, J.; Yang, J.; Vitery, M.D.C.; Qiu, Z. Molecular Biology and Physiology of Volume-Regulated Anion Channel (VRAC). Curr. Top. Membr. 2018, 81, 177–203. [Google Scholar] [CrossRef]
- Strange, K.; Yamada, T.; Denton, J.S. A 30-year journey from volume-regulated anion currents to molecular structure of the LRRC8 channel. J. Gen. Physiol. 2019, 151, 100–117. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Hao, Y.; Schwartz, S.; Plested, A.J.; Stauber, T. A FRET sensor of C-terminal movement reveals VRAC activation by plasma membrane DAG signaling rather than ionic strength. eLife 2019, 8, e45421. [Google Scholar] [CrossRef]
- Pervaiz, S.; Kopp, A.; Von Kleist, L.; Stauber, T. Absolute Protein Amounts and Relative Abundance of Volume-regulated Anion Channel (VRAC) LRRC8 Subunits in Cells and Tissues Revealed by Quantitative Immunoblotting. Int. J. Mol. Sci. 2019, 20, 5879. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Stauber, T. The Volume-Regulated Anion Channel LRRC8/VRAC Is Dispensable for Cell Proliferation and Migration. Int. J. Mol. Sci. 2019, 20, 2663. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Dubin, A.E.; Mathur, J.; Tu, B.; Reddy, K.; Miraglia, L.J.; Reinhardt, J.; Orth, A.P.; Patapoutian, A. SWELL1, a Plasma Membrane Protein, Is an Essential Component of Volume-Regulated Anion Channel. Cell 2014, 157, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Voss, F.K.; Ullrich, F.; Münch, J.; Lazarow, K.; Lutter, D.; Mah, N.; Andrade-Navarro, M.A.; von Kries, J.P.; Stauber, T.; Jentsch, T.J. Identification of LRRC8 Heteromers as an Essential Component of the Volume-Regulated Anion Channel VRAC. Science 2014, 344, 634–638. [Google Scholar] [CrossRef]
- Kumar, A.; Xie, L.; Stainier, D.Y.; Hinton, A.O.; Gunasekar, S.K.; Minerath, R.A.; Shen, K.; Maurer, J.M.; Grueter, C.E.; Abel, E.D.; et al. SWELL1 regulates skeletal muscle cell size, intracellular signaling, adiposity and glucose metabolism. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Bendahhou, S.; Donaldson, M.R.; Plaster, N.M.; Tristani-Firouzi, M.; Fu, Y.-H.; Ptácek, L.J. Defective Potassium Channel Kir2.1 Trafficking Underlies Andersen-Tawil Syndrome. J. Biol. Chem. 2003, 278, 51779–51785. [Google Scholar] [CrossRef]
- Yoon, G.; Oberoi, S.; Tristani-Firouzi, M.; Etheridge, S.; Quitania, L.; Kramer, J.; Miller, B.; Fu, Y.; Ptáček, L. Andersen-Tawil syndrome: Prospective cohort analysis and expansion of the phenotype. Am. J. Med Genet. Part A 2006, 140A, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Plaster, N.M.; Tawil, R.; Tristani-Firouzi, M.; Canún, S.; Bendahhou, S.; Tsunoda, A.; Donaldson, M.R.; Iannaccone, S.T.; Brunt, E.; Barohn, R.; et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell 2001, 105, 511–519. [Google Scholar] [CrossRef]
- Zaritsky, J.J.; Eckman, D.M.; Wellman, G.C.; Nelson, M.; Schwarz, T.L. Targeted Disruption of Kir2.1 and Kir2.2 Genes Reveals the Essential Role of the Inwardly Rectifying K+ Current in K+-Mediated Vasodilation. Circ. Res. 2000, 87, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Dahal, G.R.; Rawson, J.; Gassaway, B.; Kwok, B.; Tong, Y.; Ptáček, L.J.; Bates, E. An inwardly rectifying K+ channel is required for patterning. Development 2012, 139, 3653–3664. [Google Scholar] [CrossRef]
