Lytic Polysaccharide Monooxygenase from Talaromyces amestolkiae with an Enigmatic Linker-like Region: The Role of This Enzyme on Cellulose Saccharification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cloning, Production, and Purification of TamAA9A
2.2. Comparative Physicochemical Properties of TamAA9A Variants. Circular Dichroism and Thermostability
2.3. Substrate Specificity and Binding to Cellulose
2.4. Phylogeny, and Homology Modeling of TamAA9A
2.5. PASC and CMC Degradation Analysis by HPAEC-PAD
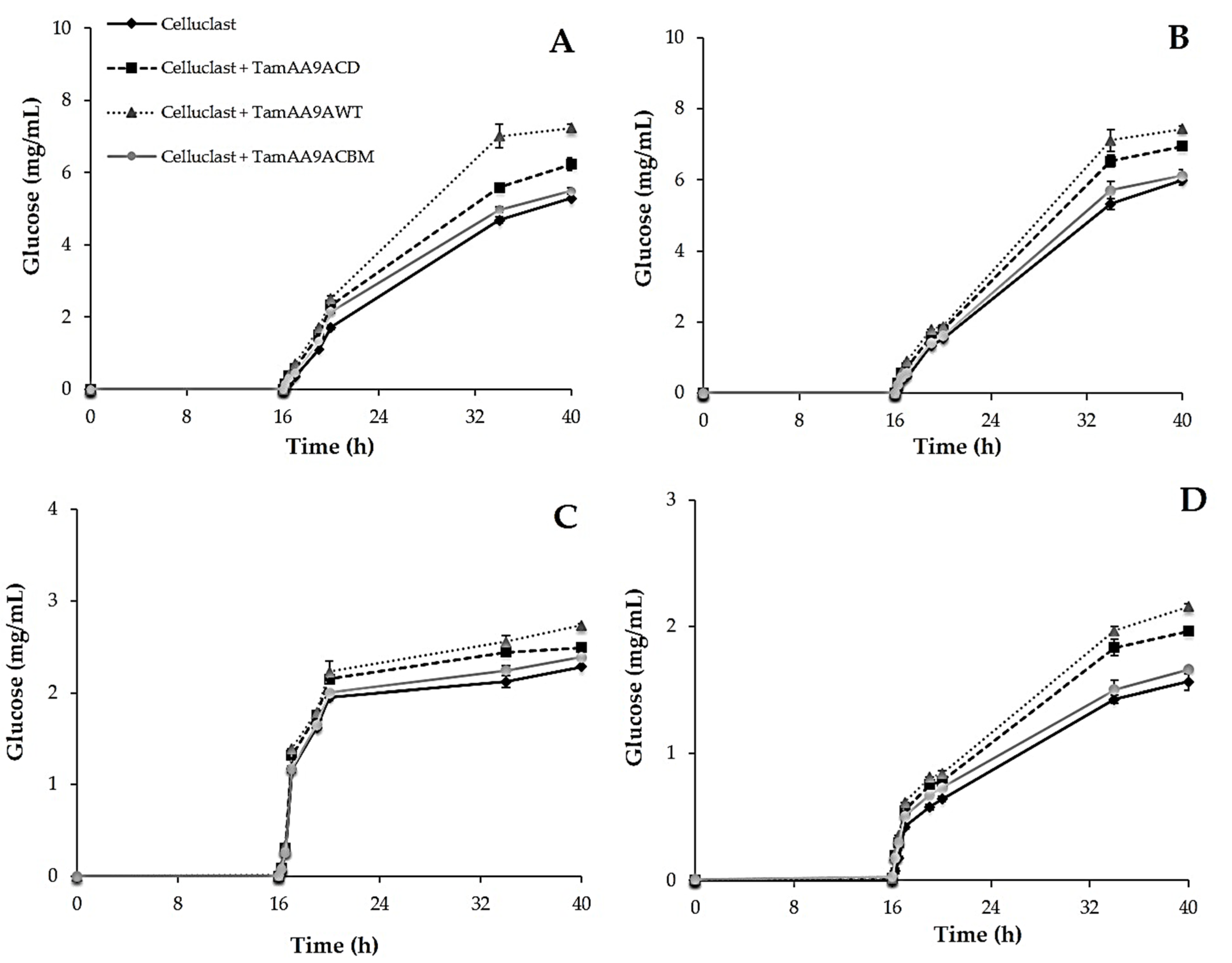
2.6. Effect of TamAA9A in Saccharification of Cellulosic Substrates
3. Materials and Methods
3.1. Microorganism and Culture Conditions
3.2. Identification of the T. amestolkiae LPMO Gene, Nucleic Acid Isolation, Cloning and Expression in P. pastoris
3.3. Production and Purification of LPMO
3.4. Protein Quantification, Enzyme Assays and Substrate Specificity
3.5. Physicochemical Properties. Cellulose Binding. Circular Dichroism
3.6. Phylogenetic and Protein Structure Analyses of the T. amestolkiae LPMO
3.7. Analysis of Degradation Products from Cellulose by High-Performance Anion-Exchange Chromatography Coupled with Pulsed Amperometric Detection (HPAEC-PAD)
3.8. Saccharification of Lignocellulosic Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, K.A.; Zhao, L.; Emptage, M. Bioethanol. Curr. Opin. Chem. Biol. 2006, 10, 141–146. [Google Scholar] [CrossRef]
- Martínez, Á.T.; Ruiz-Dueñas, F.J.; Martínez, M.J.; del Río, J.C.; Gutiérrez, A. Enzymatic delignification of plant cell wall: From nature to mill. Curr. Opin. Biotechnol. 2009, 20, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [Green Version]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Reese, E.T.; Siu, R.G.H.; Levinson, H.S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J. Bacteriol. 1950, 59, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.T. How to break down crystalline cellulose. Science 2016, 352, 1050–1051. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [Green Version]
- Aachmann, F.L.; Sørlie, M.; Skjåk-Bræk, G.; Eijsink, V.G.H.; Vaaje-Kolstad, G. NMR structure of a lytic polysaccharide monooxygenase provides insight into copper binding, protein dynamics, and substrate interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 18779–18784. [Google Scholar] [CrossRef] [Green Version]
