Recent Insights into Human Endometrial Peptidases in Blastocyst Implantation via Shedding of Microvesicles
Abstract
1. Introduction
2. Endometrial Change during Decidualization
2.1. Changes in ESCs during Decidualization
2.2. Changes in EECs during Decidualization
2.2.1. Apocrine and Holocrine Secretions from EECs during the Implantation Window
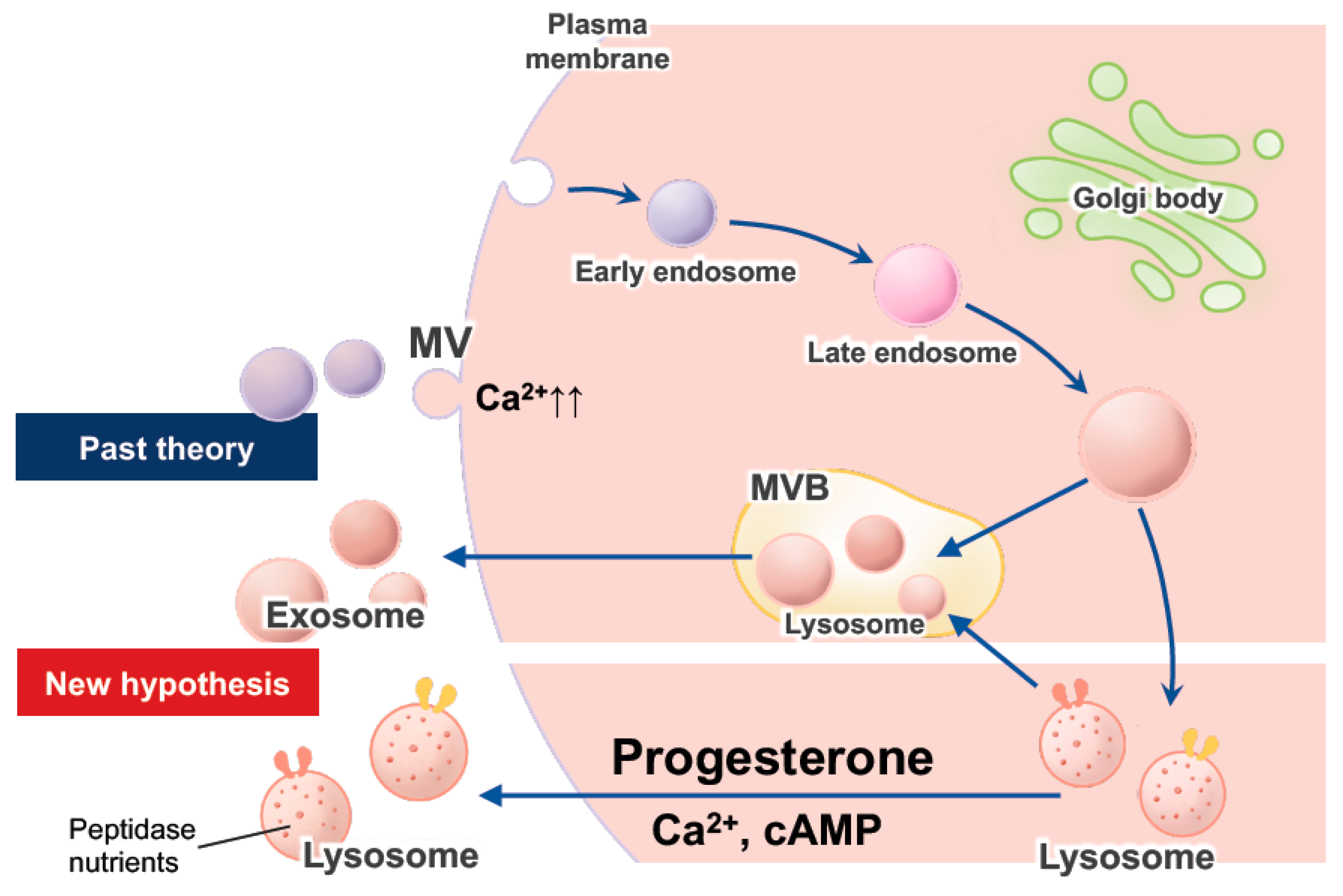
2.2.2. Exocytosis of Lysosomes, Exosomes, and EVs in EECs
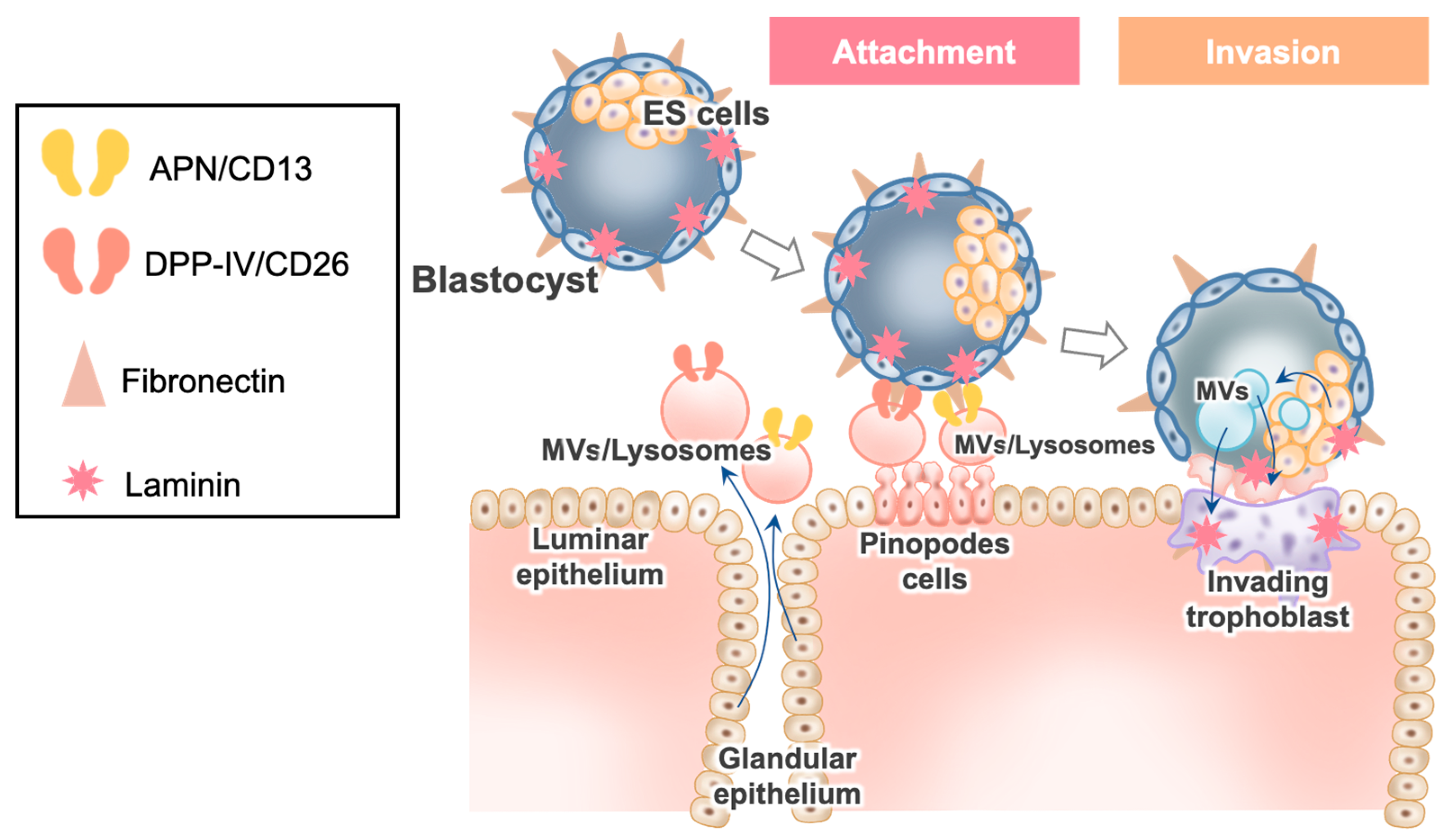
3. Implantation
3.1. Fibronectin in Blastocysts
3.2. Dynamic Aspects of EECs during the Implantation Window and Their Relation to APs
3.3. Interaction of Blastocyst Fibronectin with the Molecules in EVs/Lysosomes Derived from EECs
3.4. DPPIV/CD26 in Implantation
3.5. APN/CD13 in Implantation
3.6. Blastocyst’s EV Uptake in EECs after Interaction of Blastocyst Fibronectin with two Peptidases, EVs Derived from EECs and ES cells, and Blastocyst Attachment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mizutani, S.; Matsumoto, K.; Kato, Y.; Mizutani, E.; Mizutani, H.; Iwase, A.; Shibata, K. New insights into human endometrial aminopeptidases in both implantation and menstruation. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140332. [Google Scholar] [CrossRef]
- Ruiz-González, I.; Xu, J.; Wang, X.; Burghardt, R.C.; Dunlap, K.A.; Bazer, F.W. Exosomes, endogenous retroviruses and toll-like receptors: Pregnancy recognition in ewes. Reproduction 2015, 149, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, 40476. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Murphy, C.R.; Day, M.L. Extracellular matrix proteins secreted from both the endometrium and the embryo are required for attachment: A study using a co-culture model of rat blastocysts and Ishikawa cells. J. Morphol. 2013, 274, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Maeda, M.; Fujiwara, H.; Kariya, M.; Takakura, K.; Kanzaki, H.; Mori, T. Dipeptidyl peptidase IV as a differentiation marker of the human endometrial glandular cells. Hum. Reprod. 1992, 7, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Ando, H.; Furugori, K.; Kajiyama, H.; Suzuki, M.; Iwase, A.; Mizutani, S.; Kikkawa, F. Possible involvement of crosstalk cell-adhesion mechanism by endometrial CD26/dipeptidyl peptidase IV and embryonal fibronectin in human blastocyst implantation. Mol. Hum. Reprod. 2006, 12, 491–495. [Google Scholar] [CrossRef]
- Aplin, J.D.; Ruane, P.T. Embryo-epithelium interactions during implantation at a glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef]
- Möckl, L. The emerging role of the mammalian glycocalyx in functional membrane organization and immune system regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef]