- Stiber, J.; Hawkins, A.; Zhang, Z.-S.; Wang, S.; Burch, J.; Graham, V.; Ward, C.C.; Seth, M.; Finch, E.; Malouf, N.; et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 2008, 10, 688–697. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Braun, A.; Kleinschnitz, C.; Bender, M.; Pleines, I.; Pham, M.; Renné, T.; Stoll, G.; Nieswandt, B. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J. Exp. Med. 2008, 205, 1583–1591. [Google Scholar] [CrossRef]
- Li, T.; Finch, E.A.; Graham, V.; Zhang, Z.-S.; Ding, J.-D.; Burch, J.; Oh-Hora, M.; Rosenberg, P. STIM1-Ca2+ Signaling Is Required for the Hypertrophic Growth of Skeletal Muscle in Mice. Mol. Cell. Biol. 2012, 32, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Gwack, Y.; Srikanth, S.; Oh-Hora, M.; Hogan, P.G.; Lamperti, E.D.; Yamashita, M.; Gelinas, C.; Neems, D.S.; Sasaki, Y.; Feske, S.; et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 2008, 28, 5209–5222. [Google Scholar] [CrossRef]
- Vig, M.; DeHaven, W.I.; Bird, G.S.; Billingsley, J.M.; Wang, H.; Rao, P.E.; Hutchings, A.B.; Jouvin, M.-H.; Putney, J.; Kinet, J.-P. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release–activated calcium channels. Nat. Immunol. 2007, 9, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wei-LaPierre, L.; Carrell, E.M.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Orai1-dependent calcium entry promotes skeletal muscle growth and limits fatigue. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Chou, J.; Yee, C.S.; Borzutzky, A.; Vollmann, E.H.; Von Andrian, U.H.; Park, S.-Y.; Hollander, G.; Manis, J.P.; Poliani, P.L.; et al. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J. Exp. Med. 2014, 211, 929–942. [Google Scholar] [CrossRef]
- Przybylski, R.J.; MacBride, R.G.; Kirby, A.C. Calcium regulation of skeletal myogenesis. I. Cell content critical to myotube formation. In Vitro Cell. Dev. Biol. 1989, 25, 830–838. [Google Scholar] [CrossRef]
- Bijlenga, P.; Liu, J.-H.; Espinos, E.; Haenggeli, C.-A.; Fischer-Lougheed, J.; Bader, C.R.; Bernheim, L. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc. Natl. Acad. Sci. USA 2000, 97, 7627–7632. [Google Scholar] [CrossRef] [PubMed]
- Constantin, B.; Cognard, C.; Raymond, G. Myoblast fusion requires cytosolic calcium elevation but not activation of voltage-dependent calcium channels. Cell Calcium 1996, 19, 365–374. [Google Scholar] [CrossRef]
- Przybylski, R.J.; Szigeti, V.; Davidheiser, S.; Kirby, A.C. Calcium regulation of skeletal myogenesis. II. Extracellular and cell surface effects. Cell Calcium 1994, 15, 132–142. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, L.; Liu, L.; Cheung, C.F.; Li, X.; Yee, S.-P.; Yang, X.-J.; Wu, Z. p38 Mitogen-activated Protein Kinase-, Calcium-Calmodulin–dependent Protein Kinase-, and Calcineurin-mediated Signaling Pathways Transcriptionally Regulate Myogenin Expression. Mol. Biol. Cell 2002, 13, 1940–1952. [Google Scholar] [CrossRef]
- Porter, G.; Makuck, R.F.; Rivkees, S.A. Reduction in Intracellular Calcium Levels Inhibits Myoblast Differentiation. J. Biol. Chem. 2002, 277, 28942–28947. [Google Scholar] [CrossRef]
- Bidaud, I.; Monteil, A.; Nargeot, J.; Lory, P. Properties and role of voltage-dependent calcium channels during mouse skeletal muscle differentiation. J. Muscle Res. Cell Motil. 2006, 27, 75–81. [Google Scholar] [CrossRef]
- Spangenburg, E.E.; Bowles, D.; Booth, F.W. Insulin-Like Growth Factor-Induced Transcriptional Activity of the Skeletal α-Actin Gene Is Regulated by Signaling Mechanisms Linked to Voltage-Gated Calcium Channels during Myoblast Differentiation. Endocrinology 2004, 145, 2054–2063. [Google Scholar] [CrossRef]