- Bissaro, B.; Røhr, Å.K.; Müller, G.; Chylenski, P.; Skaugen, M.; Forsberg, Z.; Horn, S.J.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [Google Scholar] [CrossRef]
- Rieder, L.; Petrović, D.; Väljamäe, P.; Eijsink, V.G.H.; Sørlie, M. Kinetic Characterization of a Putatively Chitin-Active LPMO Reveals a Preference for Soluble Substrates and Absence of Monooxygenase Activity. ACS Catal. 2021, 11, 11685–11695. [Google Scholar] [CrossRef]
- Westereng, B.; Loose, J.S.M.; Vaaje-Kolstad, G.; Aachmann, F.L.; Sørlie, M.; Eijsink, V.G.H. Analytical Tools for Characterizing Cellulose-Active Lytic Polysaccharide Monooxygenases (LPMOs). In Cellulases: Methods and Protocols; Lübeck, M., Ed.; Springer: New York, NY, USA, 2018; pp. 219–246. ISBN 978-1-4939-7877-9. [Google Scholar]
- Frommhagen, M.; Westphal, A.H.; van Berkel, W.J.H.; Kabel, M.A. Distinct Substrate Specificities and Electron-Donating Systems of Fungal Lytic Polysaccharide Monooxygenases. Front. Microbiol. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Kracher, D.; Scheiblbrandner, S.; Felice, A.K.G.; Breslmayr, E.; Preims, M.; Ludwicka, K.; Haltrich, D.; Eijsink, V.G.H.; Ludwig, R. Extracellular electron transfer systems fuel cellulose oxidative degradation. Science 2016, 352, 1098–1101. [Google Scholar] [CrossRef]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M.; et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [Green Version]
- Crouch, L.I.; Labourel, A.; Walton, P.H.; Davies, G.J.; Gilbert, H.J. The Contribution of Non-catalytic Carbohydrate Binding Modules to the Activity of Lytic Polysaccharide Monooxygenases. J. Biol. Chem. 2016, 291, 7439–7449. [Google Scholar] [CrossRef] [Green Version]
- Forsberg, Z.; Sørlie, M.; Petrović, D.; Courtade, G.; Aachmann, F.L.; Vaaje-Kolstad, G.; Bissaro, B.; Røhr, Å.K.; Eijsink, V.G.H. Polysaccharide degradation by lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 2019, 59, 54–64. [Google Scholar] [CrossRef]
- Rani Singhania, R.; Dixit, P.; Kumar Patel, A.; Shekher Giri, B.; Kuo, C.-H.; Chen, C.-W.; Di Dong, C. Role and significance of lytic polysaccharide monooxygenases (LPMOs) in lignocellulose deconstruction. Bioresour. Technol. 2021, 335, 125261. [Google Scholar] [CrossRef]