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef]
- Whiteman, E.L.; Liu, C.; Fearon, E.R.; Margolis, B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 2008, 27, 3875–3879. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C.; Maleysson, E.; Canis, M.; Mage, G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J. Clin. Endocrinol. Metab. 2010, 95, 3437–3445. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Hwang, K.; Choi, K. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J. Steroid Biochem. Mol. Biol. 2016, 158, 1–8. [Google Scholar] [CrossRef]
- van der Horst, P.H.; Wang, Y.; Vandenput, I.; Kühne, L.C.; Ewing, P.C.; van Ijcken, W.F.J.; van der Zee, M.; Amant, F.; Burger, C.W.; Blok, L.J. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS ONE 2012, 7, e30840. [Google Scholar] [CrossRef]
- Zuo, L.; Li, W.; You, S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Res. 2010, 12, R34. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.D. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995, 154, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Dsupin, B.A.; Irwin, J.C. Steroid and peptide regulation of insulin-like growth factor-binding proteins secreted by human endometrial stromal cells is dependent on stromal differentiation. J. Clin. Endocrinol. Metab. 1992, 75, 1235–1241. [Google Scholar] [CrossRef]
- Tebbs, C.; Pratten, M.K.; Pipkin, F.B. Angiotensin II is a growth factor in the peri-implantation rat embryo. J. Anat. 1999, 195, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Pijacka, W.; Hunter, M.G.; Pipkin, F.B.; Luck, M.R. Expression of renin-angiotensin system components in the early bovine embryo. Endocr. Connect. 2012, 1, 22–30. [Google Scholar] [CrossRef]
- Han, H.J.; Heo, J.S.; Lee, Y.J. ANG II increases 2-deoxyglucose uptake in mouse embryonic stem cells. Life Sci. 2005, 77, 1916–1933. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.W. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000, 10, 316–321. [Google Scholar] [CrossRef]
- Rodríguez, A.; Webster, P.; Ortego, J.; Andrews, N.W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 1997, 137, 93–104. [Google Scholar] [CrossRef]
- Pillay, C.S.; Elliott, E.; Dennison, C. Endolysosomal proteolysis and its regulation. Biochem. J. 2002, 363, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Luzio, J.P.; Hackmann, Y.; Dieckmann, N.M.G.; Griffiths, G.M. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol. 2014, 6, a016840. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dammer, E.B.; Ren, R.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Grondin, G.; Beaudoin, A.R. Immunocytochemical and cytochemical demonstration of a novel selective lysosomal pathway (SLP) of secretion in the exocrine pancreas. J. Histochem. Cytochem. 