- Arnaudeau, S.; Holzer, N.; König, S.; Bader, C.R.; Bernheim, L. Calcium sources used by post-natal human myoblasts during initial differentiation. J. Cell. Physiol. 2006, 208, 435–445. [Google Scholar] [CrossRef]
- Nakanishi, K.; Kakiguchi, K.; Yonemura, S.; Nakano, A.; Morishima, N. Transient Ca2+ depletion from the endoplasmic reticulum is critical for skeletal myoblast differentiation. FASEB J. 2015, 29, 2137–2149. [Google Scholar] [CrossRef]
- Araya, R.; Riquelme, M.A.; Brandan, E.; Sáez, J.C. The formation of skeletal muscle myotubes requires functional membrane receptors activated by extracellular ATP. Brain Res. Rev. 2004, 47, 174–188. [Google Scholar] [CrossRef]
- Seigneurin-Venin, S.; Parrish, E.; Marty, I.; Rieger, F.; Romey, G.; Villaz, M.; García, L. Involvement of the Dihydropyridine Receptor and Internal Ca2+ Stores in Myoblast Fusion. Exp. Cell Res. 1996, 223, 301–307. [Google Scholar] [CrossRef]
- Stiber, J.A.; Tabatabaei, N.; Hawkins, A.F.; Hawke, T.; Worley, P.F.; Williams, R.S.; Rosenberg, P. Homer modulates NFAT-dependent signaling during muscle differentiation. Dev. Biol. 2005, 287, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Antigny, F.; Konig, S.; Bernheim, L.; Frieden, M. Inositol 1,4,5 trisphosphate receptor 1 is a key player of human myoblast differentiation. Cell Calcium 2014, 56, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kelu, J.J.; Webb, S.E.; Parrington, J.; Galione, A.; Miller, A.L. Ca2+ release via two-pore channel type 2 (TPC2) is required for slow muscle cell myofibrillogenesis and myotomal patterning in intact zebrafish embryos. Dev. Biol. 2017, 425, 109–129. [Google Scholar] [CrossRef]
- Kelu, J.J.; Chan, H.L.; Webb, S.E.; Cheng, A.H.; Ruas, M.; Parrington, J.; Galione, A.; Miller, A.L. Two-Pore Channel 2 activity is required for slow muscle cell-generated Ca2+ signaling during myogenesis in intact zebrafish. Int. J. Dev. Biol. 2015, 59, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Luik, R.M.; Wu, M.M.; Buchanan, J.; Lewis, R.S. The elementary unit of store-operated Ca2+ entry: Local activation of CRAC channels by STIM1 at ER–plasma membrane junctions. J. Cell Biol. 2006, 174, 815–825. [Google Scholar] [CrossRef]
- Wu, M.M.; Buchanan, J.; Luik, R.M.; Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006, 174, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Jousset, H.; Frieden, M.; Demaurex, N. STIM1 Knockdown Reveals That Store-operated Ca2+ Channels Located Close to Sarco/Endoplasmic Ca2+ ATPases (SERCA) Pumps Silently Refill the Endoplasmic Reticulum. J. Biol. Chem. 2007, 282, 11456–11464. [Google Scholar] [CrossRef]
- Choi, J.H.; Jeong, S.Y.; Oh, M.R.; Allen, P.D.; Lee, E.H. TRPCs: Influential Mediators in Skeletal Muscle. Cells 2020, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lu, J.; Li, Z.; Yu, X.; Chen, L.; Xu, T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun. 2006, 350, 969–976. [Google Scholar] [CrossRef]
- Lopez, J.J.; Salido, G.M.; Pariente, J.A.; Rosado, J. Interaction of STIM1 with Endogenously Expressed Human Canonical TRP1 upon Depletion of Intracellular Ca2+ Stores. J. Biol. Chem. 2006, 281, 28254–28264. [Google Scholar] [CrossRef]