- Eijsink, V.G.H.; Petrovic, D.; Forsberg, Z.; Mekasha, S.; Røhr, Å.K.; Várnai, A.; Bissaro, B.; Vaaje-Kolstad, G. On the functional characterization of lytic polysaccharide monooxygenases (LPMOs). Biotechnol. Biofuels 2019, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, Y.; Zheng, Y.; Hsieh, Y.S.Y. Recent Advances in Screening Methods for the Functional Investigation of Lytic Polysaccharide Monooxygenases. Front. Chem. 2021, 9, 119. [Google Scholar] [CrossRef]
- Breslmayr, E.; Hanžek, M.; Hanrahan, A.; Leitner, C.; Kittl, R.; Šantek, B.; Oostenbrink, C.; Ludwig, R. A fast and sensitive activity assay for lytic polysaccharide monooxygenase. Biotechnol. Biofuels 2018, 11, 79. [Google Scholar] [CrossRef]
- Kittl, R.; Kracher, D.; Burgstaller, D.; Haltrich, D.; Ludwig, R. Production of four Neurospora crassa lytic polysaccharide monooxygenases in Pichia pastoris monitored by a fluorimetric assay. Biotechnol. Biofuels 2012, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- de Eugenio, L.I.; Méndez-Líter, J.A.; Nieto-Domínguez, M.; Alonso, L.; Gil-Muñoz, J.; Barriuso, J.; Prieto, A.; Martínez, M.J. Differential β-glucosidase expression as a function of carbon source availability in Talaromyces amestolkiae: A genomic and proteomic approach. Biotechnol. Biofuels 2017, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, A.; de Eugenio, L.; Méndez-Líter, J.A.; Nieto-Domínguez, M.; Murgiondo, C.; Barriuso, J.; Bejarano-Muñoz, L.; Martínez, M.J. Fungal glycosyl hydrolases for sustainable plant biomass valorization: Talaromyces amestolkiae as a model fungus. Int. Microbiol. 2021, 24, 545–558. [Google Scholar] [CrossRef]
- Méndez-Líter, J.A.; de Eugenio, L.I.; Nieto-Domínguez, M.; Prieto, A.; Martínez, M.J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresour. Technol. 2021, 324, 124623. [Google Scholar] [CrossRef]
- Vaishnav, N.; Singh, A.; Adsul, M.; Dixit, P.; Sandhu, S.K.; Mathur, A.; Puri, S.K.; Singhania, R.R. Penicillium: The next emerging champion for cellulase production. Bioresour. Technol. Rep. 2018, 2, 131–140. [Google Scholar] [CrossRef]