1996, 44, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, S.; Yoshimura, Y.; Nagamatsu, S.; Sawa, H.; Hanashi, H.; Oda, T.; Katsumata, Y.; Koyama, N.; Nakamura, Y. Expression of beta 1 integrins in human endometrial stromal and decidual cells. J. Clin. Endocrinol. Metab. 1996, 81, 1533–1540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turpeenniemi-Hujanen, T.; Feinberg, R.F.; Kauppila, A.; Puistola, U. Extracellular matrix interactions in early human embryos: Implications for normal implantation events. Fertil. Steril. 1995, 64, 132–138. [Google Scholar] [CrossRef]
- Klentzeris, L.D. Adhesion molecules in reproduction. Br. J. Obstet. Gynaecol. 1997, 104, 401–409. [Google Scholar] [CrossRef]
- Bartosch, C.; Lopes, J.M.; Beires, J.; Sousa, M. Human endometrium ultrastructure during the implantation window: A new perspective of the epithelium cell types. Reprod. Sci. 2011, 18, 525–539. [Google Scholar] [CrossRef]
- Lenzen, R.; Stark, P.; Kolb-Bachofen, V.; Strohmeyer, G. Glucagon effect on intracellular proteolysis and pericanalicular location of hepatocyte lysosomes in isolated perfused guinea pig livers. Hepatology 1995, 21, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Bentin-Ley, U. Relevance of endometrial pinopodes for human blastocyst implantation. Hum. Reprod. 2000, 15 (Suppl. 6), 67–73. [Google Scholar] [CrossRef][Green Version]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.; Paule, S.G.; Li, Y.; Rombauts, L.J.; Vollenhoven, B.; Salamonsen, L.A.; Nie, G. Posttranslational removal of α-dystroglycan N terminus by PC5/6 cleavage is important for uterine preparation for embryo implantation in women. FASEB J. 2015, 29, 4011–4022. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.T.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: Insights into endometrial-embryo interactions. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef]
- George, E.L.; Georges-Labouesse, E.N.; Patel-King, R.S.; Rayburn, H.; Hynes, R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 1993, 119, 1079–1091. [Google Scholar] [CrossRef]
- Strieter, R.M.; Belperio, J.A.; Keane, M.P. Cytokines in innate host defense in the lung. J. Clin. Investig. 2002, 109, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kühn-Wache, K.; Manhart, S.; Hoffmann, T.; Hinke, S.A.; Gelling, R.; Pederson, R.A.; McIntosh, C.H.; Demuth, H.U. Analogs of glucose-dependent insulinotropic polypeptide with increased dipeptidyl peptidase IV resistance. Adv. Exp. Med. Biol. 2000, 477, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Abdel-Ghany, M.; Pauli, B.U. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J. Biol. Chem. 2003, 278, 24600–24607. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, S.; Goto, K.; Itakura, A.; Furuhashi, M.; Kurauchi, O.; Kikkawa, F.; Tomoda, Y. Physiological role of placental proteases: Interaction between pregnancy-induced bioactive peptides and proteases. Endocr. J. 1994, 41, S93–S104. [Google Scholar] [CrossRef]