- Yuan, J.P.; Zeng, W.; Huang, G.N.; Worley, P.F.; Muallem, S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 2007, 9, 636–645. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; Ceroni, D.; Bader, C.R.; Konig, S.; Bernheim, L. Human Muscle Economy Myoblast Differentiation and Excitation-Contraction Coupling Use the Same Molecular Partners, STIM1 and STIM2. J. Biol. Chem. 2010, 285, 22437–22447. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.; Zanou, N.; Van Schoor, M.; Gailly, P. TRPC1 regulates skeletal myoblast migration and differentiation. J. Cell Sci. 2008, 121, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Schakman, O.; Louis, P.; Ruegg, U.; Dietrich, A.; Birnbaumer, L.; Gailly, P. Trpc1 Ion Channel Modulates Phosphatidylinositol 3-Kinase/Akt Pathway during Myoblast Differentiation and Muscle Regeneration. J. Biol. Chem. 2012, 287, 14524–14534. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L.; Sassoli, C.; Squecco, R.; Bini, F.; Martinesi, M.; Chellini, F.; Luciani, G.; Sbrana, F.; Zecchi-Orlandini, S.; Francini, F.; et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J. Cell Sci. 2009, 122, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Bini, F.; Sassoli, C.; Martinesi, M.; Squecco, R.; Chellini, F.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L. Functional interaction between TRPC1 channel and connexin-43 protein: A novel pathway underlying S1P action on skeletal myogenesis. Cell. Mol. Life Sci. 2010, 67, 4269–4285. [Google Scholar] [CrossRef]
- Antigny, F.; Koenig, S.; Bernheim, L.; Frieden, M. During post-natal human myogenesis, normal myotube size requires TRPC1 and TRPC4 mediated Ca2+ entry. J. Cell Sci. 2013, 126, 2525–2533. [Google Scholar] [CrossRef]
- Antigny, F.; Sabourin, J.; Saüc, S.; Bernheim, L.; Koenig, S.; Frieden, M. TRPC1 and TRPC4 channels functionally interact with STIM1L to promote myogenesis and maintain fast repetitive Ca2+ release in human myotubes. Biochim. Biophys. Acta Bioenerg. 2017, 1864, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Park, J.-Y.; Kim, K. NMDA receptor-mediated calcium influx plays an essential role in myoblast fusion. FEBS Lett. 2004, 578, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Shapovalov, G.; Louis, M.; Tajeddine, N.; Gallo, C.; Van Schoor, M.; Anguish, I.; Cao, M.L.; Schakman, O.; Dietrich, A.; et al. Role of TRPC1 channel in skeletal muscle function. Am. J. Physiol. Physiol. 2010, 298, C149–C162. [Google Scholar] [CrossRef]
- Timchenko, N.A.; Iakova, P.; Cai, Z.-J.; Smith, J.R.; Timchenko, L. Molecular Basis for Impaired Muscle Differentiation in Myotonic Dystrophy. Mol. Cell. Biol. 2001, 21, 6927–6938. [Google Scholar] [CrossRef] [PubMed]
- Tajhya, R.B.; Hu, X.; Tanner, M.; Huq, R.; Kongchan, N.; Neilson, J.R.; Rodney, G.; Horrigan, F.T.; Timchenko, L.T.; Beeton, C. Functional KCa1.1 channels are crucial for regulating the proliferation, migration and differentiation of human primary skeletal myoblasts. Cell Death Dis. 2016, 7, e2426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meola, G.; Cardani, R. Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim. et Biophys. Acta Mol. Basis Dis. 2015, 1852, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yin, J.; Yue, T.; Liu, L.; Zhang, H. The CLIC5 (chloride intracellular channel 5) involved in C2C12 myoblasts proliferation and differentiation. Cell Biol. Int. 2010, 34, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Stauber, T.; Jentsch, T.J. Chloride in Vesicular Trafficking and Function. Annu. Rev. Physiol. 