- Méndez-Líter, J.A.; Gil-Muñoz, J.; Nieto-Domínguez, M.; Barriuso, J.; de Eugenio, L.I.; Martínez, M.J. A novel, highly efficient β-glucosidase with a cellulose-binding domain: Characterization and properties of native and recombinant proteins. Biotechnol. Biofuels 2017, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Líter, J.A.; de Eugenio, L.I.; Prieto, A.; Martínez, M.J. The β-glucosidase secreted by Talaromyces amestolkiae under carbon starvation: A versatile catalyst for biofuel production from plant and algal biomass. Biotechnol. Biofuels 2018, 11, 123. [Google Scholar] [CrossRef]
- Méndez-Líter, J.A.; Nieto-Domínguez, M.; Fernández de Toro, B.; González Santana, A.; Prieto, A.; Asensio, J.L.; Cañada, F.J.; de Eugenio, L.I.; Martínez, M.J. A glucotolerant β-glucosidase from the fungus Talaromyces amestolkiae and its conversion into a glycosynthase for glycosylation of phenolic compounds. Microb. Cell Fact. 2020, 19, 127. [Google Scholar] [CrossRef]
- Nieto-Domínguez, M.; Prieto, A.; Fernández de Toro, B.; Cañada, F.J.; Barriuso, J.; Armstrong, Z.; Withers, S.G.; de Eugenio, L.I.; Martínez, M.J. Enzymatic fine-tuning for 2-(6-hydroxynaphthyl) β-d-xylopyranoside synthesis catalyzed by the recombinant β-xylosidase BxTW1 from Talaromyces amestolkiae. Microb. Cell Fact. 2016, 15, 171. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Domínguez, M.; Fernández de Toro, B.; de Eugenio, L.I.; Santana, A.G.; Bejarano-Muñoz, L.; Armstrong, Z.; Méndez-Líter, J.A.; Asensio, J.L.; Prieto, A.; Withers, S.G.; et al. Thioglycoligase derived from fungal GH3 β-xylosidase is a multi-glycoligase with broad acceptor tolerance. Nat. Commun. 2020, 11, 4864. [Google Scholar] [CrossRef]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Várnai, A.; Ishida, T.; Sunagawa, N.; Petrovic, D.M.; Igarashi, K.; Jellison, J.; Goodell, B.; Alfredsen, G.; Westereng, B.; et al. A Lytic Polysaccharide Monooxygenase with Broad Xyloglucan Specificity from the Brown-Rot Fungus Gloeophyllum trabeum and Its Action on Cellulose-Xyloglucan Complexes. Appl. Environ. Microbiol. 2016, 82, 6557–6572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, H.; Karkehabadi, S.; Mikkelsen, N.; Douglas, N.R.; Kim, S.; Lam, A.; Kaper, T.; Kelemen, B.; Meier, K.K.; Jones, S.M.; et al. High-resolution structure of a lytic polysaccharide monooxygenase from Hypocrea jecorina reveals a predicted linker as an integral part of the catalytic domain. J. Biol. Chem. 2017, 292, 19099–19109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquero, M.E.; Barriuso, J.; Medrano, F.J.; Prieto, A.; Martínez, M.J. Heterologous expression of a fungal sterol esterase/lipase in different hosts: Effect on solubility, glycosylation and production. J. Biosci. Bioeng. 