- Corti, A.; Curnis, F. Isoaspartate-dependent molecular switches for integrin-ligand recognition. J. Cell Sci. 2011, 124, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Y.; Chen, L.; Lin, Y.; Li, F. A unified mechanism for aminopeptidase N-based tumor cell motility and tumor-homing therapy. J. Biol. Chem. 2014, 289, 34520–34529. [Google Scholar] [CrossRef]
- Ghosh, M.; Lo, R.; Ivic, I.; Aguilera, B.; Qendro, V.; Devarakonda, C.; Shapiro, L.H. CD13 tethers the IQGAP1-ARF6-EFA6 complex to the plasma membrane to promote ARF6 activation, β1 integrin recycling, and cell migration. Sci. Signal. 2019, 12, eaav5938. [Google Scholar] [CrossRef]
- Su, R.W.; Fazleabas, A.T. Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. Adv. Anat. Embryol. Cell Biol. 2015, 216, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.W.; Yao, S.N.; Powell, L.C.; Griffiths, S.; Berquand, A.; Piasecki, T.; Howe, W.; Gazze, A.S.; Farach-Carson, M.C.; Constantinou, P.; et al. Highly glycosylated MUC1 mediates high affinity L-selectin binding at the human endometrial surface. J. Nanobiotechnol. 2021, 19, 50. [Google Scholar] [CrossRef]
- Aplin, J.D.; Spanswick, C.; Behzad, F.; Kimber, S.J.; Vićovac, L. Integrins beta 5, beta 3 and alpha v are apically distributed in endometrial epithelium. Mol. Hum. Reprod. 1996, 2, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Apparao, K.B.; Murray, M.J.; Fritz, M.A.; Meyer, W.R.; Chambers, A.F.; Truong, P.R.; Lessey, B.A. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J. Clin. Endocrinol. Metab. 2001, 86, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.S.; Xuan, J.W.; Hota, C.; Chambers, A.F.; Harris, J.F. Inhibition of Arg-Gly-Asp (RGD)-mediated cell adhesion to osteopontin by a monoclonal antibody against osteopontin. J. Biol. Chem. 1994, 269, 23280–23285. [Google Scholar] [CrossRef]
- Qu, H.; Brown, L.F.; Dvorak, H.F.; Dvorak, A.M. Ultrastructural immunogold localization of osteopontin in human gastric mucosa. J. Histochem. Cytochem. 1997, 45, 21–33. [Google Scholar] [CrossRef]
- Lessey, B.A. Adhesion molecules and implantation. J. Reprod. Immunol. 2002, 55, 101–112. [Google Scholar] [CrossRef]