2013, 75, 453–477. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Panza, E.; Barrese, V.; Viggiano, D.; Soldovieri, M.V.; Taglialatela, M. Expression, Localization, and Pharmacological Role of Kv7 Potassium Channels in Skeletal Muscle Proliferation, Differentiation, and Survival after Myotoxic Insults. J. Pharmacol. Exp. Ther. 2009, 332, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Silvestri, C.; Mazzarella, E.; Martella, A.; Calvigioni, D.; Piscitelli, F.; Ambrosino, P.; Petrosino, S.; Czifra, G.; Biro, T.; et al. The endocannabinoid 2-AG controls skeletal muscle cell differentiation via CB1 receptor-dependent inhibition of Kv7 channels. Proc. Natl. Acad. Sci. USA 2014, 111, E2472–E2481. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Barrese, V.; Formisano, L.; Miceli, F.; Taglialatela, M. Specification of skeletal muscle differentiation by repressor element-1 silencing transcription factor (REST)-regulated Kv7.4 potassium channels. Mol. Biol. Cell 2013, 24, 274–284. [Google Scholar] [CrossRef]
- Wedhas, N.; Klamut, H.J.; Dogra, C.; Srivastava, A.K.; Mohan, S.; Kumar, A. Inhibition of mechanosensitive cation channels inhibits myogenic differentiation by suppressing the expression of myogenic regulatory factors and caspase-3 activity. FASEB J. 2005, 19, 1986–1997. [Google Scholar] [CrossRef]
- Formigli, L.; Meacci, E.; Sassoli, C.; Squecco, R.; Nosi, D.; Chellini, F.; Naro, F.; Francini, F.; Zecchi-Orlandini, S. Cytoskeleton/stretch-activated ion channel interaction regulates myogenic differentiation of skeletal myoblasts. J. Cell. Physiol. 2007, 211, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Cho, C.-H.; Kim, D.H.; Lee, E.H. TRPC3 cation channel plays an important role in proliferation and differentiation of skeletal muscle myoblasts. Exp. Mol. Med. 2010, 42, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Langlois, S.; Xiang, X.; Young, K.; Cowan, B.J.; Penuela, S.; Cowan, K.N. Pannexin 1 and Pannexin 3 Channels Regulate Skeletal Muscle Myoblast Proliferation and Differentiation. J. Biol. Chem. 2014, 289, 30717–30731. [Google Scholar] [CrossRef]
- Araya, R.; Eckardt, D.; Maxeiner, S.; Krüger, O.; Theis, M.; Willecke, K.; Sáez, J.C. Expression of connexins during differentiation and regeneration of skeletal muscle: Functional relevance of connexin43. J. Cell Sci. 2005, 118, 27–37. [Google Scholar] [CrossRef]
- Araya, R.; Eckardt, D.; Riquelme, M.A.; Willecke, K.; Sáez, J.C. Presence and importance of connexin43 during myogenesis. Cell Commun. Adhes. 2003, 10, 451–456. [Google Scholar] [CrossRef]
- Afzali, A.M.; Ruck, T.; Herrmann, A.M.; Iking, J.; Sommer, C.; Kleinschnitz, C.; Preuβe, C.; Stenzel, W.; Budde, T.; Wiendl, H.; et al. The potassium channels TASK2 and TREK1 regulate functional differentiation of murine skeletal muscle cells. Am. J. Physiol. Physiol. 2016, 311, C583–C595. [Google Scholar] [CrossRef]
- Krause, R.M.; Hamann, M.; Bader, C.R.; Liu, J.H.; Baroffio, A.; Bernheim, L. Activation of nicotinic acetylcholine receptors increases the rate of fusion of cultured human myoblasts. J. Physiol. 1995, 489, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, M.; Ogura, Y.; Funabashi, T.; Akema, T. Involvement of Transient Receptor Potential Cation Channel Vanilloid 1 (TRPV1) in Myoblast Fusion. J. Cell. Physiol. 