2015, 120, 637–643. [Google Scholar] [CrossRef]
- Petrović, D.M.; Bissaro, B.; Chylenski, P.; Skaugen, M.; Sørlie, M.; Jensen, M.S.; Aachmann, F.L.; Courtade, G.; Várnai, A.; Eijsink, V.G.H. Methylation of the N-terminal histidine protects a lytic polysaccharide monooxygenase from auto-oxidative inactivation. Protein Sci. 2018, 27, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Breslmayr, E.; Daly, S.; Požgajčić, A.; Chang, H.; Rezić, T.; Oostenbrink, C.; Ludwig, R. Improved spectrophotometric assay for lytic polysaccharide monooxygenase. Biotechnol. Biofuels 2019, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Macauley-Patrick, S.; Fazenda, M.L.; McNeil, B.; Harvey, L.M. Heterologous protein production using the Pichia pastoris expression system. Yeast 2005, 22, 249–270. [Google Scholar] [CrossRef]
- Liu, B.; Olson, Å.; Wu, M.; Broberg, A.; Sandgren, M. Biochemical studies of two lytic polysaccharide monooxygenases from the white-rot fungus Heterobasidion irregulare and their roles in lignocellulose degradation. PLoS ONE 2017, 12, e0189479. [Google Scholar] [CrossRef]
- Maruyama, N.; Katsube, T.; Wada, Y.; Oh, M.H.; Barba De La Rosa, A.P.; Okuda, E.; Nakagawa, S.; Utsumi, S. The roles of the N-linked glycans and extension regions of soybean beta-conglycinin in folding, assembly and structural features. Eur. J. Biochem. 1998, 258, 854–862. [Google Scholar] [CrossRef]
- Frommhagen, M.; Westphal, A.H.; Hilgers, R.; Koetsier, M.J.; Hinz, S.W.A.; Visser, J.; Gruppen, H.; van Berkel, W.J.H.; Kabel, M.A. Quantification of the catalytic performance of C1-cellulose-specific lytic polysaccharide monooxygenases. Appl. Microbiol. Biotechnol. 2018, 102, 1281–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.K.; Blossom, B.M.; Russo, D.A.; van Oort, B.; Croce, R.; Jensen, P.E.; Felby, C.; Bjerrum, M.J. Thermal unfolding and refolding of a lytic polysaccharide monooxygenase from Thermoascus aurantiacus. RSC Adv. 2019, 9, 29734–29742. [Google Scholar] [CrossRef] [Green Version]