- Iwamoto, R.; Higashiyama, S.; Mitamura, T.; Taniguchi, N.; Klagsbrun, M.; Mekada, E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994, 13, 2322–2330. [Google Scholar] [CrossRef]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; Milito, A.D.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, H.M.; Masoumi, N.; Witwer, K.W.; Pegtel, D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. 2016, 80, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Shui, L.J.; Meng, Y.; Huang, C.; Qian, Y.; Liu, J.Y. Aminopeptidase N expression in the endometrium could affect endometrial receptivity. Biochem. Biophys. Res. Commun. 2019, 514, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.T.; Simpson, R.J.; Salamonsen, L.A.; Greening, D.W. Extracellular vesicles in the intrauterine environment: Challenges and potential functions. Biol. Reprod. 2016, 95, 109. [Google Scholar] [CrossRef] [PubMed]
- Armant, D.R.; Kaplan, H.A.; Lennarz, W.J. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev. Biol. 1986, 116, 519–523. [Google Scholar] [CrossRef]
- Turpeenniemi-Hujanen, T.; Rönnberg, L.; Kauppila, A.; Puistola, U. Laminin in the human embryo implantation: Analogy to the invasion by malignant cells. Fertil. Steril. 1992, 58, 105–113. [Google Scholar] [CrossRef]
- Denker, H.W. Implantation: A cell biological paradox. J. Exp. Zool. 1993, 266, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Bischof, P.; Campana, A. A model for implantation of the human blastocyst and early placentation. Hum. Reprod. Update 1996, 2, 262–270. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshihara, M.; Mizutani, S.; Kato, Y.; Matsumoto, K.; Mizutani, E.; Mizutani, H.; Fujimoto, H.; Osuka, S.; Kajiyama, H. Recent Insights into Human Endometrial Peptidases in Blastocyst Implantation via Shedding of Microvesicles. Int. J. Mol. Sci. 2021, 22, 13479. https://doi.org/10.3390/ijms222413479
Yoshihara M, Mizutani S, Kato Y, Matsumoto K, Mizutani E, Mizutani H, Fujimoto H, Osuka S, Kajiyama H. Recent Insights into Human Endometrial Peptidases in Blastocyst Implantation via Shedding of Microvesicles. International Journal of Molecular Sciences. 2021; 22(24):13479. https://doi.org/10.3390/ijms222413479
Chicago/Turabian StyleYoshihara, Masato, Shigehiko Mizutani, Yukio Kato, Kunio Matsumoto, Eita Mizutani, Hidesuke Mizutani, Hiroki Fujimoto, Satoko Osuka, and Hiroaki Kajiyama. 2021. "Recent Insights into Human Endometrial Peptidases in Blastocyst Implantation via Shedding of Microvesicles" International Journal of Molecular Sciences 22, no. 24: 13479. https://doi.org/10.3390/ijms222413479
APA StyleYoshihara, M., Mizutani, S., Kato, Y., Matsumoto, K., Mizutani, E., Mizutani, H., Fujimoto, H., Osuka, S., & Kajiyama, H. (2021). Recent Insights into Human Endometrial Peptidases in Blastocyst Implantation via Shedding of Microvesicles. International Journal of Molecular Sciences, 22(24), 13479. https://doi.org/10.3390/ijms222413479