2016, 231, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Obi, S.; Nakajima, T.; Hasegawa, T.; Nakamura, F.; Sakuma, M.; Toyoda, S.; Tei, C.; Inoue, T. Heat induces myogenic transcription factors of myoblast cells via transient receptor potential vanilloid 1 (Trpv1). FEBS Open Bio 2018, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Mandai, S.; Furukawa, S.; Kodaka, M.; Hata, Y.; Mori, T.; Nomura, N.; Ando, F.; Mori, Y.; Takahashi, D.; Yoshizaki, Y.; et al. Loop diuretics affect skeletal myoblast differentiation and exercise-induced muscle hypertrophy. Sci. Rep. 2017, 7, 46369. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nat. Cell Biol. 2013, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Hippenmeyer, S.; Saadat, L.V.; Luo, L.; Weissman, I.L.; Ardehali, R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 8850–8855. [Google Scholar] [CrossRef]
- Später, D.; Hansson, E.M.; Zangi, L.; Chien, K.R. How to make a cardiomyocyte. Development 2014, 141, 4418–4431. [Google Scholar] [CrossRef]
- Barreto, S.; Hamel, L.; Schiatti, T.; Yang, Y.; George, V. Cardiac Progenitor Cells from Stem Cells: Learning from Genetics and Biomaterials. Cells 2019, 8, 1536. [Google Scholar] [CrossRef] [PubMed]
- Zwi, L.; Caspi, O.; Arbel, G.; Huber, I.; Gepstein, A.; Park, I.-H.; Gepstein, L. Cardiomyocyte Differentiation of Human Induced Pluripotent Stem Cells. Circulation 2009, 120, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Kleger, A.; Seufferlein, T.; Malan, D.; Tischendorf, M.; Storch, A.; Wolheim, A.; Latz, S.; Protze, S.; Porzner, M.; Proepper, C.; et al. Modulation of Calcium-Activated Potassium Channels Induces Cardiogenesis of Pluripotent Stem Cells and Enrichment of Pacemaker-Like Cells. Circulation 2010, 122, 1823–1836. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; Liu, H.; Meng, Y.; Lin, F.; Gu, Y.; Wang, H.; Shang, M.; Tong, C.; Sachinidis, A.; et al. ERG1 Plays an Essential Role in Rat Cardiomyocyte Fate Decision by Mediating AKT Signaling. Stem Cells 2021, 39, 443–457. [Google Scholar] [CrossRef]
- Jeziorowska, D.; Korniat, A.; Salem, J.-E.; Fish, K.; Hulot, J.-S. Generating patient-specific induced pluripotent stem cells-derived cardiomyocytes for the treatment of cardiac diseases. Expert Opin. Biol. Ther. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Fu, J.; Yu, H.-M.; Wang, R.; Liang, J.; Yang, H.-T. Developmental regulation of intracellular calcium transients during cardiomyocyte differentiation of mouse embryonic stem cells1. Acta Pharmacol. Sin. 2006, 27, 901–910. [Google Scholar] [CrossRef]
- Janowski, E.; Cleemann, L.; Sasse, P.; Morad, M. Diversity of Ca2+ Signaling in Developing Cardiac Cells. Ann. New York Acad. Sci. 2006, 1080, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.; Miranda, A.; Chen, C.-M.; Davidson, S.; Srinivas, S.; Riley, P.R. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLife 2016, 5, e17113. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; Han, M.-D.; Artman, M.; Ludwig, C.A. Sodium-calcium exchanger (NCX-1) and calcium modulation: NCX protein expression patterns and regulation of early heart development. Dev. Dyn. 2001, 221, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Reppel, M.; Sasse, P.; Malan, D.; Nguemo, F.; Reuter, H.; Bloch, W.; Hescheler, J.; Fleischmann, B.K. Functional expression of the Na+/Ca2+ exchanger in the embryonic mouse heart. J. Mol. Cell. Cardiol. 2007, 42, 121–132. [Google Scholar] [CrossRef]