- Kadowaki, M.A.S.; Várnai, A.; Jameson, J.-K.; Leite, A.E.T.; Costa-Filho, A.J.; Kumagai, P.S.; Prade, R.A.; Polikarpov, I.; Eijsink, V.G.H. Functional characterization of a lytic polysaccharide monooxygenase from the thermophilic fungus Myceliophthora thermophila. PLoS ONE 2018, 13, e0202148. [Google Scholar] [CrossRef]
- Agrawal, D.; Basotra, N.; Balan, V.; Tsang, A.; Chadha, B.S. Discovery and Expression of Thermostable LPMOs from Thermophilic Fungi for Producing Efficient Lignocellulolytic Enzyme Cocktails. Appl. Biochem. Biotechnol. 2020, 191, 463–481. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Zhang, Y.; Feng, D.; Hou, S.; Guo, W.; Niu, K.; Jiang, Y.; Han, L.; Sindhu, L.; et al. Identification of a thermostable fungal lytic polysaccharide monooxygenase and evaluation of its effect on lignocellulosic degradation. Appl. Microbiol. Biotechnol. 2019, 103, 5739–5750. [Google Scholar] [CrossRef]
- Tuveng, T.R.; Jensen, M.S.; Fredriksen, L.; Vaaje-Kolstad, G.; Eijsink, V.G.H.; Forsberg, Z. A thermostable bacterial lytic polysaccharide monooxygenase with high operational stability in a wide temperature range. Biotechnol. Biofuels 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Wang, J.; Yu, Z. Truncation of the cellulose binding domain improved thermal stability of endo-beta-1,4-glucanase from Bacillus subtilis JA18. Bioresour. Technol. 2009, 100, 345–349. [Google Scholar] [CrossRef]
- Forsberg, Z.; Mackenzie, A.K.; Sørlie, M.; Røhr, Å.K.; Helland, R.; Arvai, A.S.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structural and functional characterization of a conserved pair of bacterial cellulose-oxidizing lytic polysaccharide monooxygenases. Proc. Natl. Acad. Sci. USA 2014, 111, 8446–8451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arfi, Y.; Shamshoum, M.; Rogachev, I.; Peleg, Y.; Bayer, E.A. Integration of bacterial lytic polysaccharide monooxygenases into designer cellulosomes promotes enhanced cellulose degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 9109–9114. [Google Scholar] [CrossRef] [Green Version]
- Borisova, A.S.; Isaksen, T.; Dimarogona, M.; Kognole, A.A.; Mathiesen, G.; Várnai, A.; Røhr, Å.K.; Payne, C.M.; Sørlie, M.; Sandgren, M.; et al. Structural and Functional Characterization of a Lytic Polysaccharide Monooxygenase with Broad Substrate Specificity. J. Biol. Chem. 2015, 290, 22955–22969. [Google Scholar] [CrossRef] [Green Version]
- Chalak, A.; Villares, A.; Moreau, C.; Haon, M.; Grisel, S.; d’Orlando, A.; Herpoël-Gimbert, I.; Labourel, A.; Cathala, B.; Berrin, J.-G. Influence of the carbohydrate-binding module on the activity of a fungal AA9 lytic polysaccharide monooxygenase on cellulosic substrates. Biotechnol. Biofuels 2019, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Vaaje-Kolstad, G.; Forsberg, Z.; Loose, J.S.M.; Bissaro, B.; Eijsink, V.G.H. Structural diversity of lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 2017, 44, 67–76. [Google Scholar] [CrossRef]
- Danneels, B.; Tanghe, M.; Desmet, T. Structural Features on the Substrate-Binding Surface of Fungal Lytic Polysaccharide Monooxygenases Determine Their Oxidative Regioselectivity. Biotechnol. J. 2019, 14, e1800211. [Google Scholar] [CrossRef]
- Olusola, O.; Anmoldeep, R.; Mayank, G.; Chandra, K.V.; Kumar, V.P.; Shams, Y.S.; Maia, K. Synergistic Action of a Lytic Polysaccharide Monooxygenase and a Cellobiohydrolase from Penicillium funiculosum in Cellulose Saccharification under High-Level Substrate Loading. Appl. Environ. Microbiol. 2021, 86, e01769-20. [Google Scholar]
- Semenova, M.V.; Gusakov, A.V.; Telitsin, V.D.; Rozhkova, A.M.; Kondratyeva, E.G.; Sinitsyn, A.P. Purification and characterization of two forms of the homologously expressed lytic polysaccharide monooxygenase (PvLPMO9A) from Penicillium verruculosum. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140297. [Google Scholar] [CrossRef]
- Müller, G.; Várnai, A.; Johansen, K.S.; Eijsink, V.G.H.; Horn, S.J. Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol. Biofuels 2015, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Song, Y.; Kim, H.M.; Bae, H.-J. Enhanced lignocellulosic biomass hydrolysis by oxidative lytic polysaccharide monooxygenases (LPMOs) GH61 from Gloeophyllum trabeum. Enzym. Microb. Technol. 2015, 77, 38–45. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Katoh, K.; Kuma, K.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [Green Version]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Cui, J.; Lynd, L.R.; Kuang, L.R. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef]