- Li, X.; Karki, P.; Lei, L.; Wang, H.; Fliegel, L. Na+/H+ exchanger isoform 1 facilitates cardiomyocyte embryonic stem cell differentiation. Am. J. Physiol. Circ. Physiol. 2009, 296, H159–H170. [Google Scholar] [CrossRef]
- Hoorntje, T.; Alders, M.; van Tintelen, P.; van der Lip, K.; Sreeram, N.; van der Wal, A.; Mannens, M.; Wilde, A. Homozygous premature truncation of the HERG protein: The human HERG knockout. Circulation 1999, 100, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.Q.; Zhao, X.; Lees-Miller, J.P.; Quinn, F.R.; Li, P.; Rancourt, D.E.; London, B.; Cross, J.C.; Duff, H.J. Homozygous Missense N629D hERG (KCNH2) Potassium Channel Mutation Causes Developmental Defects in the Right Ventricle and Its Outflow Tract and Embryonic Lethality. Circ. Res. 2008, 103, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Timothy, K.W.; Sharpe, L.M.; Decher, N.; Kumar, P.; Bloise, R.; Napolitano, C.; Schwartz, P.J.; Joseph, R.; Condouris, K.; et al. CaV1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism. Cell 2004, 119, 19–31. [Google Scholar] [CrossRef]
- Splawski, I.; Timothy, K.W.; Decher, N.; Kumar, P.; Sachse, F.; Beggs, A.; Sanguinetti, M.C.; Keating, M.T. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 8089–8096. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Owens, G.K. Molecular Determinants of Vascular Smooth Muscle Cell Diversity. Circ. Res. 2005, 96, 280–291. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental Basis of Vascular Smooth Muscle Diversity. Arter. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef]
- Xie, C.; Ritchie, R.P.; Huang, H.; Zhang, J.; Chen, Y.E. Smooth muscle cell differentiation in vitro: Models and underlying molecular mechanisms. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell. Mol. Life Sci 2020, 71, 2271–2288. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Mack, C.P. Signaling Mechanisms That Regulate Smooth Muscle Cell Differentiation. Arter. Thromb. Vasc. Biol. 2011, 31, 1495–1505. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Zhou, Y.; Chen, L.; Wang, Y.-Q.; Wang, X.; Pi, Y.; Gao, C.-Y.; Li, J.-C.; Zhang, L.-L. An overview of potential molecular mechanisms involved in VSMC phenotypic modulation. Histochem. Cell Biol. 2015, 145, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Frismantiene, A.; Filippova, M.; Erne, P.; Resink, T.J. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell. Signal. 2018, 52, 48–64. [Google Scholar] [CrossRef]
- Kudryavtseva, O.; Aalkjaer, C.; Matchkov, V. Vascular smooth muscle cell phenotype is defined by Ca2+-dependent transcription factors. FEBS J. 2013, 280, 5488–5499. [Google Scholar] [CrossRef]
- Wan, X.-J.; Zhao, H.-C.; Zhang, P.; Huo, B.; Shen, B.-R.; Yan, Z.-Q.; Qi, Y.-X.; Jiang, Z.-L. Involvement of BK channel in differentiation of vascular smooth muscle cells induced by mechanical stretch. Int. J. Biochem. Cell Biol. 2015, 59, 21–29. [Google Scholar] [CrossRef]
- Johnson, M.T.; Gudlur, A.; Zhang, X.; Xin, P.; Emrich, S.M.; Yoast, R.; Courjaret, R.; Nwokonko, R.M.; Li, W.; Hempel, N.; et al. L-type Ca2+ channel blockers promote vascular remodeling through activation of STIM proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 17369–17380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, L.; Bai, Y.; Song, J.; Cheng, J.; Ma, H.; Ma, J.; Xie, M. miR-137 and its target T-type Ca V 3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway. Cell Prolif. 2020, 53, e12774. [Google Scholar] [CrossRef] [PubMed]