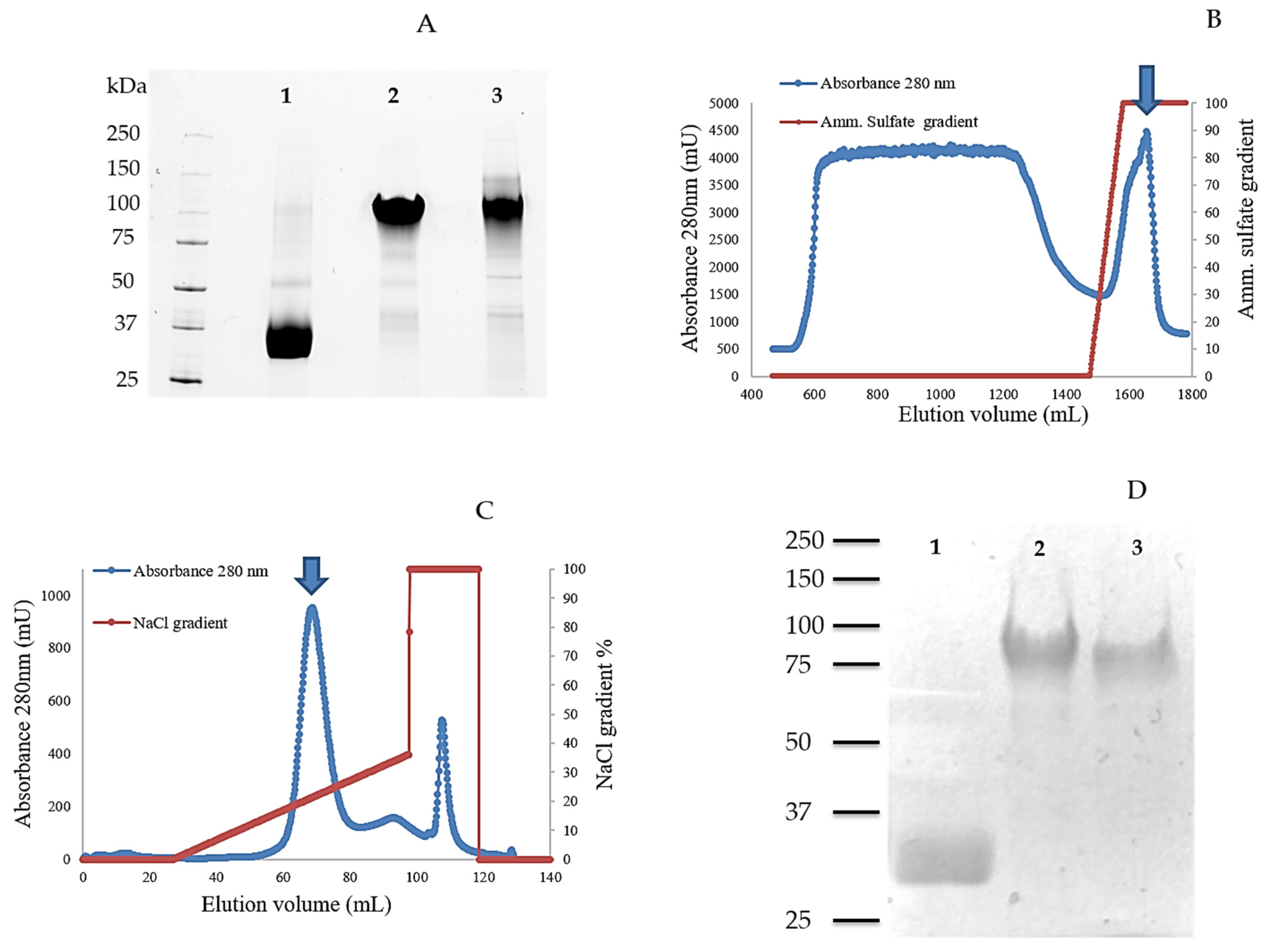

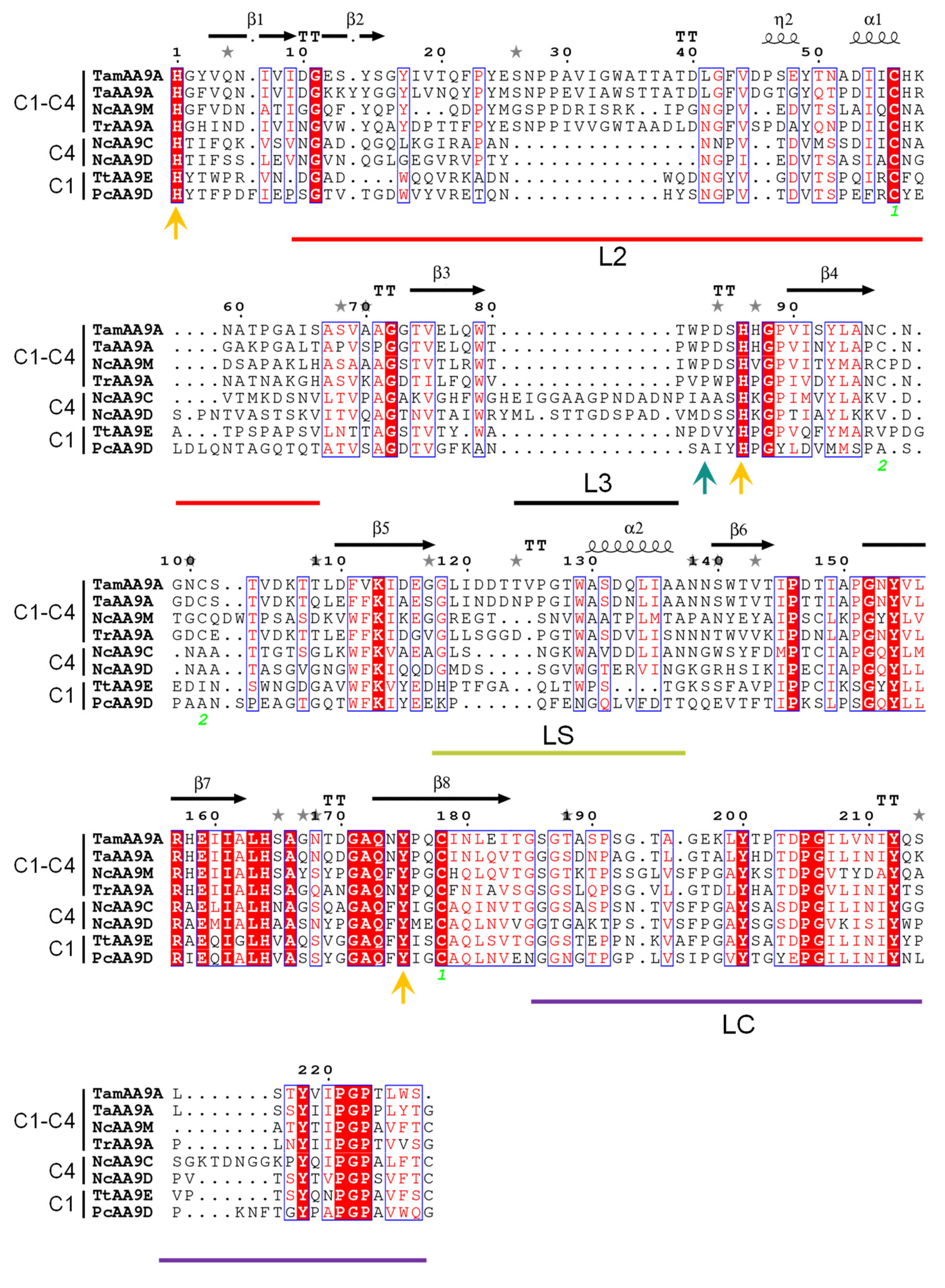
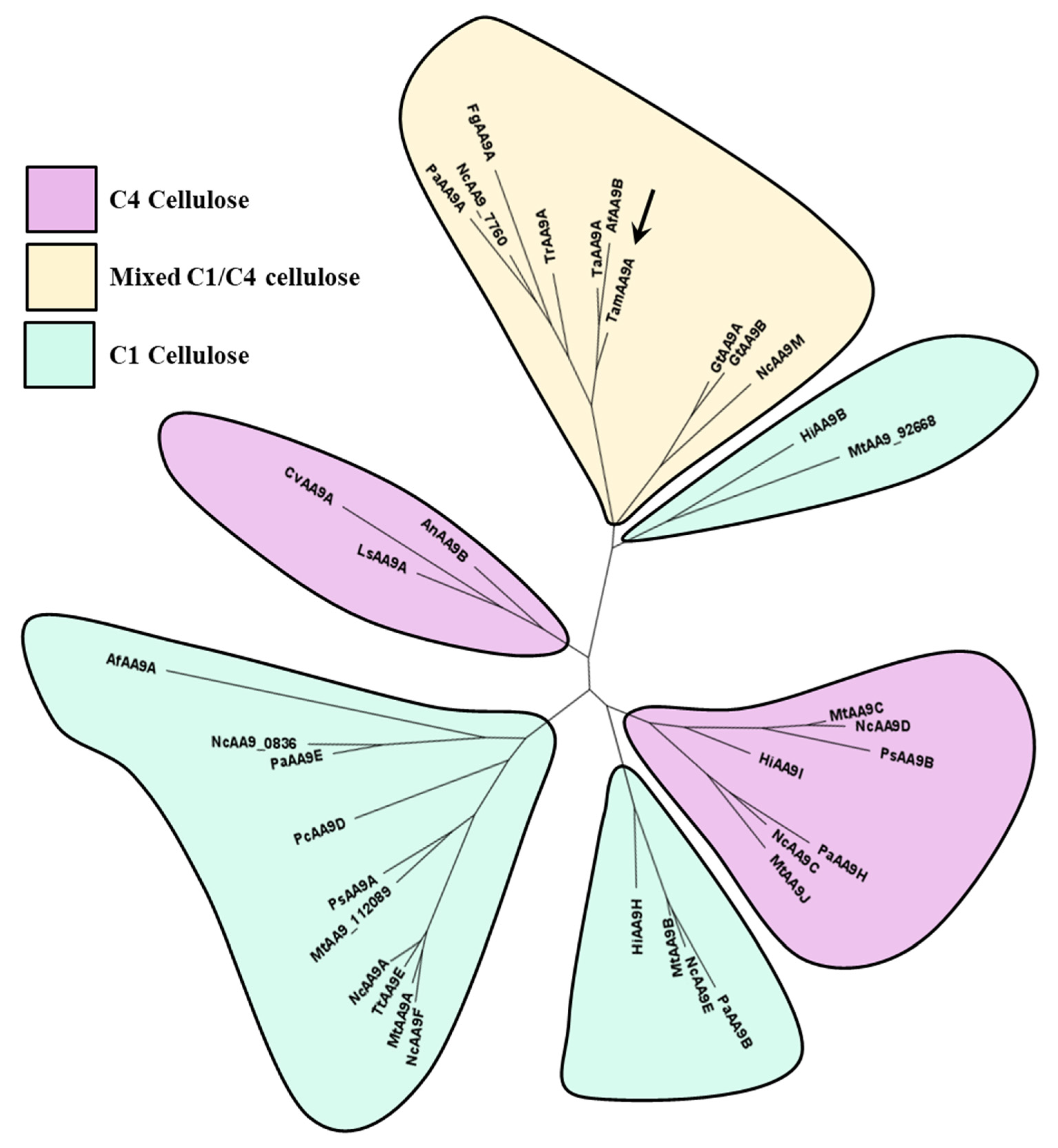
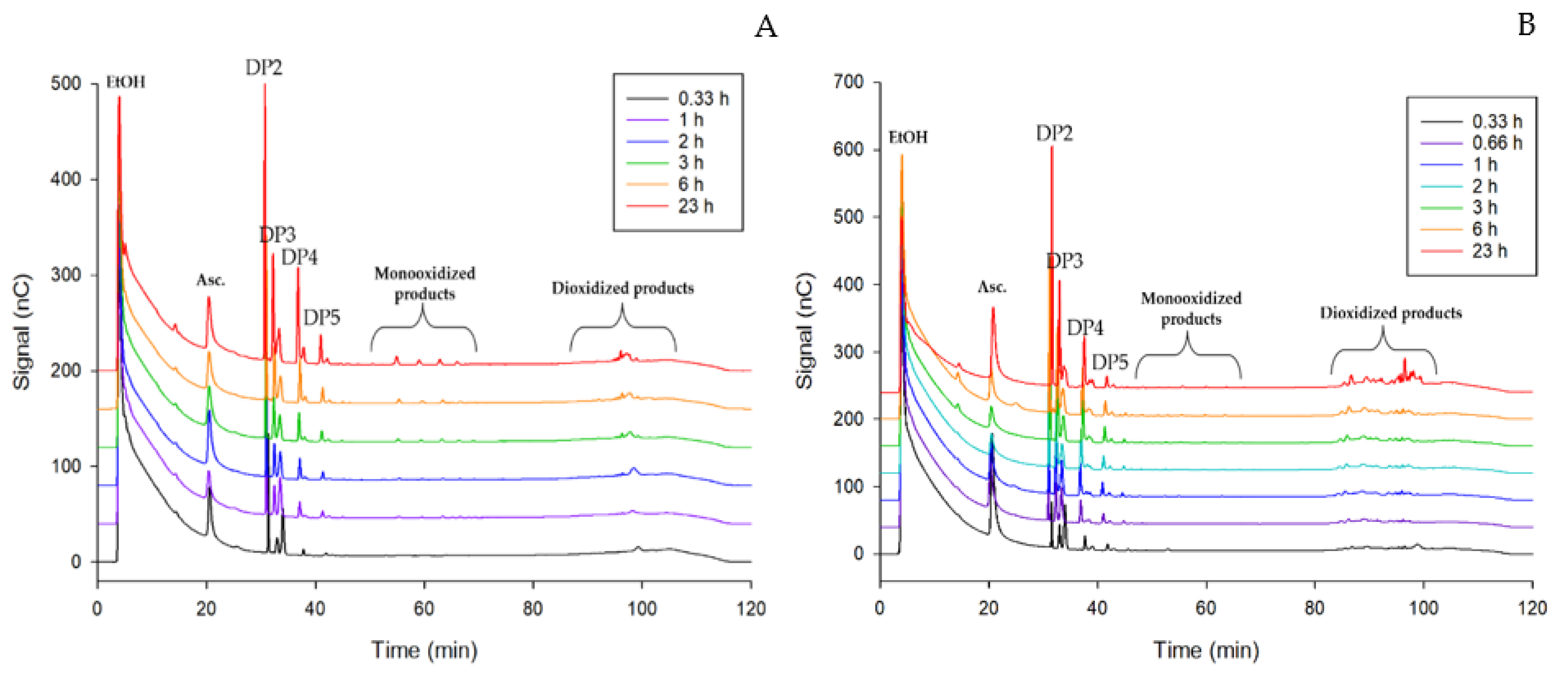
| Enzyme | Substrate | Km (mM) | kcat (s−1) | kcat/Km (s−1/mM) |
|---|---|---|---|---|
| TamAA9A-WT | 2,6-DMP | 109.8 ± 0.5 | 0.68 ± 0.03 | 0.006 |
| H2O2 | 0.28 ± 0.01 | 0.14 ± 0.01 | 0.501 | |
| TamAA9A-CD | 2,6-DMP | 87.7 ± 0.7 | 0.96 ± 0.04 | 0.011 |
| H2O2 | 0.31 ± 0.02 | 0.17 ± 0.01 | 0.548 | |
| TamAA9A-CBM | 2,6-DMP | 85.68 ± 0.7 | 0.53 ± 0.02 | 0.003 |
| H2O2 | 1.03 ± 0.06 | 0.25 ± 0.03 | 0.135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Líter, J.A.; Ayuso-Fernández, I.; Csarman, F.; de Eugenio, L.I.; Míguez, N.; Plou, F.J.; Prieto, A.; Ludwig, R.; Martínez, M.J. Lytic Polysaccharide Monooxygenase from Talaromyces amestolkiae with an Enigmatic Linker-like Region: The Role of This Enzyme on Cellulose Saccharification. Int. J. Mol. Sci. 2021, 22, 13611. https://doi.org/10.3390/ijms222413611
Méndez-Líter JA, Ayuso-Fernández I, Csarman F, de Eugenio LI, Míguez N, Plou FJ, Prieto A, Ludwig R, Martínez MJ. Lytic Polysaccharide Monooxygenase from Talaromyces amestolkiae with an Enigmatic Linker-like Region: The Role of This Enzyme on Cellulose Saccharification. International Journal of Molecular Sciences. 2021; 22(24):13611. https://doi.org/10.3390/ijms222413611
Chicago/Turabian StyleMéndez-Líter, Juan Antonio, Iván Ayuso-Fernández, Florian Csarman, Laura Isabel de Eugenio, Noa Míguez, Francisco J. Plou, Alicia Prieto, Roland Ludwig, and María Jesús Martínez. 2021. "Lytic Polysaccharide Monooxygenase from Talaromyces amestolkiae with an Enigmatic Linker-like Region: The Role of This Enzyme on Cellulose Saccharification" International Journal of Molecular Sciences 22, no. 24: 13611. https://doi.org/10.3390/ijms222413611
APA StyleMéndez-Líter, J. A., Ayuso-Fernández, I., Csarman, F., de Eugenio, L. I., Míguez, N., Plou, F. J., Prieto, A., Ludwig, R., & Martínez, M. J. (2021). Lytic Polysaccharide Monooxygenase from Talaromyces amestolkiae with an Enigmatic Linker-like Region: The Role of This Enzyme on Cellulose Saccharification. International Journal of Molecular Sciences, 22(24), 13611. https://doi.org/10.3390/ijms222413611