- Wamhoff, B.; Bowles, D.; McDonald, O.; Sinha, S.; Somlyo, A.; Owens, G. L-type Voltage-Gated Ca2+ Channels Modulate Expression of Smooth Muscle Differentiation Marker Genes via a Rho Kinase/Myocardin/SRF–Dependent Mechanism. Circ. Res. 2004, 95, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Thakore, P.; Pritchard, H.A.T.; Griffin, C.S.; Yamasaki, E.; Drumm, B.T.; Lane, C.; Sanders, K.M.; Earley, Y.F.; Earley, S. TRPML1 channels initiate Ca2+ sparks in vascular smooth muscle cells. Sci. Signal. 2020, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Durlu-Kandilci, N.T.; Ruas, M.; Chuang, K.-T.; Brading, A.; Parrington, J.; Galione, A. TPC2 Proteins Mediate Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP)- and Agonist-evoked Contractions of Smooth Muscle. J. Biol. Chem. 2010, 285, 24925–24932. [Google Scholar] [CrossRef] [PubMed]
- Berra-Romani, R.; Mazzocco-Spezzia, A.; Pulina, M.V.; Golovina, V.A. Ca2+handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am. J. Physiol. Physiol. 2008, 295, C779–C790. [Google Scholar] [CrossRef] [PubMed]
- Boie, S.; Chen, J.; Sanderson, M.J.; Sneyd, J. The relative contributions of store-operated and voltage-gated Ca2+ channels to the control of Ca2+ oscillations in airway smooth muscle. J. Physiol. 2016, 595, 3129–3141. [Google Scholar] [CrossRef]
- Kim, B.; Molina, R.; Jensen, G.; Poburko, D. A differentiated Ca2+ signalling phenotype has minimal impact on myocardin expression in an automated differentiation assay using A7r5 cells. Cell Calcium 2021, 96, 102369. [Google Scholar] [CrossRef]
- Teng, G.; Zhao, X.; Lees-Miller, J.P.; Belke, D.; Shi, C.; Chen, Y.; O’Brien, E.R.; Fedak, P.W.; Bracey, N.; Cross, J.C.; et al. Role of mutation and pharmacologic block of human KCNH2 in vasculogenesis and fetal mortality: Partial rescue by transforming growth factor-β. Circulation. Arrhythmia Electrophysiol. 2015, 8, 420–428. [Google Scholar] [CrossRef]
- Numaga-Tomita, T.; Shimauchi, T.; Oda, S.; Tanaka, T.; Nishiyama, K.; Nishimura, A.; Birnbaumer, L.; Mori, Y.; Nishida, M. TRPC6 regulates phenotypic switching of vascular smooth muscle cells through plasma membrane potential-dependent coupling with PTEN. FASEB J. 2019, 33, 9785–9796. [Google Scholar] [CrossRef]
- Bates, E. Ion Channels in Development and Cancer. Annu. Rev. Cell Dev. Biol. 2015, 31, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Role of Membrane Potential in the Regulation of Cell Proliferation and Differentiation. Stem Cell Rev. Rep. 2009, 5, 231–246. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Hassani Nia, F.; Stauber, T. Ion Channels and Transporters in Muscle Cell Differentiation. Int. J. Mol. Sci. 2021, 22, 13615. https://doi.org/10.3390/ijms222413615
Chen L, Hassani Nia F, Stauber T. Ion Channels and Transporters in Muscle Cell Differentiation. International Journal of Molecular Sciences. 2021; 22(24):13615. https://doi.org/10.3390/ijms222413615
Chicago/Turabian StyleChen, Lingye, Fatemeh Hassani Nia, and Tobias Stauber. 2021. "Ion Channels and Transporters in Muscle Cell Differentiation" International Journal of Molecular Sciences 22, no. 24: 13615. https://doi.org/10.3390/ijms222413615
APA StyleChen, L., Hassani Nia, F., & Stauber, T. (2021). Ion Channels and Transporters in Muscle Cell Differentiation. International Journal of Molecular Sciences, 22(24), 13615. https://doi.org/10.3390/ijms222413615






